Effect of Biofloc-based Diet on Hepatic Enzymes of Ctenopharyngodon idella Fingerlings
Effect of Biofloc-based Diet on Hepatic Enzymes of Ctenopharyngodon idella Fingerlings
Ghazala Nasreen1, Ali Hussain2, Komal Tayyab1, Muhammad Rashid3 and Sumaira Aslam1*
1Department of Zoology, Government College Women University, Faisalabad, Pakistan
2Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore, Pakistan
3Faculty of Fisheries and Wildlife, University of Veterinary and Animal Sciences, Lahore, Pakistan
ABSTRACT
The impact of bioflocs on the hepatic enzymes under variable diet and water exchange conditions was investigated in the fingerlings of Ctenopharyngodon idella in this study. For this purpose, the fingerlings of C. idella were fed on different concentrations of bioflocs bacteria enriched on banana peels) under different diet treatments for a period of 50 days. The diet treatments devised in this study were, T1 (bioflocs only with zero water exchange), T2 (bioflocs only with 10 % weekly water exchange), T3 (bioflocs + 50 % commercial diet and with zero water exchange) and T4 (bioflocs + 50 % commercial diet and with 10 % weekly water exchange). A control experiment C (commercial diet with daily water exchange) was run parallel. At the end of the experimental trial, the cultured fish were processed for the determination of hepatosomatic indices, levels of total soluble proteins and hepatic enzymes being marker of hepatic injury for all the treatment and control groups. The highest levels of acid phosphatase (ACP, 11.75 ± 0.23 IU L−1), alkaline phosphatase (ALP, 81.50 ± 1.49 IU L−1), aspartate aminotransferase (AST, 30.25 ± 1.62 IU L−1) and superoxide dismutase (SOD, 19.75 ± 1.78 IU L−1) were recorded in the liver samples of the fingerlings of the treatment group T1, while the lowest corresponding figures were noted for the fingerlings of the treatment group T4. These values were lower than found in the control group having 10.50±0.23, 77.25 ± 1.19, 24.50 ± 0.96 and 18.25± 1.19 IU L−1 levels for the ACP, ALP, AST and SOD, respectively. The hyperproduction of the hepatic enzymes revealed dysfunction of the livers in the fingerlings of the treatment group T1. However, the moderate production of hepatic enzymes, elevated levels of total soluble proteins (8073.00 ± 848 mg mL−1) and high hepatosomatic indices (0.913) for the fingerlings of the treatment group T4, thus, declare the productive utility of bioflocs-amended commercial fish feed with 10 % weekly water exchange to achieve optimum growth of the grass carp.
Article Information
Received 27 June 2020
Revised 05 March 2022
Accepted 22 March 2022
Available online 20 May 2022
(early access)
Published 21 December 2022
Authors’ Contribution
GN and KT conducted the research and compiled the results. AH analyzed the data and wrote the manuscript. MR critically reviewed the article. SA supervised the study.
Key words
Agro-industrial waste valorization, Aquaculture biotechnology, Biofloc technology, Fish nutrition, Hepatosomatic indices, Liver enzymes
DOI: https://dx.doi.org/10.17582/journal.pjz/20200627110650
* Corresponding author: [email protected]
0030-9923/2023/0002-707 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Increasing demand of proteins to meet nutritional needs of rapidly increasing human population urges economical, innovative and sustainable technologies for the production of proteinaceous diets. Proteins make a very important part of the fish meal for their necessary roles in growth maintenance and reproduction. Essential and non-essential amino acids both are critical in building body tissues of the fish. Non-essential amino acids are synthesized from nitrogen source present in the dietary proteins of the fish, while essential amino acids must be supplied in fish diet. However, protein levels must be managed to raise healthy fish and achieve optimum production (Pelczar et al., 1993).
A major requirement for an efficient fish culture is the assimilation of dietary proteins to the formation of tissue proteins, growth and repair rather than dissimilation to the energy (Weatherley and Gill, 1987; Jauncey, 1998). The activity of the enzymes involved in energy metabolism has been found to be linked to the growth rates in fish (Ahmad et al., 2012). Variation in the concentration levels of liver enzymes-Acid phosphatase (ACP), alkaline phosphatase (ALP); aspartate aminotransferase (AST0, superoxide dismutase (SOD) is considered as important index of growth and metabolic disturbances (Ahmad et al., 2012; Mir et al., 2016). The effects of dietary nutrients on liver metabolism have been studied in different perspectives. Dietary carbohydrates are found to be directly linked to the lipid contents of the liver (Zhou et al., 2013). For instance, lipid vacuoles of larger size and peripheral nuclei in hepatocytes were found in livers of juvenile Labeo rohita and fry fed on non-gelatinized and gelatinized carbohydrates, respectively (Mohapatra et al., 2003; Kumar et al., 2005; Ahmad et al., 2012). The pathological effects of nutrition may result in structural modifications of nuclei within hepatocytes (Caballero et al., 1999). Limited ability of the fish to digest and metabolize nutrients may result in nutritional problems such as poor feed consumption and hence poor growth when they are taken in excess (Hemre et al., 2002). Maximum activity of ACP enzymes under the effects of dietary proteins in fingerlings of Labeo rohita was observed when a diet with optimum (20.54 mg) protein was fed (Ahmad et al., 2012).
The biofloc (BF) technology is an advanced and innovative approach for improved aquaculture production and involves the use of microbial aggregates as a rich source of protein. The technology is mainly based on the principle of waste nutrients recycling, in particular nitrogen, into microbial biomass that can be used in situ by the cultured animals (Avnimelech, 2009; Kuhn et al., 2010). Heterotrophic microorganisms are allowed to grow by maintaining the C/N ratio in the water through the modification of the carbohydrate content in the feed or by the addition of an external C source in the water (Avnimelech, 1999), so that the bacteria can assimilate the waste ammonium for new biomass production. Hence, ammonium/ammonia can be maintained at a low and non-toxic concentration so that water replacement is no longer required (Bossier and Ekasari, 2017).
Beneficial effects of BFT on fish growth, water quality, liver condition, digestive enzymes, and immunity have been studied in tilapia (Avnimelech, 2007), common carp (Najdegerami et al., 2016), rahu (Verma et al., 2016) and catfish (Ekasari et al., 2015). The bioflocs are reported to increase the growth performance by stimulating digestive enzyme activities (Xu et al., 2012), and improving the immune response (Kim et al., 2016).
Nutritional contribution of the BF-based diets to the metabolic functions and enzymes of the fish is very scarce. Pradhan and Das (2015) reported a marked effect of blue green algae (Microcystis aeruginosa) supplemented diet on ALP levels of the carp liver and its survival rate. The higher ALP levels indicate the higher reserved energy breakdown utilized for growth and survival of fish. A recent study by Najdegerami et al. (2016) reported significantly improved hepatocellular quantification and health of the liver of common carp fed on diets partially replaced with BFs. Thus, the present study was designed to determine the effect of BFs utilizing agri-wastes as C source, on the liver functions of the grass carp (Ctenopharyngodon idella) and to optimize the BF concentration for healthy liver functions.
MATERIALS AND METHODS
Production of BFs
Microorganisms for the development of BFs were enriched on the medium containing banana peels as carbon source. For this purpose, 20 g of dried and finely ground banana peels were weighed on electrical balance and added into 1 L distilled water in screw capped glass bottles to obtain a 2% (w/v) concentration. The pH was adjusted to 8. The medium containing bottle was then autoclaved at 121°C under pressure 15 psi for 15 min. The sterile medium was then inoculated with 1 g of soil sample (obtained from the fish pond) under hygienic conditions. An air pipe provided with a cotton plug to supply sterile oxygen into the medium bottle was used for the continuous aeration of the medium. This medium was incubated at room temperature for 10 days for the enrichment of heterotrophic microorganisms growing on banana peels. All the experiments for enrichment culture development were performed in triplicates.
The experiment was then up-scaled to the fiberglass aquaria (90 L capacity) for the production of BFs. Each aquarium was filled with 60 L water and provided with the banana peels’ powder (2 %). Then 500 mL of the microbial growth was taken from the previously enriched microbial culture and inoculated to each of the aquaria allocated for the treatments, T1, T2, T3 and T4. The control group was kept un-inoculated and without the addition of any feed ingredients. The BF growth was further developed for 30 days in these fiber glass aquaria before stocking the fish. The neutral to alkaline pH was maintained during this period. Two air pipe aerators attached with hollow plastic tubes were used for the rich and continuous aeration of the aquaria. The C/N ratio of 15:1 was maintained for the experiment. All of the experiments were performed in triplicates. Once the bacterial counts reached upto 108 to 109 C.F.U. mL−1 in the aquaria, the fish feed and water exchange were managed according to the required treatment conditions.
Experimental trial
The experiment was conducted to find the effect of BF-supplemented fish feed on the liver metabolic enzymes of the grass carp fingerlings under different experimental conditions. The experiment was performed in Microbiology and Biotechnology Laboratory, Department of Zoology, Government College Women University, Faisalabad, Pakistan. The experiment was comprised of 4 different feeding treatments, T1, T2, T3 and T4 (Table I) on the basis of diet and water exchange and compared against the control group utilizing commercial fish feed. Fingerlings of the C. idella were obtained from the Fish Seed Hatchery, Faisalabad, Pakistan. The fish were stocked in a fibreglass aquarium for 15 days for acclimation and adaption to the new environment. After acclimation for 15 days, 7 fish individuals were distributed in each of the 5 aquaria representing 4 experimental and 1 control groups. The feeding and water exchange conditions for these rearing groups were maintained as described in Table I. All the experiments for this purpose were conducted in triplicates and maximally for a period of 50 days at room temperature. The aquaria were equipped with aerators to maintain maximum availability of oxygen for the fish throughout the experimental trial.
Determination of HSI
At the beginning of the experiment, the fish were weighed following 24-h starvation. At the termination of the experiment, the fish from each experiment were weighed again following 24-h starvation. The fish were then dissected, their livers were weighed and the HSI were calculated by the following formula:
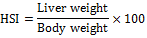
The liver samples were then placed in clean plastic vials and stored in a freezer till further use.
Fish liver enzyme activity estimation
The frozen liver samples were homogenized finely and the resulting supernatant was used for measurement of subsequent enzymes’ activity as described by Jaroli and Sharma (2005). The effect of control and BF-based feeds on the liver of C. idella fingerlings was thus determined by monitoring different enzymes in the liver homogenate.
For the estimation of ACP and ALP activity, the methods proposed by the Jaffe and Badansky (1943) were followed. The ACP and ALP activity was calculated as:
ACP activity (IU L−1) = 122 A
ALP activity (IU L−1) = 3300 A
Where, A= Change in absorbance per minute (Jaffe and Badansky, 1943).
The AST activity was estimated using Randox kits (Reitman and Frankel, 1957). The AST activity was calculated as:
AST activity (nm min−1)= 1746 × ΔA 340 nm min−1
Where; 1746 = Extinction coefficient; ΔA 340 nm min−1 = Change in absorbance per minute for the homogenate sample.
The hepatic SOD activity was measured by the xanthine oxides method (Fridovich, 1974). The assay is based on the competitive use of superoxide ions (generated by XOD during the conversion of xanthene into hydrogen peroxide and uric acid) by SOD. The level of TSPs in the supernatant was measured by the method as described by Lowry et al. (1951).
Statistical analysis
Statistical analysis of the data was performed through analysis of variance and students’ t- test using Prism 5 Software. Differences between means were considered significant at P ≤ 0.05.
RESULTS
Production of BFs
The microbial counts in the bioflocculated aquaria running under different feeding conditions were
Table I. Effect of four different diets (T1, T2, T3, T4) and water exchange on total and specific activity (IU L-1) of different hepatic enzymes in the livers of C. idellla fingerlings.
|
Control |
T1 |
T2 |
T3 |
T4 |
|
|
HSI |
0.50±0.03 |
0.44±0.07 |
0.54±0.09 |
0.48±0.05 |
0.91±0.06*** |
|
ACP |
10.50±0.23 (0.0009) |
11.75±0.23** (0.0013) |
10.50±0.35 (0.0003) |
11.00±0.21 (0.0009) |
10.00±0.12 (0.0015) |
|
ALP |
77.50±1.22 (0.0341) |
81.50±1.49 (0.0776) |
77.25±1.19 (0.0096) |
80.75±1.71 (0.0389) |
75.5±0.94 (0.0497) |
|
AST |
24.50±0.96 (0.0118) |
30.25±1.62* (0.0199) |
24.50±1.21 (0.003) |
26.25±1.78 (0.0116) |
22.00±2.90 (0.0210) |
|
SOD |
18.25±1.29 (0.0120) |
19.75±1.78 (0.0188) |
16.00±2.88 (0.0070) |
19.50±1.98 (0.0024) |
13.25±2.37 (0.0064) |
|
TSP |
2075.04±60 |
1049.99±107**** |
2269.92±777 |
1517.88±405 |
8073.00±848**** |
Values are Mean ± SEM of replicates (n=21) and are significantly different from the control value of respective parameter in the row. Significance levels are *, P≤ 0.05; **, P≤ 0.01; ***, P≤ 0.001; ****, P≤ 0.0001.
HIS, histosomatic index; ACP (IU L−1), acid phosphatase (IU L−1); ALP, alkaline phosphatase (IU L−1); SOD (IU L−1), superoxide dismutase; TSP (mg mL−1), total soluble proteins. Values in parentheses are specific activity (IU L-1) of the respective enzymes. Composition of feed: Bacteria (CFU mL−1): 102 in control and 108 to 109 in all treated feeds. Biofloc volume: 0.2 mL L−1 in all treatment groups. Commercial feed (%): 5% of body weight twice daily in control; 2.5% of body weight thrice in a week in T3 and T4. Water exchange (%): 90 % every second day throughout the experiment in control; 0 % in T1 and T3, 10% in T2 and T4 weekly. Composition of commercial feed (%): Fish meal, 12; Protein, 38; Rice polish, 12; whaet flour, 10, fish oil, 6; vitamin premix, 1; minerals,1; ascorbic acid, 1; chromic oxide, 1
determined and are presented in Table I. The bacterial counts recorded from the aquaria kept for different experimental diet treatments (T1, T2, T3 and T4), appeared almost the same and were in the range of 108−109 C.F.U. mL−1. The bacterial counts in all the experimental groups were significantly higher as compared to those of the control group (102) utilizing commercial protein fish feed only. The bioflocs were comprised of heterotrophic bacteria of the genera Alcaligenes, Halomonas, Enterobacter and Pseudomonas (unpublished data).
Determination of HSI
The body weight gain by the fingerlings of C. idella differed significantly among different diet treatments and the maximum weight was achieved by the fingerlings feeding on the BFs only diet with 10 % weekly water exchange (T2). However, the value of HSI appeared maximum (0.913) for the fingerlings reared in T4. This value was significantly higher than those obtained for the treatment groups T1, T2, T3 and the control group (Table I).
Among the different treatments T1, T2, T3 and T4, the hepatic enzymes levels were found lowest in the liver samples of the fingerlings reared in the T4 group feeding on the mixture of BFs and commercial diet with 10% weekly water exchange. The mean values of 10.00 ± 0.12 ,75.50±0.94 and 22.00±2.9 IU per liter were recorder for the ACP, ALP and AST, respectively. The SOD levels were also found minimum ((13.25 ± 2.37 IU L−1) in the treatment group T4.
The treatment group T1 showed maximum values of the hepatic enzymes when compared with the all other three treatment groups T2, T3, T4 and the control group (C). The highest ACP levels found in the liver samples of the fingerlings reared on BFs only with zero water exchange (T1) were 11.75 ± 0.23 IU L−1, while 81.50 ± 1.49, 30.25 ± 1.62 and 19.75 ± 1.78 IU of the ALP, AST and SOD per liter were recorded. The hepatic enzymes in the livers of the fingerlings reared in the treatment T3 feeding on the mixture of BFs and commercial diet with zero water exchange were comparable with the T4 and showed 11 ± 0.21, 80.75 ± 1.71, 19.50 ± 1.98 IU of the ACP, ALP and SOD per liter, respectively. The AST values were also higher than those of the fingerlings reared treatmnets T2, T4 and control groups.
The treatment group T2 showed hepatic responses in the liver comparable with the control C group fingerlings and gave 10.50±0.35 and 10.50±0.23 for ACP, 77.50 ± 1.22 and 77.25 ± 1.19 IU L−1 levels of ALP, 24.50 ± 2.13 and 24.50 ± 0.96 IU L−1 for AST. The SOD levels were, however, lower in T2 than in the control group.
The specific activity (U mg−1 of protein) of the ACP is also presented in Table I. Maximum specific activity of ACP was shown by the fingerlings reared in the treatment group T4 showing specific activity of 0.0015 U mg−1 of the TSPs, while the minimum specific activity (0.0009 U mg−1) was given by the fingerlings reared in the control group.
The maximum specific activity of ALP was given by the fingerlings reared in the treatment group T1 with 0.0776 U mg−1 of the TSPs, while the minimum specific activity (0.0096 U mg−1 of protein) was given by the fingerlings reared in treatment group T2. The ALP specific activities of the treatment groups T3 and T4 were 0.0389 and 0.0497 U mg−1 of the protein, respectively (Table I).
The maximum specific activity of AST (0.0210 U mg−1) was shown by the fingerlings reared in the treatment group T4, while the minimum specific activity (0.0030 U mg−1 of protein) was shown by the fingerlings reared in treatment group T2. The AST specific activities of the groups T3, T4 and C were 0.0116, 0.0210 and 0.0118 U mg−1 of the protein, respectively (Table I).
The maximum specific activity of SOD (0.0188 U mg−1 of protein) was shown by the fingerlings reared in the treatment group T1, while the minimum specific activity (0.0024 U mg−1 of protein) was shown by the fingerlings reared in treatment group T3. The SOD specific activities of the study groups T2, T4 and C were 0.0070, 0.0064 and 0.0120 U mg−1 of the protein, respectively (Table I).
Estimation of TSPs
The level of TSPs varied significantly among different diet treatments (Table I). The levels of TSPs found in the liver samples of the fingerlings for the study treatments T1, T2, T3, T4 and C were 1049.99 ± 107, 2269.92 ± 777, 1517.88 ± 405, 8073.00 ± 848 and 2075.04 ± 60 mg mL−1 (Table I).
DISCUSSION
The impact of BF supplemented feed on the production of hepatic enzymes in C. idella fingerlings was evaluated in this study. The higher carbon to nitrogen ratios in the BF system bring about the enrichment of beta-polyhydroxyalkanoates accumulating bacteria. These PHB containing heterotrophic bacteria are not only known for bioflocculant-production but also exert antibacterial activity against pathogens (Russel, 1992; Salehizadeh et al., 2001; Kumaresan et al., 2002; Sinha et al., 2008; Emerenciano et al., 2013). These attributes of the biofloc community support them to be employed in a sustainable aquaculture.
The present study’s results revealed that the values of HSI differed significantly among all the feeding groups. The highest value of HSI was noted for the treatment group T4. The size of an organ proportional to the body weight is determined by taking the relation of the organ to body weight. Wilber and Gilchrist (1965) suggested the use of these ratios a valuable criterion in evaluating the correlation between certain experimental conditions and the biological response of a particular organism. The liver is the main organ performing various metabolic processes and detoxification. Its ability to regenerate makes it unique among the vital organs of the body. Organ weight is a direct response of an experimental condition thus making it most sensitive indicator. Significant differences in an animal’s organ weight may occur under different experimental conditions even when no apparent morphological changes are observed (Bailey et al., 2004). This makes the organ weight analysis an important parameter to find the effect of an experimental diet. Afuang et al. (2003) correlated HSI range of 1.5−2.7 with incorporation of body lipids which is greatly influenced by the dietary components. Thus, a lipid-rich diet gives a higher HSI owing to its relation to the lipid contents (Ogunji et al., 2008). These reports clearly explain the lower levels of HSI when a protein-rich diet is used as represented by the present study results (0.436−0.913) of the C. idella fingerlings feeding on protein-rich microbial diet.
The ACP and ALP levels in the liver samples of the fingerlings belonging to the different treatment groups were non-significantly different from those of the control groups. These results are consistent with the findings of Mohapatra et al. (2014) where the levels of ACP and ALP were reduced in the probiotics-fed group of Labeo rohita as compared to the non-probiotics-fed group. For endoplasmic reticulum and plasma membrane, ALP is marker enzyme (Muhammad, 2007). Thus, it is an ectoenzyme of plasma membrane which is used to determine the plasma membrane integrity oftenly (Akanji et al., 1993; Shanhjahan et al., 2004). Its high levels in the serum and tissue indicate damage to the external cell boundaries. Elevated levels of ALP may enhance the chances of membrane damage because it is an enzyme bounded to the membrane (Rao, 2006).
The AST activity in the present study ranged between 22-30 IU L−1 in all the study groups. The decreased level of the enzyme in the fingerlings of the treatment group T4 in comparison with the control and the remaining treatment groups is attributed to the balanced nutritional contents of the fish feed as reported by the Metón et al. (1999). Some other researchers, however, relate these differences in the activity of the AST to the fish species rather than diet compositions (Nagai and Ikeda, 1972; Cowey and Walton, 1989). Increased levels of AST and ALT in the Nile Tilapia and other fish species have been reported previously when the fish were fed with the probiotic-supplemented diets (El-Rhman et al., 2009; Harikrishnan et al., 2011). The activities of AST and ALT in the heart and liver tissues are markers for their functions and integrity (Adeniyi et al., 2010). They reorganize the proteins’ building blocks. These enzymes are reported to be released from the damaged liver tissue (Nelson and Cox, 2000). Increased levels in the serum are indications of cellular damage (necroting tissues). Myocardial infarction and increased risk of cardiovascular disease are attributed to increase of ALT and AST levels (Ioannou et al., 2006).
The SOD levels among different treatment groups ranged between 13.25−19.75 IU L−1 which were non-significantly different from those of the control group. Slightly higher levels of SOD in the fingerlings of the treatment groups T1, T2 and T3 as compared to those fed on BF-supplemented commercial diet indicated a light stress of the live microbial culture on the hepatocytes. Our results are supported by the previous findings where L. vannamei reared in a BF-based system showed improved physiological functions as reported by Becerra-Dórame et al. (2014). This improved performance was indicated by the SOD activity. Similarly, Jang et al. (2011) reported enhanced expression of an oxidase-activating enzyme (lvPPAE1) in hemocytes of L. vannamei reared for a long term in a BF-based system. The much higher level of TSPs was noted in the treatment group T4. The enhancement of TSPs in different cells under potentially suitable diet treatments has been reported by many researchers (Gupta and Sharma, 2016; Rajkumar et al., 2016; Kumar et al., 2017).
Conclusions and Recommendations
The present study arrived at the conclusion that utilization of BF-based fish diet in aquaculture is a promising approach to achieve much better results of the fish weight gain. Since higher levels of the hepatic enzymes are markers for the tissue damage thus lower levels of the liver enzymes in the treatment group T4 of the present study clearly demonstrate that the mixture of BFs and commercial diet is beneficial and economic for rearing of the grass carp fingerlings and fulfill needs of the nutrients for the carp culture. However, the highest levels of the hepatic enzymes in the treatment group T1 depicted hepatic stress and revealed that only BFs are not enough to support optimum growth of the grass carp. Our findings of the present study will be helpful for developing economical fish farming and valorization of agricultural wastes for the development of nutritional supplements (BFs) in aquaculture. Future studies on different other agro-industrial wastes like wheat straw, rice husk, rice straw, cotton sticks, maize waste, sugarcane bagasse and fruit wastes, are recommended for their potential utility in developing BFs. The microscopic examinations and the microbial community analyses of the BFs are further needed to be explored in future studies.
Acknowledgements
The support of Fish Seed Hatchery, Faisalabad, Pakistan for providing fingerlings of the grass carp (C. idella), is highly acknowledged.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Adeniyi, A.F., Adeleye, J.O. and Adeniyi, C.Y., 2010. Diabetes, sexual dysfunction and therapeutic exercise: A 20 years review. Curr. Diabetes Rev., 6: 201–206. https://doi.org/10.2174/157339910791658907
Afuang, W., Siddhuraju. P. and Becker, K., 2003. Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of moringa (Moringa oleifera Lam.) leaves on growth performance and feed utilization in Nile tilapia (Oreochromis niloticus L.). Aquacult. Res., 34: 1147–1159. https://doi.org/10.1046/j.1365-2109.2003.00920.x
Ahmad, M., Qureshi, T.A., Singh, A.B., Manohar, S., Borana, K. and Chalko, S.R., 2012. Effect of dietary protein, lipid and carbohydrate contents on the growth, feed efficiency and carcass composition of Cyprinus carpio communis fingerlings. Int. J. Farm. All. Sci., 4: 30–40.
Akanji, M.A., Olagoke, O.A. and Oloyede, O.B., 1993. Effect of chronic consumption of metabisulphite on the integrity of the kidney cellular system. Toxicology, 81: 173–179. https://doi.org/10.1016/0300-483X(93)90010-P
Avnimelech, Y., 1999. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture, 176: 227–235. https://doi.org/10.1016/S0044-8486(99)00085-X
Avnimelech, Y., 2007. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds, Aquaculture, 264: 1–4. https://doi.org/10.1016/j.aquaculture.2006.11.025
Avnimelech, Y., 2009. Biofloc technology-a practical guide book. The world Aquaculture Society, Baton Rouge.
Bailey, S.A., Zidell, R.H. and Perry, R.W., 2004. Relationship between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol. Pathol., 32: 448–466. https://doi.org/10.1080/01926230490465874
Becerra-Dórame, M.J., Martinez-Cordova, L.R., Martínez-Porchas, M., Hernández-López, J., Lopez-Elías, J.A. and Mendoza-Cano, F., 2014. Effect of using autotrophic and heterotrophic microbial based systems for the pre-grown of Litopenaeus vannamei, on the production performance and selected haemolymph parameters. Aquacult. Res., 45: 944–948. https://doi.org/10.1111/are.12033
Ekasari, J., Zairin, M., Putri, D.U., Sari, N.P., Surawidjaja, E.H., and Bossier, P., 2015. Biofloc-based reproductive performance of Nile tilapia Oreochromis niloticus L. broodstock. Aquacult. Res., 46: 509-512. https://doi.org/10.1111/are.12185
Bossier, P. and Ekasari, J., 2017. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol., 10: 1012–1016. https://doi.org/10.1111/1751-7915.12836
Caballero, M.J., López-Calero, G., Socorro, J., Roo, F.J., Izquierdo, M.S. and Fenández, A.J., 1999. Combined effect of lipid level and fish meal quality on liver histology of gilthead sea bream (Sparus aurata). Aquaculture, 179: 277–290. https://doi.org/10.1016/S0044-8486(99)00165-9
Cowey, C.B. and Walton, M.J., 1989. Intermediary metabolism. In: Fish nutrition (ed. J.E. Halver). Academic Press, San Diego, pp. 260–329.
El-Rhman, A.M.A., Khattab, Y.A.E., Adel, M.E. and Shalaby, A.M.E., 2009. Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia (Oreochromis niloticus). Fish Shellf. Immunol., 27: 175–180. https://doi.org/10.1016/j.fsi.2009.03.020
Emerenciano, M., Gaxiola, G. and Cuzon, G., 2013. Biofloc Technology (BFT): A review for aquaculture application and animal food industry. In: Biomass now cultivation and utilization (ed. M.D. Matovic). Intech Open. https://doi.org/10.5772/53902
Fridovich, I., 1974. Superoxide dismutase. In: Advances in enzymology and related areas of molecular biology (ed. A. Meister).
Gupta, A. and Sharma, B., 2016. Cypermethrin impact on total protein in muscle and liver of the freshwater fish Channa punctatus. Int. J. Adv. chem. Eng. biol. Sci., 3: 222–224. https://doi.org/10.15242/IJACEBS.AE0616302
Harikrishnan, R., Kim, M.C., Kim, J.S., Balasundaramb, C. and Heo, M.S., 2011. Immunomodulatory effect of probiotics enriched diets on Uronema marinum infected olive flounder. Fish Shellf. Immunol., 30: 964–971. https://doi.org/10.1016/j.fsi.2011.01.030
Hemre, G.I., Mommsen, T.P. and Krogdahl, Å., 2002. Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquacult. Nutr., 8: 175–219. https://doi.org/10.1046/j.1365-2095.2002.00200.x
Ioannou, G.N., Weiss, N.S., Boyko, E.J., Mozaffarian, D. and Lee, S.P., 2006. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology, 43: 1145–1151. https://doi.org/10.1002/hep.21171
Jaffe, H. L., and Bodansky, A., 1943. Diagnostic significance of serum alkaline and acid phosphatase values in relation to bone disease. Bull. N. Y. Acad. Med., 19: 831–848.
Jang, I.K., Pang, Z., Yu, J., Kim, S.K., Seo, H.C. and Cho, Y.R., 2011. Selectively enhanced expression of prophenoloxidase activating enzyme 1 (PPAE1) at a bacteria clearance site in the white shrimp, Litopenaeus vannamei. BMC Immunol., 12: 70–80. https://doi.org/10.1186/1471-2172-12-70
Jaroli, D. and Sharma, B., 2005. Effect of organophosphate insecticide on the organic constituents in liver of Channa punctatuus. Asian J. exp. Sci., 19: 121–129.
Jauncey, K., 1998. Tilapia feeds and feeding. Pisces Press Ltd. Stirling, Scotland, pp. 240–285.
Kim, Y.S., Sasaki, T., Awa, M., Inomata, M., Honryo, T., Agawa, Y. M. and Sawada, Y., 2016. Effect of dietary taurine enhancement on growth and development in red sea bream Pagrus major larvae. Aquacult. Res., 47: 1168-1179. https://doi.org/10.1111/are.12573
Kuhn, D.D., Lawrence, A.L., Boardman, G.D., Patnaik, S., Marsh, L. and Flick, G.J. Jr., 2010. Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp Litopenaeus vannamei. Aquaculture, 303: 28–33. https://doi.org/10.1016/j.aquaculture.2010.03.001
Kumar, A., Singh, S. and Sharma, H.N., 2017. Changes in total protein in liver and kidney of freshwater fish, Channa punctatus (Bloch.) after intoxication of carbaryl. J. Adv. Lab. Res. Biol., 8: 41–43.
Kumar, S., Sahu, N.P., Pal, A.K., Choudhury, D., Yengkokpam, S. and Mukherjee, S.C., 2005. Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish Shellf. Immunol., 19: 331–344. https://doi.org/10.1016/j.fsi.2005.03.001
Kumaresan, V. and Suryanarayanan, T.S., 2002. Endophyte assemblages in young, mature and senescent leaves of Rhizophora apiculata: Evidence for the role of endophytes in mangrove litter degradation. Fungal Divers., 9: 81-91.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. biol. Chem., 193: 265-275. https://doi.org/10.1016/S0021-9258(19)52451-6
Metón, I., Mediavilla, D., Caseras, A., Canto, E., Fernández, F. and Baanante, I.V., 1999. Effect of diet composition and ration size on key enzyme activities of glycolysis-gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of gilthead sea bream (Sparus aurata). Br. J. Nutr., 82: 223–232. https://doi.org/10.1017/S0007114599001403
Mir, G.H., Tharani, M., Hussain, A., Ahmad, Y. and Rashid, A., 2016. Variations in acid phosphatase (ACP) and alkaline phosphatase (ALP) activities in liver and kidney of a fresh water fish Labeo rohita exposed to heavy metal concentrations. Eur. J. Pharm. med. Res., 3: 398–401.
Mohapatra, M., Sahu, N.P. and Chaudhari, A., 2003. Utilization of gelatinized carbohydrate in diets of Labeo rohita fry. Aquacult. Nutr., 9: 189–196. https://doi.org/10.1046/j.1365-2095.2003.00243.x
Mohapatra, S., Chakraborty, T., Prusty, A.K., PaniPrasad, K. and Mohanta, K.N., 2014. Beneficial effects of dietary probiotics mixture on hemato-immunology and cell apoptosis of Labeo rohita fingerlings reared at higher water temperatures. PLoS One, 9: e100929. https://doi.org/10.1371/journal.pone.0100929
Muhammad, N.O., 2007. Studies on the nutritional and toxicological aspects of Terminalia catappa seeds fermented Aspergillus niger. Ph.D. thesis. University of llorin.
Nagai, M. and Ikeda, S., 1972. Carbohydrate metabolism in fish III. Effects of dietary composition of metabolism of glucose-U-14C and glutamate-U-14C in carp. Bull. Jap. Soc. Sci. Fish., 38: 137–143. https://doi.org/10.2331/suisan.38.137
Najdegerami, E.H., Bakhshi, F. and Lakani, F.B., 2016. Effects of biofloc on growth performance, digestive enzyme activities and liver histology of common carp (Cyprinus carpio L.) fingerlings in zero-water exchange system. Fish Physiol. Biochem., 42: 457–465. https://doi.org/10.1007/s10695-015-0151-9
Nelson, D.L. and Cox, M.M., 2000. Lehninger principles of biochemistry, 3rd Ed, Worth Publishers, New York.
Ogunji, J., Toor, R.U.A.S. and Schulz, C.K.W., 2008. Growth performance, nutrient utilization of Nile tilapia Oreochromis niloticus fed housefly maggot meal (magmeal) diets. Turk. J. Fish. aquat. Sci., 8: 141–147.
Pelczar, M.J.J., Chan, E.C.S. and Krieg, N.R., 1993. Microbiology concepts and application. 5th Ed. McGraw-Hill. New York.
Pradhan, J. and Das, B.K., 2015. Effect of supplementation diet containing Microcystis aeroginosa on hematological and biochemical changes in Labeo rohita infected with Aeromonas hydrophila. J. Aquacult. Res. Dev., 6: 871–876.
Rajkumar, M., Pandey, P.K., Aravind, R., Vennila, A., Bharti, V. and Purushothaman, C.S., 2016. Effect of different biofloc system on water quality, biofloc composition and growth performance in Litopenaeus vannamei (Boone, 1931). Aquacult. Res., 47: 3432–3444. https://doi.org/10.1111/are.12792
Rao, M.N., 2006. Medical biochemistry: For medical, dental, nursing, physiotherapy, pharmacy, food science, nutrition and science students. 2nd Revised Ed, New Age International (P) Limited Publishers, New Delhi, pp. 743–780.
Reitman S, and Frankel S., 1957. A colorimetric method for the determination of glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. clin. Pathol., 33: 1–13. https://doi.org/10.1093/ajcp/28.1.56
Russell, J., 1992. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. appl. Bact., 73: 363-370. https://doi.org/10.1111/j.1365-2672.1992.tb04990.x
Salehizadeh, H. and Shojaosadati, S.A., 2001. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv., 19: 371-385. https://doi.org/10.1016/S0734-9750(01)00071-4
Shanhjahan, M., Sabitha, K.E., Mallika, J. and Hyamala-Devi, C.S., 2004. Effect of Solanum tribatum against carbon tetrachloride induced hepatic damage in albino rats. Ind. J. med. Res., 120: 194–198.
Sinha, A.K., Baruah, K., and Bossier, P., 2008. Horizon Scanning: The potential use of biofloc as an anti-infective strategy in aquaculture. An overview. Aquac. Hlth. Int., 13: 8-10.
Verma, A.K. Rani, A.B., Rathore, G., Saharan N., and Gora, A.H., 2016. Growth, non-specific immunity and disease resistance of Labeo rohita against Aeromonas hydrophila in biofloc systems using different carbon sources. Aquaculture, 457: 61-67. https://doi.org/10.1016/j.aquaculture.2016.02.011
Weatherley, A.H. and Gill, H.S., 1987. The biology of fish growth. Academic Press, London and New York.
Widanarni, D.W., Puspita, F., 2012. Aplikasi bakteri probiotik melalui pakan buatan untuk meningkatkan kinerja pertumbuhan udang windu Penaeus monodon. J. Sains Terapan Edisi, 2: 2012.https://doi.org/10.29244/jstsv.2.1.19-29
Wilber, C.G. and Gilchrist, R.D., 1965. Organ weight: body weight ratios in the Mongolian gerbil Meriones unguiculatus. Chesapeake Sci., 6: 109–114. https://doi.org/10.2307/1351327
Xu, W.J., Pan, L.Q., Sun, X.H. and Huang, J., 2012. Effects of bioflocs on water quality, and survival, growth and digestive enzyme activities of Litopenaeus vannamei (Boone) in zero-water exchange culture tanks. Aquacult. Res., 44: 1093-1102. https://doi.org/10.1111/j.1365-2109.2012.03115.x
Zhou, C.P., Ge, X.P., Liu, B., Xie, J. and Miao, L.H., 2013. Effect of high dietary carbohydrate on the growth performance and physiological responses of juvenile Wuchang bream, Megalobrama amblycephala. Asian-Austral. J. Anim. Sci., 26: 1598–1608. https://doi.org/10.5713/ajas.2012.12659
To share on other social networks, click on any share button. What are these?









