Effect of Irrigation With Household Detergent on Germination, Activities of Oxidative Stress Enzymes and Chlorophyll Content of Pod Maize
Research Article
Effect of Irrigation With Household Detergent on Germination, Activities of Oxidative Stress Enzymes and Chlorophyll Content of Pod Maize
Ngele Blessing Alfred, Agba Mary-Ibenreh Ogaboh*, Bassey Rosemary Anietie and Egeh Ajah Egwu
Department of Botany, University of Calabar, PMB 1115, Calabar, Nigeria.
Abstract | Detergents, used for cleaning in both domestic and industrial settings, contain surfactants and other compounds that can affect crops through discharge and environmental runoff. In many underdeveloped areas, untreated wastewater is used to irrigate crops, potentially harming them. This study investigated the impact of irrigation with various concentrations of a common commercial laundry detergent in Nigeria (Ariel) on maize seedlings’ germination, growth, and physiological processes, including oxidative stress enzymes activities and chlorophyll content. A mixture of sawdust and sand (1:1) was utilized as the growth medium. Detergent solutions with concentrations of 1g/l, 2.5g/l, and 5.0g/l were prepared, with deionized water as the control (0g/l). Maize seeds were sown in germination trays and irrigated with these solutions to assess the effects on germination, growth performance, oxidative stress enzyme activities, and chlorophyll content. The detergent concentrations did not significantly affect the germination and growth of maize. However, fresh and dry weights of the plants decreased significantly (P≤0.05) as detergent concentration increased, with fresh and dry weights at 0 g/l being 1.68g and 0.69g, respectively, and at 5g/l being 0.50g and 0.69g, respectively. Seedlings irrigated with 5 g/l detergent solution showed higher peroxidase and polyphenol oxidase activities (1714.14 µmol product/L/min and 40.43 µmol product/L/min, respectively), while catalase activity decreased with higher detergent concentrations (1860.15 µmol product/L/min at 0g/l and 861.54 µmol product/L/min at 5g/l). Total chlorophyll content was higher (2313.01 mgg-1fw) in seedlings irrigated with the 5 g/l detergent concentration. The study concluded that high detergent concentrations in irrigation water induce oxidative stress in maize seedlings.
Received | April 28, 2024; Accepted | August 13, 2024; Published | September 11, 2024
*Correspondence | Agba Mary-Ibenreh Ogaboh, Department of Botany, University of Calabar, PMB 1115, Calabar, Nigeria; Email: agbamary03@gmail.com
Citation | Alfred, N.B., A.M.I. Ogaboh, B.R.Anietie and E.A. Egwu. 2024. Effect of irrigation with household detergent on germination, activities of oxidative stress enzymes and chlorophyll content of pod maize. Pakistan Journal of Agricultural Research, 37(3): 290-299.
DOI | https://dx.doi.org/10.17582/journal.pjar/2024/37.3.290.299
Keywords | Detergent, Germination, Oxidative stress enzymes, Chlorophyll
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The use of detergents as cleaning agents by households has significantly increased over the years and there are concerns regarding pollution caused by them, or their components when contained in sewage sludge used for irrigation. Recently, detergents have been a massive contributor to environmental pollution, associated with their high production and use rate in our everyday lives (Issayeva et al., 2015). Also, the recycling of wastewater is essential in water resources planning, particularly in arid and semi-arid regions (Abedi-Koupai and Bakhtiarifar, 2003).
Reports on the pollution of water bodies by detergents in on the increase, often these sources of water are used to irrigate crops (Ehilen et al., 2017). There have been reports of many polluted rivers and lakes in Nigeria, as a result of detergents (Adekola and Eletta, 2006). Unfortunately, environmental pollution arising from detergent use may get worse overtime as a result of high rates of production and their daily use (Issayeva et al., 2015). Detergents affect seed germination and subsequent seedling growth in various ways. Detergents and their components may induce stress in crops, by delaying germination, and reducing germination rates (Khaje-Hosseini et al., 2003; Ashfrat et al., 2005; Janmohamadi et al., 2008). Although there have been reports of low concentrations of detergent enhancing some aspects of plant growth (Ehilen et al., 2017), other researchers reported a significant decrease in germination and subsequent plant growth caused by high concentration of detergent in irrigation water (Barua et al., 2011; Ehiagbonare et al., 2011; Heidari, 2013; Sawadogo et al., 2014; Sharifzadeh et al., 2006; Ehilen et al., 2017). Also, most detergents contain high concentrations of salts making them saline; this may in turn induce salinity stress in plants during germination and growth (Sharma et al., 2004; Saboora and Kiarostami, 2006).
Different stresses, including salt stress which can be caused by high concentrations of detergent, can reduce chlorophyll content in plants (Li et al., 2010; Yang et al., 2011), and has been reported for different crops (Arfan et al., 2007; Pinheiro et al., 2008; Perveen et al., 2010). The degree of chlorophyll reduction depends on the tolerance level of plant species; chlorophyll increases in salt tolerant species but reduces in salt sensitive species (Khan et al., 2014; Akram et al., 2011; Ngele et al., 2020). Therefore a buildup of chlorophyll has been suggested as an indicator for salinity tolerance in various crops (Arfan et al., 2007; Monirifari and Barghi, 2009; Sabir et al., 2011; Noreen et al., 2010). However, Noreen and Ashraf (2009) reported that the buildup of chlorophyll during stress is not always associated with tolerance in plants.
During stress, plants respond by generating and building up substantial amounts of reactive oxygen species (ROS) (Choudhury et al., 2017). These ROS molecules are vital in plant development because of their signaling role in response to developmental stimuli (Choudhury et al., 2017; Mittler, 2017). The production of ROS and other redox regulations associated with its production is a common response in crops during stress. An excessive buildup of ROS is injurious to living cells by causing oxidative damage which may lead to an irreversible cell destruction and eventual death of cells. However, plant cells have an antioxidant defense system consisting of antioxidants and reactive oxygen species scavenging enzymes that are able to deactivate reactive oxygen species (Sewelam et al., 2016; Kao, 2017). Although a mild increase in reactive oxygen species is necessary for a few of plants’ responses to stress, a continued ROS production may overwhelm plant’s defense ability resulting in oxidative harm (Hui et al., 2017).
Although the effects of household detergents have been reported in many crops, its effects on the activities of oxidative stress enzymes in pod maize, an important cereal crop, has not been documented. Therefore this study investigated the effects of irrigation with solutions, containing varying concentrations of a synthetic household detergent (Ariel, manufactured by Procter and Gamble) on the germination and subsequent seedling growth of pod maize (Zea mays L.), and the effect on chlorophyll content and activity of some oxidative stress enzymes. Finds from this research will encourage best practices in the use of wastewater in irrigation.
Materials and Methods
Research area/ experimental site and layout
This research was carried out at the University of Calabar, Calabar, Cross River State, Nigeria. The laboratory analysis was carried out in the Research Laboratory of Botany of the same University, while the germination studies were done in a screen house of the same Department under ambient conditions. The experimental design was a complete randomized design replicated thrice.
Detergent used in study
Ariel, a commercial detergent in Nigeria manufactured by procter and gamble, was used in this investigation. Based on the information on the detergent pack, it contained surfactants, amine oxide, carboxymethyl, sodium carbonate, sodium disilicate, LAS, STPP, hydrogen peroxide, enzymes, perfume and optical brighteners.
Growth medium collection and preparation
The growth medium used was a mixture of fine sawdust and sea sand in a ratio of 1:1. The choice of growth medium was based on the need to use a relatively inert material so that observed effects would be as a result of treatments applied. Sea sand is relatively inert after drainage, while sawdust was to serve as a moisture retainer. Ten kilograms (10 kg) of the growth medium were weighed into twenty-four germination trays and watered with deionized water to field capacity and allowed over night to drain. Watering and draining was continued for two weeks to leach out possible contaminant and unwanted constituents.
Germination studies
The effect of four levels of detergent concentrations (1.0 g/l, 2.5 g/l and 5.0 g/l) on maize germination was investigated. These levels of concentration were chosen because they are sufficiently different from each other and increase the likelihood of detecting statistically significant differences; also representing lower and higher concentrations. Twelve germination trays were used for the germination studies. Three of each of the trays was used for each treatment level by irrigation with 500ml of the respective detergent solution before planting; subsequently irrigation was done by watering with 200ml of solution twice a week. The pH, salinity and conductivity of treatment solutions were determined before use.
In another setup, seeds were sown in twelve germination trays and irrigated with deionized until they were germinated and established (two weeks). The treatment solutions, containing detergent, were then applied accordingly after growth to determine the effect of detergent on established maize seedlings.
Maize seeds were seeped in water for 12 hours at ambient conditions before planting. Ten seeds were sown in each germination tray; planted seeds were observed daily for germination, and germination counts were recorded. Germination records were used in computing Percentage Maximum Germination (Gmax.) (Abbasi et al., 2019) and Germination Rate (GR) (Commander et al., 2017) as:
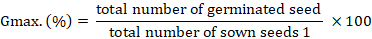
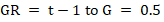
Where; t = time to germination in days; G = 0.5 (50 % germination).
Growth performance of seedlings
Seedlings were grown for three weeks, after which representative samples were picked randomly from each treatment group to compare growth performances. Growth parameters studied are leaf area, root length, fresh weight and dry weight.
Biochemical studies on seedlings
The activity of peroxidase, catalase, and polyphenol oxidase in maize seedlings was investigated using a modified enzyme extraction and assay method described by Nkang and Chandler (1989). Also, foliar chlorophyll a chlorophyll b contents were also determined using the method described by Ngele et al. (2020).
Enzyme extraction
Enzymes were extracted in a mixed phosphate buffer. The extraction buffer contained 50mM monobasic potassium phosphate (KH2PO4), 50mM dibasic potassium phosphate (K2HPO4), and 1 % (w/v) of a phenolic adsorbent (polyvinyl polypyrrolidone -PVPP). This was prepared by dissolving 2.613g of K2HPO4 in 300ml distilled water, and dissolving 2.042g of KH2PO4 in 300 ml distilled water. The solutions of the two salts were then mixed together and 6g of PVPP was added to the mixture. The mixture was stirred using a magnetic stirrer and the pH was adjusted to 7.0 at 30oC. The extraction buffer was stored under refrigerated conditions. Leaves (0.5g) of each treatment group were homogenized in 5 ml of extraction buffer. The homogenate was centrifuged at 6000 rotation per minute for 5 min. The filtrate was re-centrifuged at 6000 rotation per minute for 5 min. The supernatant fraction was stored on ice and used as enzyme source.
Enzyme assay
Enzyme assay was carried out using a mixed phosphate buffer containing 50mM monobasic potassium phosphate salt and 50mM dibasic potassium phosphate salt and pH adjusted to 7.0. Assay buffer was prepared same way as extraction buffer but without the addition of a phenolic absorbent, PVPP. Assays were carried out spectrophotometrically by measuring the change in extinction at 30oC using a Unico S-2150 Series spectrophotometer. Absorbance readings were used in computing enzyme activity as follows:
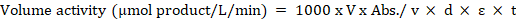
Where: V = Total assay volume; Abs = Absorbance; v = volume of enzyme extract; d = internal diameter of cuvette; ε = extinction coefficient; t = time in minutes.
Peroxidase assay
To 2mls of assay buffer in a quartz cuvette, was added 0.6mls of enzyme extract, and 0.1ml 10mM guaiacol; 10mM guaiacol was prepared by adding 6.207ml of guaiacol to 5ml distilled water. These were mixed and the reaction initiated with the addition of 0.1ml 10mM H2O2, prepared by adding 2.5 ml H2O2 to 5ml of distilled water. Absorbance was read at 436nm after 30 seconds and used in calculating enzyme activity (µmol product/L/min), using an extinction coefficient of 6.39 mMcm-1 for tetraguaiacol (the guaiacol dehydrogenation product) (Nkang et al., 2000).
Polyphenol oxidase assay
The reaction mixture included 1.4mls mixed phosphate buffer, 0.5ml of 20mM phloroglucinol, and 0.6ml of enzyme extract; another mixture was prepared as described but without enzyme extract, this served as blank. The mixture was allowed to incubate for 10 minutes at 30oC after which absorbance was read at 475nm and absorbance reading was used in calculating polyphenol oxidase activity (µmol product/L/min) using an extinction coefficient of 1433 mMcm-1 for the quinone product (Jimenez and Garcia-Corona, 1996; Nkang et al., 2000).
Catalase assay
The enzyme for catalase assay was further purified by bringing 5ml of the enzyme extract to 80% saturation with ammonium sulfate (NH4)2SO4). To 2ml of assay buffer in a quartz cuvette was added 0.6mls of the partially purified enzyme preparation. The reaction was initiated with the addition of 0.2ml 10mMH2O2. Absorbance was read at 240nm after 10 seconds and used in calculating catalase activity (µmol product/L/min) using an extinction coefficient of 43.6 M-1cm-1 for hydrogen peroxide (Nkang and Chandler, 1989).
Chlorophyll estimation
Foliar chlorophyll content was determined by extraction with 80% ethanol. Chlorophyll was extracted by pulverizing 1g fresh leaf weight in 10mls of 80% acetone. This was centrifuged at 2000 rpm for 2 mins., after which the supernatant was decanted into sample bottles for subsequent chlorophyll estimation. Chlorophyll estimation was done spectrophotometrically by taking absorbance readings at 663 and 643 nm. Absorbance readings were used in computing chlorophyll a, chlorophyll b and total chlorophyll as:
Chl. a = (11.6 A663 – 1.3 A643) VX-1
Chl. b = (19.1 A643 – 4.7 A663) VX-1
Total Chl. a and b = Chl. a + Chl. b
Where; Chl. a and Chl. b contents are in mgg-1fw; A663andA664 = absorbance readings at 663 and 643 nm; V = volume (ml) of 80 % acetone; X = fresh weight of sample used.
Statistical analysis
All data obtained from this study were in triplicate and were statistically analyzed using the Statistical Package for Social Sciences (SPSS) for Windows (Version 20.1). Differences between means were determined by analysis of variance (ANOVA) and least significant difference (LSD) was used to compare and separate means at p ≤ 0.05.
Results and Discussion
The investigation carried out on the physicochemical properties of the detergent solutions used in this study showed that salinity, conductivity, and pH of the solutions increased with increasing detergent concentration (Table 1). This could be attributed to the fact that ariel detergent contains various concentrations of salts and alkali as revealed in its contents. The concentration of these salts and alkali increases with increase in detergent concentration, which in turn increases salinity, conductivity and pH. High concentrations of detergents in irrigation solution impact plants in various ways; Sawadogo et al. (2014) observed that detergent in water, particularly at higher concentrations impacted negatively on the growth and yield of okra and lettuce. Saeed et al. (2015) also observed that while lower concentrations of soap water promoted growth in Sesbania grandiflora (a medicinal plant), higher concentration inhibited growth. Generally, the different concentrations of detergent solutions had varying effects on germination and growth. These findings are presented below:
Table 1: Physicochemical properties of treatment solutions.
|
Concentration (g/L) |
Salinity (%) |
Electrical conductivity ms/cm |
Ph |
|
0.00 |
0.00 |
2.40 |
6.43 |
|
1.00 |
0.54 |
4.46 |
7.9 |
|
2.50 |
1.12 |
13.00 |
9.1 |
|
5.00 |
1.22 |
21.00 |
10.3 |
Table 2: Effect of detergent solution on germinability of maize seeds.
|
Concentration (g/L) |
GMax (%) |
Germination rate |
|
0 |
*76.67a±8.82 |
0.20a±0.00 |
|
1.0 |
73.33a±3.33 |
0.20a±0.00 |
|
2.5 |
83.33a±8.82 |
0.20a±0.00 |
|
5.0 |
76.67a±8.82 |
0.20a±0.00 |
*Means of three replicates ± standard error (SE). Means followed by different letters in each column are significantly different (P≤0.05).
Influence of household detergent on germination and growth performance of maize
Irrigation with detergent solutions had no significant effect on the germination of maize seeds (Table 2). Cereals especially are known to possess one of the best adaptability features against environmental stress and inducements; such observations in maize have been recorded by Guan et al. (2015) and Uzma et al. (2018). Plant height, fresh and dry weights were significantly lower (P ≤ 0.05) in seedlings irrigated with a 5g/l concentration of detergent solution; leaf area was noticeably higher (P ≤ 0.05) in the control seedlings; while there were no significant differences in root length of control seedlings and those treated with detergent solutions (Table 3).
However, for seedlings treated after plants were established, there were no significant differences (P ≤ 0.05) in the height and leaf area of maize seedlings at the various levels of detergent concentrations investigated (Table 4). Root length was significantly higher (P ≤ 0.05) in seedlings irrigated with 5g/l detergent solution, while fresh and dry weights were significantly lower (P ≤ 0.05) at the same concentration. Although plant height was not significantly affected, as suggested by Kang et al. (2000), at lower concentrations, height, fresh and dry weights were significantly reduced at 5g/l concentration. This reduction could be due to the synergistic effect of salinity and alkalinity stresses conferred on the treatment solution by the detergents. A combination of saline and alkaline stresses results in salinity stress, pH stress, and their interaction. A study by Shi and Wang (2005) found that various salt-alkaline mixed stresses significantly inhibited the growth of Aneurolepidium chinense, a grass species native to saline-alkaline soils. Alkalinity stress brought about by high pH levels injures root cells severely, often damaging root cells and inhibiting seedling growth.
Influence of detergent on foliar chlorophyll content of maize seedlings
The control plants had significantly higher (P ≤ 0.05) chlorophyll a content, while chlorophyll b
Table 3: Effect of detergent solution on growth performance of maize seedlings.
|
Concentration (g/L) |
Plant Height (cm) |
Leaf area (cm2) |
Root length (cm) |
Fresh weight (g) |
Dry weight (g) |
|
0.0 |
*10.10a±1.07 |
19.65a±0.26 |
10.18a±3.77 |
1.68a±0.15 |
0.69a±0.07 |
|
1.0 |
8.67ab±0.96 |
14.42b±1.31 |
12.76a±3.41 |
1.42a±0.03 |
0.26b±0.04 |
|
2.5 |
7.80ab±0.23 |
12.38b±0.31 |
13.95a±1.54 |
1.64b±0.06 |
0.19bc±0.01 |
|
5.0 |
7.07b±0.41 |
13.82b±1.52 |
11.90a±1.00 |
0.50c±0.02 |
0.05c±0.01 |
*Means of three replicates ± standard error (SE). Means followed by different letters in each column are significantly different (P≤0.05).
Table 4: Effect of detergent solution on growth performance of established maize seedlings.
|
Treatment (g/l) |
Height (cm) |
Root length (cm) |
Leaf area (cm2) |
Fresh weight (g) |
Dry weight (g) |
|
0.00 |
*10.10a±1.07 |
19.17ab±0.09 |
10.17a±3.77 |
1.48ab±0.02 |
0.73a±0.06 |
|
1.00 |
9.50a±0.90 |
13.38b±0.02 |
13.74a±4.69 |
1.21b±0.04 |
0.46b±0.05 |
|
2.50 |
10.23a±0.50 |
17.61b±0.01 |
16.17a±3.89 |
1.85a±0.36 |
0.15c±0.04 |
|
5.00 |
11.97a±0.50 |
24.57a±4.08 |
5.86a±0.23 |
0.35c±0.03 |
0.12c±0.01 |
*Means of three replicates ± standard error (SE). Means followed by different letters in each column are significantly different (P≤0.05).
was significantly higher (P ≤ 0.05) in seedlings treated with 5.0 g/l solution of detergent (Figure 1). Also, total chlorophyll was significantly higher (P≤0.05) in control seedlings. But in maize seedlings irrigated with detergent solution after the seedlings were established, Chlorophyll a and total chlorophyll were significantly higher (P≤0.05) in seedlings treated with 5.0 g/l detergent solution (Figure 2), while seedlings from the control group had a significantly higher (P≤0.05) chlorophyll b content. Stress-induced alterations in a leaf’s chlorophyll content could be caused by impaired biosynthesis of the pigments, or accelerated pigment degradation caused by the stress. However, during the process of chlorophyll degradation, chlorophyll b may be converted into chlorophyll a, thus resulting in the increased content of chlorophyll a (Eckardt, 2009). Although stress reduces chlorophyll content, the extent of chlorophyll decline is influenced by the tolerance of plant species to stress. For instance, it is generally known that chlorophyll contents increases in tolerant species, while decreasing in sensitive species.
Juan et al. (2005) observed in their study an increase in the chlorophyll content of salt-tolerant cultivars of tomato. Also, Khan et al. (2014) also reported that salinity stress adversely affected photosynthetic efficiency, as indicated by reductions in chlorophyll content, while tolerant genotypes showed less reduction. Therefore a buildup of chlorophyll has been proposed as a potential indicator of tolerance in plants. Given this, an accumulation of chlorophyll has been proposed as one of the potential biochemical indicators of tolerance in different crops, although it has also been reported by Mittler (2006) that chlorophyll buildup is not always an indication of tolerance. Thus, using chlorophyll buildup as an indicator for stress tolerance is dependent on the plants traits.
Influence of detergent on enzyme activity in maize seedlings
There was a significant (P ≤ 0.05) decline in peroxidase (Figure 3), catalase (Figure 4) and polyphenol oxidase activity (Figure 5) following an increase in the concentration of treatment solutions, but peroxidase
Table 5: Effect of detergent on oxidative stress enzymes activity (µmol product/L/min) in established maize seedlings.
|
Treatment |
Peroxidase |
Catalase |
Polyphenol Oxidase |
|
0.00 |
1663.30d±0.02 |
1860.27b±0.08 |
18.88c±0.06 |
|
1.00 |
1095.57c±0.46 |
724.46d±0.01 |
18.83c±0.05 |
|
2.50 |
1059.70d±0.00 |
1370.60c±0.04 |
32.36b±0.02 |
|
5.00 |
2247.84a±0.01 |
1879.72a±0.02 |
74.24a±0.05 |
*Means of three replicates ± standard error (SE). Means followed by different letters in each column are significantly different (P≤0.05).
and polyphenol oxidase activity increased noticeably (P ≤ 0.05) in seedlings treated with 5g/ldetergent solution. Also, in seedlings treated with detergent solutions after the plants were established, those irrigated with 5.0 g/l detergent solution had a significantly higher (P ≤ 0.05) peroxidase, catalase and polyphenol oxidase activity in comparison to other treatment groups (Table 5). One of the protective systems developed in plants to reduce the effects, or eradicate reactive oxygen species is the development of an enzymatic antioxidant (Beak and Skinner, 2003). Srivalli et al. (2003) reported that stress was associated with increased antioxidant enzyme response. Also, the antioxidant activities of enzymes like catalase and peroxidase have been reported to increase during stress exposure in some plants (Hui et al., 2017). Maize Plants showed greater sensitivity to 5.0g/L detergent concentration, evidenced by the reduced leaf area, chlorophyll content, fresh and dry weights, increased peroxidase and polyphenol oxidase activity, indicating that high detergent concentration reduces maize plants’ growth performance and physiological processes.
Conclusions and Recommendations
The findings obtained from this study revealed that germination in maize was not affected significantly by the different concentrations of detergent solutions studied. However higher concentrations reduced seedling growth performance. Although there are reports of low concentrations of detergents in irrigation water enhancing growth in plants by adding to nutrient levels, a continuous use of such solutions may lead to a buildup causing nutrient toxicity. Similarly an accumulation of salts found in detergents in the soil may result in an increase in soil electrical conductivity, soil pH, soil water repellence and a gradual degradation of soil structure. This may in turn result in a drastic reduction in plant growth and development, thereby limiting agricultural productivity as well as environmental sustainability.
Total chlorophyll was higher in seedlings grown before detergent solution application (5g/ml) in comparison with the control (0g/l); while it was lower in seedling irrigated from seed level. This suggests that tolerance is enhanced in already grown seedlings, as compared to seeds or very tender seedlings. Likewise the increase in oxidative stress enzymes activities, particularly when irrigated with 5g/l of detergent solution shows an increase in oxidative stress which may be due to an increased production of reactive oxygen species. Therefore there is need for careful management and treatment of wastewater used in irrigation, particularly those containing detergents, to minimize adverse effects on crops. Moreover there is need for further molecular research on the genes responsible for conferring some level of tolerance in already grown maize seedlings.
Acknowledgement
The authors would like to express their gratitude to the Department of Botany, Faculty of Biological Sciences, University of Calabar, for providing a conducive laboratory environment for conducting the analysis required for this research.
Novelty Statement
This study specifically investigated the impact of irrigation with household detergent on the germination, oxidative stress enzymatic activities and chlorophyll content of pod maize. By examining these specific biochemical and physiological responses, this research provides new insights into the potential risks of using detergent -contaminated water in agriculture on plants health and resilience.
Author’s Contribution
Ngele Blessing Alfred: Conceptualization, funding. methodology, data collection and editing of draft.
Agba Mary-Ibenreh Ogaboh: Editing of draft manuscript.
Bassey Rosemary Anietie: Data curation.
Egeh Ajah Egwu: Funding and writing of original draft.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbasi, K.M., A. Ghorbani and F. Dadjou. 2019. Influence of nano-priming on Festuca ovina seed germination and early seedling traits under drought stress, in laboratory conditions. J. Ecol. Environ., 7(3): 133-139.
Abedi-Koupai, J. and A. Bakhtiarifar. 2003. Investigation of the effect of treated wastewater on hydraulic properties of emitters in a trickle irrigation system. In: 20th European Regional Conference, CD International Workshop, Irrigation technologies and method: Research, development and testing, Montpellier, France.
Adekola, B.N. and O.A. Eletta. 2006. A study of heavy metal pollution of Asa River, Ilorin, Nigeria. Environ. Monit. Assess, 125: 157- 163. https://doi.org/10.1007/s10661-006-9248-z
Akram, N.A., M. Ashraf and F. Al-Qurainy. 2011. Aminolevulinic acid-induced changes in yield and seed-oil characteristics of sunflower (Helianthus annuus L.) plants under salt stress. Pak. J. Bot., 43: 2845-2852.
Arfan, M., H.R. Athar and M. Ashraf. 2007. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol., 164(6): 685-694. https://doi.org/10.1016/j.jplph.2006.05.010
Ashraf, M.Y., K. Akhtar, G. Sarwar and M. Ashraf. 2005. Role of rooting system in salt tolerance potential of different guar accessions. Agron. Sustain Dev., 25: 243–249. https://doi.org/10.1051/agro:2005019
Barua, D., J. Buragohain and S.K. Sarma. 2011. Impact of Assam petroleum crude oil on the germination of four crude oil resistant species. Asian J. Pl. Sci. Res., 1(3): 68-76.
Beak, K.H. and D.Z. Skinner. 2003. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci., 165(6): 1221-1227. https://doi.org/10.1016/S0168-9452(03)00329-7
Choudhury, F.K., R.M. Rivero, E. Blumwald and R. Mittler. 2017. Review: Reactive oxygen species, abiotic stress and stress combination. The Plant J., 90(5): 856-867. https://doi.org/10.1111/tpj.13299
Commander, L., P. Golos, B. Miller and D. Merritt. 2017. Seed germination traits of desert perennials. Plant Ecol., 218(10): 11258-017-0753-7. https://doi.org/10.1007/s11258-017-0753-7
Eckardt, N.A., 2009. A new chlorophyll degradation pathway. Plant Cell, 21(3): 700. https://doi.org/10.1105/tpc.109.210313
Ehiagbonare, J.E., S. Obayuwana, W.T. Aborisade and I. Asogwa. 2011. Effect of unspent and spent diesel fuel on two agricultural crop plants: Arachis hypogea and Zea mays. Sci. Res. Essays, 6: 2296-2301.
Ehilen, O.E., B.O. Obadoni, F.N. Imade, D. Eseigbe and J.K. Mensah. 2017. The effect of detergents on the germination and growth of Amaranthus hybridus L. and Solanum lycopersicon L. Niger. Ann. Nat. Sci., 16(1): 100–108.
Guan, Y., Li, Z., He, F., Huang, Y., Song, W., & Hu, J. (2015). “On-off” thermoresponsive coating agent containing salicylic acid applied to maize seeds for chilling tolerance. PLOS one, 10(3). https://doi.org/10.1371/JOURNAL.PONE.012069
Heidari, H., 2013. Effect of irrigation with contaminated water by cloth detergent on seed germination traits and early growth of sunflower (Helianthus annuus L.). Not. Sci. Biol., 5(1): 86-89. https://doi.org/10.15835/nsb519003
Hui, Z., L. Xiao-Long, Z. Rui-Xue, Y. Hai-Yan, W. Ming-Ming, Y. Hao-Yu, M. Hong-Yuan, L. Duo, J. Chang-Jie and L. Zheng-Wei. 2017. Root damage under alkaline stress in association with reactive oxygen species accumulation in rice (Oryza sativa L.). Front. Plant Sci., 8: 1580.
Issayeva, A.U., E. Syrlybayeva, A.I.Z. Zhymadullayeva and A. Balgabekova. 2015. The effect of detergents on the anatomical changes in the roots of beans. J. Educ. Policy Entr. Res., 2(2): 18-22.
Janmohammadi, M., P.M. Dezfuli and F. Sharifzadeh. 2008. Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress. Gen. Appl Plant Physiol., 34(3-4): 215-226.
Jiménez, M., and García-Carmona, F. 1996. The effect of sodium dodecyl sulphate on polyphenol oxidase. Phytochemistry, 42(6), 1503-1509, https://doi.org/10.1016/0031-9422(96)00175-6
Juan, M., R.M. Rivero, L. Romero and J.M. Ruiz. 2005. Evaluation of some nutritional and biochemical indicators in selecting salt-tolerant tomato cultivars. Environ. Exp. Bot., 54(3): 193–201. https://doi.org/10.1016/j.envexpbot.2004.07.004
Kang, S., Z. Liang, Y. Pan and J. Zhang. 2000. Alternate furrow irrigation for maize production in arid areas. Agric. Water Manage, 45(3): 267-274. https://doi.org/10.1016/S0378-3774(00)00072-X
Kao, C.H., 2017. Mechanisms of salt tolerance in rice plants: reactive oxygen species scavenging-systems. J. Taiwan Agric. Res., 66(1): 1–8.
Khaje-Hosseini, M., A.A. Powell and I.J. Bingham. 2003. The interaction between salinity stress and seed vigor during germination of soybean seeds. Semicond. Sci. Technol., 33(3): 715-725. https://doi.org/10.15258/sst.2003.31.3.20
Khan, A., M.U. Shirazi, W. Mahboob and S.A.I. Mujtaba. 2014. Morpho-physiological adaptations of wheat genotypes to salinity stress. Pak. J. Bot., 46(6): 1981-1985.
Li, R., F. Shi and K. Fukuda. 2010. Interactive effects of various salt and alkali stresses on growth, organic solutes, and cation accumulation in a halophyte Spartina alterniflora (Poaceae). Environ. Exp. Bot., 68(1): 66–74. https://doi.org/10.1016/j.envexpbot.2009.10.004
Mittler, R., 2006. Abiotic stress, the field environment and stress combination. Trends Plant Sci., 11(1): 15-19. https://doi.org/10.1016/j.tplants.2005.11.002
Mittler, R., 2017. Review: ROS are good. Trends Plant Sci., 22(1): 11-19. https://doi.org/10.1016/j.tplants.2016.08.002
Monirifari, H. and M. Barghi. 2009. Identification and selection for salt tolerance in alfalfa (Medicago sativa L.) ecotypes via physiological traits. Notulae Sci. Biol., 1(1). https://doi.org/10.15835/nsb113498
Ngele, B.A., A.E. Nkang, I.E. Okon and E.A. Effa. 2020. Foliar nutrient yield, chlorophyll content and mycorrhizal colonization of Parkia biglobosa (Jacq.) inoculated with arbuscular mycorrhizal fungus and rhizobium strain. Int. J. Emerg. Technol. Adv. Eng., 10(4): 102-107.
Nkang, A., and Chandler, G. 1989. Changes during Germination in Rainforest Seeds with Orthodox and Recalcitrant Viability Characteristics. J. Plant Physiol., 134(1), 9-16. https://doi.org/10.1016/S0176-1617(89)80194-4
Nkang, A., Omokaro, D., and Egbe, A. 2000. Effects of desiccation on the lipid peroxidation and activities of peroxidase and polyphenoloxidase in seeds of Telfairia occidentalis. Seed Sci. Technol., 28(1): 1-9.
Noreen, Z. and M. Ashraf. 2009. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J. Plant Physiol., 166(16): 1764–1774. https://doi.org/10.1016/j.jplph.2009.05.005
Noreen, Z., M. Ashraf and N.A. Akram. 2010. Salt-induced modulation in some key physio-biochemical processes and their use as selection criteria in potential vegetable crop pea (Pisum sativum L.). Crop Past. Sci., 61: 369-378. https://doi.org/10.1071/CP09255
Perveen, S., M. Shahbaz and M. Ashraf. 2010. Regulation in gas exchange and quantum yield of photosystem II (PSII) salt stress and non-stressed wheat plants raised from seed treated with triacontanol. Pak. J. Bot., 42: 3073-3081.
Pinheiro, H.A., J.V. Silva, L. Endres, V.M. Ferreira, C.A. Câmara, F.F. Cabral, J.F. Oliveira, L.W.T. Carvalho and J.M. Santos. 2008. Leaf gas exchange, chloroplastic pigments and dry matter accumulation in castor bean (Ricinus communis L) seedlings subjected to salt stress conditions. Ind. Crops Prod., 27(3): 385–392. https://doi.org/10.1016/j.indcrop.2007.10.003
Sabir P., M. Ashraf and N.A. Akram. 2011. Accession variation for salt tolerance in proso millet (Panicum miliaceum L.) using leaf proline content and activities of some key antioxidant enzymes. J. Am. Chem. Soc., 197(5): 340–347. https://doi.org/10.1111/j.1439-037X.2011.00471.x
Saboora, A. and K. Kiarostami. 2006. Salinity (NaCl) tolerance of wheat genotypes at germination and early seedling growth. Pak. J. Bot., 9(11): 2009-2021. https://doi.org/10.3923/pjbs.2006.2009.2021
Saeed, R., A.A. Mirbahar, B. Jahan and A. Zehra. 2015. Effect of greywater (soap water) irrigation on growth and root nodules of medicinal plant (Sesbania grandiflora) L. Fuuast J. Biol., 5(1): 115-121.
Sawadogo, B., M. Sou, N. Hijikata, D. Sangare, A. Hama and N. Funamizu. 2014. Effect of detergents from grey water on irrigated plants: Case of okra (Abelmoschus esculentus) and lettuce (Lactuca sativa). J. Arid Land Stud., 24(1): 117-120.
Sewelam, N., K. Kazan and P.M. Schenk. 2016. Review: Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci., 7: 187. https://doi.org/10.3389/fpls.2016.00187
Sharifzadeh, F., H. Heidari, H. Mohamadi and M. Janmohamadi. 2006. Study of osmotic priming effects on wheat (Triticum aestivum) germination in different temperature and local seed masses. J. Agron., 5(4): 647-650. https://doi.org/10.3923/ja.2006.647.650
Sharma, A.D., M. Thakur, M. Rana and K. Singh. 2004. Effect of plant growth hormones and abiotic stresses on germination, growth and phosphatase activities in Sorghum bicolor (L.) moench seeds. Afr. J. Biotechnol., 3(6): 308-312. https://doi.org/10.5897/AJB2004.000-2057
Shi, D.C. and D.L. Wang. 2005. Effects of various salt-alkaline mixed stresses on Aneurolepidium Chinense (Trin.) Kitag. Plant Soil, 271: 15–26. https://doi.org/10.1007/s11104-004-1307-z
Srivalli, B., G. Sharma and R. Khanna-Chopra. 2003. Antioxidative defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Plant Physiol., 119: 503-512. https://doi.org/10.1046/j.1399-3054.2003.00125.x
Uzma S., S. Khan, W. Murad, N. Taimur and A. Azizullah. 2018. Phytotoxic effects of two commonly used laundry detergents on germination, growth, and biochemical characteristics of maize (Zea mays L.) seedlings. Environ. Monit. Assess, 190(11): 651. https://doi.org/10.1007/s10661-018-7031-6
Yang, J.Y., W. Zheng, Y. Tian, Y. Wu and D.W. Zhou. 2011. Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica, 49: 275–284. https://doi.org/10.1007/s11099-011-0037-8
To share on other social networks, click on any share button. What are these?







