Effect of Fermentation Time and Organic Acid Level on Organoleptic Quality and Chemical Components of Black Soldier Fly Prepupae Silage
Research Article
Effect of Fermentation Time and Organic Acid Level on Organoleptic Quality and Chemical Components of Black Soldier Fly Prepupae Silage
Yunilas1*, Muheri Indra Aja Nasution2, Edhy Mirwandhono1, Adi Fathul Qohar3
1Department of Animal Science, Faculty of Agriculture, Universitas Sumatera Utara, Medan, Indonesia; 2Graduate Student, Department of Animal Production and Technology, Faculty of Animal Science, IPB University, Bogor, Indonesia; 3Department of Animal Science, Faculty of Agriculture and Animal Science, Universitas Ma’arif Nahdlatul Ulama Kebumen, Kebumen, Indonesia.
Abstract | Black Soldier Fly (BSF) is an insect with a high nutritional content, especially protein, used as a source of animal feed. However, in the prepupal phase (BSFP) there is chitin which is a limiting factor in livestock rations because the bodies of poultry and monogastric livestock cannot digest it. Chitin is found in the BSF exoskeleton which is bound to proteins, minerals, and pigments, so it is necessary to do processing to reduce the chitin content first by fermentation using organic acids. Therefore, this study aimed to determine the effect of using organic acids (propionic acid and formic acid) and various fermentation times in reducing the BSFP chitin content. Completely Randomized Design (CRD), factorial 3 x 3 with three replications. Factor I was various doses of organic acid (3%, 6%, 9%), and Factor II was different fermentation times (5 d, 10 d, 15 d). The treatment had a significant effect (P<0.05) on color, odor, texture, and total titrated acid. In addition, crude protein, nitrogen, crude fat, and ash had no significant effect (P>0.05). The treatment had a very significant effect (P<0.01) on chitin and pH. There is an interaction between organic acid dosage and fermentation time for chitin. Using a mixture of organic acids at a dose of 9% and a fermentation time of 15 days resulted in the highest reduction in chitin content from 18.05% to 11.60% (a decrease of 35.74%).
Keywords | Black soldier fly prepupae, Chemical component, Formic acid, Organoleptic quality, Propionic acid
Received | June 02, 2023; Accepted | June 20, 2023; Published | September 15, 2023
*Correspondence | Yunilas, Department of Animal Science, Faculty of Agriculture, Universitas Sumatera Utara, Medan, Indonesia; Email: [email protected]
Citation | Yunilas, Nasution MIA, Mirwandhono E, Qohar AF (2023). Effect of fermentation time and organic acid level on organoleptic quality and chemical components of black soldier fly prepupae silage. Adv. Anim. Vet. Sci. 11(10): 1651-1658.
DOI | http://dx.doi.org/10.17582/journal.aavs/2023/11.10.1651.1658
ISSN (Online) | 2307-8316

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Proteins are essential components in animal feed formulas, and the Black Soldier Fly (BSF) is a potential insect-based protein source for this purpose. This is due to its short life cycle, which lasts about 38-41 days and includes five life stages: eggs, larvae, prepupae, pupae, and adult flies. Each stage contains high levels of nutrients, particularly proteins, with the larval phase containing 44.26% protein and the prepupa phase containing 39.9% - 43.1% (Beski et al., 2015; Oliveira et al., 2015; Spranghers et al., 2016; Wardhana, 2016). In addition, these insects have been produced widely as a source of insect-based protein, and these insects are more economical, environmentally friendly and do not compete with humans, so they have the potential to be used as animal feed ingredients (Kawasaki et al., 2019; Van Huis, 2013).
BSF in the prepupa phase (BSFP) contains 18.05% chitin which has anti-nutritional properties, meaning it cannot be digested by the bodies of poultry and monogastric livestock, thereby inhibiting its application in animal feed (Eggink and Dalsgaard 2023; Nasution et al., 2020). Sanchez-Muros et al. (2014) suggested that the digestive tract of chickens has chitinase, but its capacity to digest chitin is restricted. The digestibility level of insect nutrients in broilers differs and depends on various factors, such as the insect species, life cycle, and the process of chitin removal (De Marco et al., 2015; Schiavone et al., 2017). Chitin is the second most abundant polysaccharide in nature after cellulose, chitin is also the main structural component of the exoskeleton, and chitin can be isolated from insects, including the stages of demineralization, deproteination and depigmentation (Erdogan and Kaya 2016; Mirwandhono et al., 2022; Yadav et al., 2019). In addition, organic acids are acids that are generally used in the degradation of chitin (Su et al., 2019).
Efforts to stretch the limiting factor on BSFP are processing it into silage chemically using organic acids because this type of acid is safe to use and easy to obtain on the market. The use of organic acids as much as 3% of the raw material with a ratio of propionic and formic acids 1: 1 is a good quality silage, and if the use of acids less than 3% will be easily attacked by fungi (Jutavia 2013). In addition, using only propionic and formic acids does not produce stable silage because the properties of organic acids are different. Namely, propionic acid is fungicidal. In contrast, formic acid is bactericidal, reducing clostridia microbes’ activity in silage (Lv et al., 2020).
Nasution et al. (2020) researched the use of organic acids to decrease chitin content in BSFP, which showed that fermentation using a combination of 30% propionic acid and 30% formic acid with 40% distilled water at a 3% dose for 9 days resulted in a 14.36% decrease in chitin content. This finding sparked the authors’ interest in investigating the effect of organic acid fermentation on BSFP chitin content with different fermentation durations.
MATERIALS AND METHODS
Material
The material used in this study, using BSFP (age 18-25 days with fruit waste media) from Magot Medan Teratai (MMT), propionic acid product of Merck, formic acid product of Brataco Chemical, Whatman paper No.40 size 125 mm, aquades, HCl 1N, NaOH 3.5%, aceton, and NaOCl 0.315%.
The equipment used is a glass jar, oven, analytical balance, thermometer, titration tool, erlenmeyer, measuring cup, beaker glass, hotplate, pH meter, and laboratory equipment for chemical components.
Method
The experimental research method used was BSFP fermentation with organic acids (propionic acid and formic acid). Completely Randomized Design (CRD) factorial 3 x 3 with three replications. Factor I was doses of organic acid, namely: DA1 = 3% organic acid; DA2 = 6% organic acid; DA3 = 9% organic acid; Factor II is the fermentation time, namely: FT1 = 5 d; FT2 = 10 d and, FT3 = 15 d.
Bsfp Fermentation With Organic Acids
In this BSFP, fermentation uses organic acids consisting of propionic acid and formic acid. Propionic acid is a product of Merck, while formic acid is a product of Brataco Chemical. This fermentation refers to Nasution et al. (2020) BSFP is cleaned of foreign matter with clean water, then boiled with boiling water 100oC for 10-15 minutes. Then BSFP is dried for 24 hours in an oven at 60oC. After drying, BSFP is put into glass jars that have been mixed with organic acids (30% propionic acid and 30% formic acid + 40% aquadest) as much as 3%, 6%, and 9%, then stored for 5 days, 10 days and 15 days. Every 2 days the BSFP is shaken so that it is homogeneous. After 15 days the jar is opened and aerated, then dried for 24 hours in an oven at 60oC.
Measurement Of Organoleptic Quality And Chemical Components
Isolation and measurement of chitin yield, the process described by Mirwandhono et al. (2022) involves isolating fermented BSFP by drying it for 24 hours at 60oC and then placing 2 g of the dried BSFP into an Erlenmeyer flask. The BSFP was then demineralized by dissolving it in 1N HCl at a 1:20 (w/v) ratio, using 20 ml of HCl for every 1 g of dry BSFP, and heated on a hotplate for 20 minutes at 100oC. The resulting precipitate is washed with distilled water until the pH becomes (6.8-7), and then deproteinated by soaking in 3.5% NaOH at a ratio of 1:20 (w/v) for 24 hours. Afterward, the mixture is heated on a hotplate at 80oC for 1 hour, filtered with Whatman filter paper No. 40, and washed with distilled water until the pH is neutral. The resulting residue is dried in an oven at 60oC for 24 hours, and the weight of the isolated chitin is used to calculate the percent yield using a spesific formula:
% Yield = 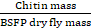 x 100
x 100
Observations of organoleptic tests include color, odor, and texture using the five senses. Measurement of chemical properties in the form of crude protein, nitrogen, crude fat, ash, pH, and total titrated acid using the AOAC method, (2005).
Variables
The parameters studied were organoleptic quality (color, odor, and texture) and chemical component (chitin, crude protein, nitrogen, crude fat, ash, pH, and total titrated acid).
After fermentation, BSFP silage was opened and evaluated for organoleptic quality (color, odor, and texture). Amount 15 panelists observed evaluation of organoleptic quality. Yunilas et al. (2021) states using a rating of each organoleptic quality observation from 1 to 9.
Organoleptic quality tests include color, odor, and texture. Classifies the colors of silage products on three criteria: dark brown (1 to 3), brown (4 to 6), and light brown (7 to 9). Classifies the odor of silage products on three criteria: not acid (1 to 3), acid (4 to 6), and very acid (7 to 9). Finally, it classifies the texture of silage products on three criteria: soft (1 to 3), moderate (4 to 6) hard (7 to 9) (Yunilas et al., 2021).
Data Analysis
The data was obtained, tabulated and analyzed using variance. If real or very real results are obtained, then the Duncan Multiple Range Test (DMRT) is carried out using SPSS software (Adinurani 2016).
RESULTS AND DISCUSSION
Organoleptic Quality
Observation of an organoleptic test is a method of testing the quality of a material in the form of color, odor, and texture using the five human senses. Unfermented and fermented BSFP show different colors, odors, and textures. The non-fermented treatment has a dark brown color, a distinctive BSFP odor, and a soft texture, while the fermented one has a variety of colors, odors, and textures. Organoleptic values before fermentation are presented in Table 1 and after fermentation are presented in Table 2 and 3.
Table 1: Chemical component and organoleptic quality of BSFP before fermentation.
| Parameters | Results |
| Chitin (%) | 18.05 |
| Crude Protein (%) | 35.18 |
| Nitrogen (%) | 5.62 |
| Crude Fat (%) | 32.17 |
| Ash (%) | 7.74 |
| Color | Dark brown |
| Odor | Typical BSFP |
| Texture | Soft |
In the DA1 treatment (3% dose) and the fermentation time of up to 15 days, the color changed from dark brown to brown, in DA2 (6% dose) and the fermentation time of up to 15 days, the color changed from brown to light brown and DA3 (9% dose). and the fermentation time of up to 15 days experienced a color change from brown to light brown. Various color changes occur due to varying doses of organic acids and fermentation time. The higher the organic acid concentration in each treatment, the brighter the different color changes. This is to the research of Yunilas et al. (2021), which reported that treatment with fermentation always causes a different color change. In addition, BSFP silage is fermented using acid, causing changes in temperature conditions during the fermentation process. Sio et al. (2022) also reported that the color changes that occur are based on changes in temperature during the fermentation process.
The analysis of variance showed that the treatment with various doses of organic acids and fermentation time had a significant (P<0.05) effect on color, odor, and texture and there was no interaction. The average color of BSFP silage was 5.47 - 7.55 (dark brown to light brown), the odor of BSFP silage was 5.27 - 6.21 (acid to very acidic) and the texture of BSFP silage was 4.82 - 5.27 (acid to very acidic). Based on this score, the quality of BSFP silage is quite good.
In the DA1 treatment (3% dose) and up to 15 days of fermentation, the odor changed, namely acid, DA2 (6% dose), and up to 15 days of fermentation, the odor changed from acidic to very acidic and DA3 (9% dose) and the longer the fermentation up to 15 days experience a change in odor from acid to very acid. Various odor changes occur due to varying doses of organic acids and fermentation time. The higher concentration of organic acids given to each treatment will produce a different odor change that is increasingly sour. This is to the research of Yunilas et al. (2021), which reported that the aroma turns more sour because the pH has decreased. Sio et al. (2022) also reported that fermentation would experience a change in aroma, namely the distinctive aroma of the fermented product, which is sour.
In the DA1 treatment (3% dose) and the fermentation time up to 15 days, the texture changed, namely soft, DA2 (6% dose) and the fermentation time up to 15 days, the texture changed from soft to moderate and DA3 (9% dose) and the fermentation time up to 15 days experienced a change in texture from soft to hard. Various textural changes occur due to varying doses of organic acids and fermentation time. The higher the concentration of organic acids given to each treatment will result in a different and harder texture change. This is to the research of Yunilas et al. (2021), who reported that a good silage texture is indicated by texture characteristics that are not crushed and not soggy.
Table 2: Organoleptic assessment of BSFP silage.
| Treatment | Organoleptic quality | |||
| Doses acid | Fermentation time | Color | Odor | Texture |
| DA1 (3%) | FT1 (5 d) | Dark brown | Acid | Soft |
| FT2 (10 d) | Brown | Acid | Soft | |
| FT3 (15 d) | Brown | Acid | Soft | |
| DA2 (6%) | FT1 (5 d) | Brown | Acid | Soft |
| FT2 (10 d) | Brown | Very acid | Moderate | |
| FT3 (15 d) | Light brown | Very acid | Moderate | |
| DA3 (9%) | FT1 (5 d) | Brown | Acid | Soft |
| FT2 (10 d) | Brown | Very acid | Moderate | |
| FT3 (15 d) | Light brown | Very acid |
Hard |
|
Table 3: Organoleptic quality of BSFP silage.
| Color | Odor | Texture | |
| Fermentation Time (FT) | |||
| 5 day (FT1) |
6.25a |
5.76a |
4.90a |
| 10 day (FT2) |
6.39b |
5.90b |
5.04b |
| 15 day (FT3) |
6.57c |
6.11c |
5.15c |
| Doses Acid (DA) | |||
| 3% (DA1) |
5.68a |
5.60a |
4.94a |
| 6% (DA2) |
6.13b |
6.02b |
5.02b |
| 9% (DA3) |
7.41c |
6.14c |
5.13c |
| Interaction (FT x DA) | |||
| FT1DA1 | 5.47 | 5.27 | 4.82 |
| FT1DA2 | 6.03 | 5.93 | 4.87 |
| FT1DA3 | 7.26 | 6.09 | 5.01 |
| FT2DA1 | 5.62 | 5.54 | 4.98 |
| FT2DA2 | 6.15 | 6.03 | 5.03 |
| FT2DA3 | 7.42 | 6.13 | 5.11 |
| FT3DA1 | 5.95 | 6.01 | 5.04 |
| FT3DA2 | 6.21 | 6.11 | 5.16 |
| FT3DA3 | 7.55 | 6.21 |
5.27 |
Means in a column with different superscripts differ at (P<0.05).
Table 4: Chemical component of BSFP silage.
| Chitin | Crude Protein | Nitrogen | Crude Fat | Ash | pH | Total Titrated Acid | |
| Fermentation Time (FT) | |||||||
| 5 day (FT1) | 13.59 | 33.94 | 5.42 | 30.81 | 8.13 |
5.4c |
0.036c |
| 10 day (FT2) | 13.19 | 33.90 | 5.42 | 30.99 | 8.25 |
5.1b |
0.026b |
| 15 day (FT3) | 12.89 | 33.31 | 5.32 | 31.89 | 8.17 |
4.8a |
0.019a |
| Doses Acid (DA) | |||||||
| 3% (DA1) | 14.39 | 33.53 | 5.36 | 30.82 | 8.21 |
5.3c |
0.036c |
| 6% (DA2) | 13.35 | 34.71 | 5.55 | 30.95 | 8.00 |
5.0b |
0.028b |
| 9% (DA3) | 11.92 | 32.91 | 5.26 | 31.92 | 8.35 |
4.9a |
0.016a |
| Interaction (FT x DA) | |||||||
| FT1DA1 |
14.60Cb |
32.93 | 5.26 | 30.72 | 8.26 | 5.5 | 0.043 |
| FT1DA2 |
14.38Cc |
34.42 | 5.50 | 30.36 | 8.27 | 5.3 | 0.036 |
| FT1DA3 |
14.21Cc |
33.26 | 5.32 | 31.38 | 8.10 | 5.1 | 0.028 |
| FT2DA1 |
13.96Bab |
36.01 | 5.76 | 30.79 | 7.82 | 5.4 | 0.036 |
| FT2DA2 |
13.52Bb |
33.83 | 5.41 | 30.19 | 8.02 | 5.0 | 0.028 |
| FT2DA3 |
12.86Bb |
34.30 | 5.48 | 31.89 | 8.16 | 4.7 | 0.014 |
| FT3DA1 |
12.23Ab |
32.88 | 5.26 | 30.94 | 8.33 | 5.2 | 0.028 |
| FT3DA2 |
11.95Aa |
33.47 | 5.35 | 32.43 | 8.47 | 4.9 | 0.021 |
| FT3DA3 |
11.60Aa |
32.39 | 5.18 | 32.40 | 8.27 | 4.6 |
0.007 |
Means in a column with different superscripts differ at (P<0.05).
In contrast, Sio et al. (2022) reported that the texture will change from slightly hard to soft during the fermentation process.
C hemical Component
Measurement of chemical properties of chitin, crude protein, nitrogen, crude fat, ash, pH, and total titrated acid. The content of BSFP chitin can be determined by measuring the yield. Crude protein, nitrogen, crude fat, and ash content can be determined by proximate analysis. The pH can be determined using a pH meter. Total titrated acid can be known using a titration tool. The chemical components of BSFP silage are presented in Table 4.
The chitin content of BSFP fermented with organic acids at various doses and fermentation time ranged from 11.60 - 14.60%. The percentage of BSFP chitin content before being fermented was 18.05%. BSFP fermentation with organic acids up to 9% dose and fermentation time up to 15 days decreased 19.12 - 35.74%. Fermentation carried out using different starters showed different results, some experienced a decrease, and some experienced an increase in the BSFP chitin content. This is evident from various studies, namely in the study of Harefa et al. (2018) reported that the chitin content of BSF maggot flour was fermented using organic acids (propionic and formic) as much as 7% with a fermentation time of 8 days, which decreased from 14.39% to 7.22%. The study by Mulyono et al. (2019) reported that fermentation using Trichoderma increased chitin content from 9.8% to 10.3%.
The analysis of variance showed that the treatment of various doses of organic acid and fermentation time had a very significant effect (P<0.01) on the chitin content of BSFP and there was an interaction between organic acid doses and fermentation time. In the DA3FT3 treatment (9% fermented dose for 15 days) there was a high reduction in chitin of 11.60%, while in the DA1FT1 treatment (3% fermented dose for 5 days) there was a low chitin reduction of 14.60%. The high reduction of chitin occurs due to the high organic acid content and the longer fermentation and the low chitin reduction occurs due to the low organic acid content and the short fermentation time. In addition, the decrease in chitin is caused by the degradation of protein, mineral, and pigment components in the BSFP exoskeleton. This is by Khong, (2013) who stated that chitin is closely related to proteins, minerals and pigments in the cuticle of crustaceans and insects.
The crude protein content of BSFP fermented with organic acids at various doses and fermentation time ranged from 32.39 - 36.01%. The percentage of BSFP crude protein content before fermentation was 35.18%. BSFP fermentation with organic acids up to a dose of 9% and a fermentation time of up to 15 days decreased by 2.31 - 7.94%. The fermentation that was carried out using different starters showed different results, some experienced a decrease, and some experienced an increase in the BSFP crude protein content. This is evident from various studies, namely Nafisah research (2019) which reported that the crude protein content of BSFP fermented using propionate decreased from 42.99% to 40.69%. Mulyono et al. (2019) also reported that fermentation using Trichoderma decreased crude protein content. Meanwhile, a study by Zhao et al., (2021) reported that fermentation using formic acid increased crude protein content from 5.89% to 6.36%.
The analysis of variance showed that the treatment with various doses of organic acids and fermentation time had no significant effect (P>0.05) on crude protein, nitrogen, crude fat, and ash and there were no interactions. The content of crude protein, nitrogen, crude fat, and BSFP ash fermented with organic acids up to a dose of 9% and a fermentation time of up to 15 days showed no significant difference in results but tended to decrease. This occurs due to the release of crude protein to the treatment between the length and dose during fermentation, the longer the fermentation process lasts, the opportunity for organic acids to break the bonds of crude protein. The release of the contribution of N to the treatment between the length and dose during fermentation, so that the protein content decreases, the nitrogen content also decreases. Nuraini et al. (2017) stated that low protein quality results in low nitrogen. Decomposition and loosening of fat bonds to the treatment between time and dose during fermentation. In addition, the DA3FT2 and DA3FT3 treatments experienced an increase in the BSFP fat before it was fermented. This is because the fat content of BSFP has a high-fat content than other treatments. But the fat content in this study is still by Spranghers et al. (2016) which states that BSFP has about 21.8% to 38.6% fat. The decrease in organic matter results in an increase in the ash content.
The nitrogen content of BSFP fermented with organic acids at various doses and fermentation time ranged from 5.18 - 5.76%. The percentage of BSFP nitrogen before fermentation was 5.62%. BSFP fermentation with organic acids up to 9% dose and fermentation time up to 15 days decreased by 2.44 - 7.83%. However, the DA2FT1 treatment experienced an increase in BSFP nitrogen content before fermentation because the nitrogen content in the BSFP DA2FT1 treatment had a higher nitrogen content than other treatments.
The fat content of BSFP fermented with organic acids at various doses and fermentation time ranged from 30.19 - 32.43%. The percentage of BSFP crude fat before fermentation was 32.17%. BSFP fermentation with organic acids up to 9% dose and fermentation time up to 15 days decreased by 0.81 - 6.16%. The fermentation that is carried out using one of the organic acids, namely formic acid or propionic acid alone, will decrease the fat content of BSFP. This is evident from Nafisah research (2019) which reported that the fat content in BSFP which was fermented using propionic acid with a fermentation time of 30 days was 35.52%, while the fat content of BSFP before it was fermented was 36.13%.
The ash content of BSFP fermented with organic acids at various doses and fermentation time ranged from 7.82 - 8.47%. The percentage of BSFP ash content before fermentation was 7.74%. BSFP fermentation with organic acids up to 9% dose and fermentation time up to 15 days decreased by 1.03 - 8.62%. Fermentation carried out using different starters showed different results, some experienced a decrease, and some experienced an increase in the BSFP ash content. This is evident from various studies, namely in the study by Zhao et al. (2021), which reported that fermentation using formic acid decreased ash content from 14.9% to 12.8%. Nafisah (2019) also reported that propionic acid fermentation resulted in an ash content of 9.74%. In contrast, the study by Mulyono et al. (2019) reported that Trichoderma fermentation increased ash content.
The pH value of BSFP fermented with organic acids at various doses and fermentation time ranges from 4.6 - 5.5. Fermentation carried out using organic acids experienced a decrease in pH. This is evident from various studies, namely in the research by Nasution et al. (2020) reported that BSFP fermentation with organic acids (30% propionic acid and 30% formic acid + 40% distilled water) at a dose of 3% and a fermentation time of 9 days resulted in a pH of 5.3. In addition, Zhao et al. (2021) reported that fermentation using formic acid decreased pH, which was around 4. Nafisah, (2019) also reported that propionic acid fermentation resulted in a pH value 6.86. Becerril-Gil et al. (2018) added that silage produces a pH of 3 - 4.
The analysis of variance showed that the treatment with various doses of organic acids and fermentation time had a very significant effect (P<0.01) and there was no interaction. The higher the dose of organic acids causes a decrease in pH and the longer the fermentation lasts, the lower the pH. Duncan’s further test showed that the pH in the DA1 treatment (3% dose) was significantly higher than in the DA2 (6% dose) and DA3 (9% dose) treatments. The above statement indicates that an increase in propionic acid and formic acid concentration during BSFP fermentation leads to a reduction in pH levels. Varying concentrations of these two acids will result in different degrees of pH reduction, and higher organic acid concentrations generally lead to more significant pH reductions.
Duncan’s further test showed that the pH in the FT1 treatment (5 days fermentation) was significantly higher than the FT2 treatment (10 days fermentation) and FT3 (15 days fermentation). This shows that the longer the fermentation, the more acidic the atmosphere. Based on the results of this study, it is also known that the addition of organic acids during fermentation and different fermentation times will produce different acidic conditions, some are pungent and some are very pungent so that the acidic atmosphere can lower the pH according to the amount of acid and the duration of fermentation. given during fermentation.
The total value of BSFP titrated acid fermented with organic acids at various doses and fermentation time ranged from 0.007 - 0.043%. Fermentation carried out using organic acids experienced an increase in the total titrated acid value. This is to the research by Nasution et al. (2020), which reported that BSFP fermentation with organic acids (30% propionic acid and 30% formic acid + 40% aquadest) at a dose of 3% and a fermentation time of 9 days resulted in a total titrated acid value of 0.028 %.
The analysis of variance results revealed that the treatment involving different doses of organic acids and fermentation time had a significant impact (P<0.05), but no interaction effect was observed. The results indicate that using higher concentrations of organic acids during BSFP fermentation increases the total titrated acid. Furthermore, utilizing varying concentrations of propionic and formic acids leads to different degrees of pH reduction and total titrated acid increase. Nasution et al. (2020) stated that fermentation with a high acid concentration would increase the total titrated acid value and decrease the pH value.
CONCLUSIONS AND RECOMMENDATIONS
Based on the study’s results, it can be concluded that BSFP fermentation using organic acids with varying fermentation times can reduce the chitin content. Using a mixture of organic acids at a dose of 9% and a fermentation time of 15 days resulted in the highest reduction in chitin content from 18.05% to 11.60% (a decrease of 35.74%).
coNFlICT OF INTEREST
The authors have declared no conflict of interest.
NOVELTY STATEMENT
This study differs from previous studies in reducing chitin content. In this study, BSFP was used, fermented by a combination of organic acids, namely propionic acid and formic acid. The combination of organic acids in this fermentation has a different role, namely propionic acid can prevent mold growth. In contrast, formic acid can extend shelf life and avoid decay during fermentation, so this research reduced the chitin content of BSFP.
AUTHOR’S CONTRIBUTION
Yunilas conceptualized and designed the study, elaborated the intellectual content, and performed the literature search, data acquisition, statistical analysis and manuscript preparation. Muheri Indra Aja Nasution carried out experimental studies performed the literature search, data acquisition, analysed data and reviewed manuscript. Edhy Mirwandhono elaborated the intellectual content performed the literature search, reviewed manuscript and guarantor. Adi Fathul Qohar performed the literature search and data acquisition.
REFERENCES
Adinurani PG (2016). Design and analysis of agro trial data: Manual and SPSS. Plantaxia, Yogyakarta, Indonesia.
AOAC (Association of Official Analytical Chemists) (2005). Official Methods of Analysis. Association of Official Analysis Chemistry. Washington.
Becerril-Gil MMN, Lopez-Gonzalez F, Estrada-Flores JG, Arriaga-Jordan CM (2018). Black oat (Avena strigosa) silage for small-scale dairy systems in the highlands of central mexico. Trop. Subtrop. Agroecosyst., 21: 467-476.
Beski SSM, Swick RA, Iji PA (2015). Specialized protein products in broiler chicken nutrition: A review. Anim. Nutrit., 1: 47-53. https://doi.org/10.1016/j.aninu.2015.05.005
De Marco M, Martinez S, Hernandez F, Madrid J, Gai F, Rotolo L, Belforti M, Bergero D, Katz H, Dabbou S (2015). Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol., 209: 211-218. https://doi.org/10.1016/j.anifeedsci.2015.08.006
Eggink KM, Dalsgaard J (2023). Chitin contents in different black soldier fly (Hermetia illucens) life stages. J. Insects Food Feed., 1-10. https://doi.org/10.3920/JIFF2022.0142
Erdogan S, Kaya M (2016). High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. Int. J. Biolog. Macromolecul.., 118-126. https://doi.org/10.1016/j.ijbiomac.2016.04.059
Harefa D, Adelina, Suharman I (2018). Pemanfaatan fermentasi tepung maggot (Hermetia illucens) sebagai sunstitusi tepung ikan dalam pakan buatan untuk benih ikan baung (Hemibagrus nemurus). J. Online Mahasiswa., 5: 1-15.
Jutavia K (2013). Pengaruh Level Campuran Asam Organik dan Lama Ensilase Silase Limbah Udang Terhadap pH, Kandungan Kitin dan Kalsium. Skripsi, Universitas Andalas, Padang, Indonesia.
Kawasaki K, Hashimoto T, Hori A, Kawasaki T, Hirayasu H, Iwasae SI, Hashizume A, Ido A, Miura C, Miura T, Nakamura S (2019). Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals., 9: 98. https://doi.org/10.3390/ani9030098
Khong TT (2013). Thang Trung Khong Vietnamese Chitin Raw M the Chitin de-N-Acetylation Reaction, and a New Chitosan-Alginate Gelling Concept. Thesis for the Degree of Philosophiae Doctor. Vietnam.
Lv J, Fang X, Feng G, Zhang G, Zhao C, Zhang Y, Li Y (2020). Effect of sodium formate and calcium propionat additives on the fermentation quality and microbial community of wet brewers grains after short-term storage. Animals., 10: 1608. https://doi.org/10.3390/ani10091608
Mirwandhono E, Nasution MIA, Yunilas (2022). Extraction of chitin and chitosan black soldier fly (Hermetia illucens) prepupa phase on characterization and yield. IOP Conference Series Earth Environ. Sci., 1114 012019. https://doi.org/10.1088/1755-1315/1114/1/012019
Mulyono M, Yunianto VD, Suthama N, Sunarti D (2019). The effect of fermentation time and Trichoderma levels on digestibility and chemical components of Black Soldier Fly (Hermetia illucens) larvae. Livest. Res. Rural Develop. 31.
Nafisah A (2019). Valorisasi Black Soldier Fly (Hermetia illucens) Fase Prepupa Rendah Kitin Sebagai Alternatif Tepung Ikan In Vitro. Thesis, Institut Pertanian Bogor, Bogor, Indonesia.
Nasution MIA, Yunilas, Mirwandhono E (2020). Black soldier fly (Hermetia illucens) prepupa phase fermentation by organic acids to decrease chitin content. J. Peternakan Integratif., 8: 159-165. https://doi.org/10.32734/jpi.v8i3.5490
Nuraini, Djulardi A, Trisna A (2017). Palm oil sludge fermented by using lignocellulolytic fungi as poultry diet. Int. J. Poult. Sci., 16: 6-10. https://doi.org/10.3923/ijps.2017.6.10
Oliveira F, Doelle K, Richard L, O’Reilly JR (2015). Assessment of Diptera: Stratiomyidae, genus Hermetia illucens (L.,1758) using electron microscopy. J. Entomol. Zool. Stud., 3: 147-152.
Sanchez-Muros MJ, Barroso FG, Manzano-Agugliaro F (2014). Insect meal as renewable source of food for animal feeding: a review. J. Cleaner Prod., 65: 16-27. https://doi.org/10.1016/j.jclepro.2013.11.068
Schiavone A, De Marco M, Martínez S, Dabbou S, Renna M, Madrid J, Gasco L (2017). Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol., 8: 51. https://doi.org/10.1186/s40104-017-0181-5
Sio S, Bira GF, Batu MS, Pardosi L, Mau RJ, Klau MO, Hoar J (2022). Organoleptic quality and nutrition of rice straw silage utilizing local microorganisms of cattle rumen fluid at different inoculum levels. J. Adv. Vet. Res., 12: 36-41.
Spranghers T, Ottoboni M, Klootwijk C, Ovyn A, Deboosere S, De Meulenaer B, Michiels J, Eeckhout M, De Clercq P, De Smet S (2016). Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substartes. J. Sci. Food Agricult., 97: 2594-2600. https://doi.org/10.1002/jsfa.8081
Su H, Wang J, Yan L (2019). Homogeneously synchronous degradation of chitin into carbon dots and organic acids in aqueous solution. ACS Sustain. Chem. Engineer., 7: 18476-18482. https://doi.org/10.1021/acssuschemeng.9b04436
Van Huis A (2013). Potential of insects as food and feed in assuring food security. Ann. Rev. Entomol., 58: 563-583. https://doi.org/10.1146/annurev-ento-120811-153704
Wardhana AH (2016). Black soldier fly (Hermetia illucens) sebagai sumber protein alternatif untuk pakan ternak. Wartazoa: Buletin Ilmu Peternakan dan Kesehatan Hewan Indonesia., 26: 69-78.
Yadav M, Goswami P, Paritosh K, Kumar M, Pareek N, Vivekanand V (2019). Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 6: 1-20. https://doi.org/10.1186/s40643-019-0243-y
Yunilas Y, Ginting N, Wahyuni TH, Zahoor M, Fati N, Wahyudi A (2021). Effect of various doses of local microorganism additives on silage physical qualityof corn (Zea mays L.) waste. Sarhad J. Agricult.., 37: 197-206.
Zhao J, Wang S, Dong Z, Li J, Jia Y, Shao T (2021). Effect of storage time and level of formic acid on fermentation characteristics, epiphytic microflora, carbohydrate components and in vitro digestibility of rice straw silage. Anim. Biosci., 34: 1038-1048.
To share on other social networks, click on any share button. What are these?





