Efficacy of Exogenous Applications of Glucose in Improving Wheat Crop (Triticum aestivum L.) Performance under Drought Stress
Efficacy of Exogenous Applications of Glucose in Improving Wheat Crop (Triticum aestivum L.) Performance under Drought Stress
Muhammad Zahid1, Naeem Iqbal1, Sohaib Muhammad2*, Summiya Faisal3, Wajid Mahboob3, Makhdoom Hussain4 and Zaheer ud din Khan2
1Government College University, Faisalabad (GCUF), Department of Botany, Faisalabad, Pakistan; 2Government College University Lahore, Department of Botany, Lahore, Pakistan; 3Nuclear Institute of Agriculture (NIA), Plant Physiology Division, Tandojam, Sindh, Pakistan; 4Ayub Agriculture Research Institute, Wheat section, Faisalabad, Pakistan.
Abstract | Restriction in crop growth and yield occurs when it encounters extreme water shortage. In order to reduce the adversities of drought, glucose was exogenously applied at various phenological stages on wheat variety “AARI-11” and desiccation tolerance potentials were assessed through growth, yield and physiological attributes. Glucose was supplied in various concentrations (0, 5, 10, 25 and 50 mM) as seed priming and foliar spray treatments at vegetative and reproductive stages. Plant growth and grain yields were reduced under drought. Two glucose treatments (10 mM and 50 mM) improved tillering and dry biomass as compared to other treatments. Foliar sprayed-glucose treated plants showed increasing trends in grain yield under drought. Physio-chemical attributes were also modulated by exogenously applied carbohydrates. Nitrate reductase activity and total soluble proteins were increased with increase in sugar treatments under drought. Osmotic and water potentials were reduced under drought but foliar glucose sprays of 10 mM and 50 mM applied at reproductive phase significantly reversed the adverse effects of drought. Gas exchange characteristics including CO2 concentration, transpiration and photosynthesis rates were raised by glucose treatments under irrigated and non-irrigated conditions. Hence, drought tolerance was slightly induced by exogenous glucose doses in terms of improved growth, yield and physiological traits.
Received | July 25, 2017; Accepted | August 26, 2018; Published | September 26, 2018
*Correspondence | Sohaib Muhammad, Government College University Lahore, Department of Botany, Lahore, Pakistan; Email: [email protected]
Citation | Zahid, M., N. Iqbal, S. Muhammad, S. Faisal, W. Mahboob, M. Hussain and Z.d. Khan. 2018. Efficacy of exogenous applications of glucose in improving wheat crop (Triticum aestivum L.) performance under drought stress. Pakistan Journal of Agricultural Research, 31(3): 264-273.
DOI | http://dx.doi.org/10.17582/journal.pjar/2018/31.3.264.273
Keywords | Foliar spray, Glucose, Grain yield, Seed priming, Soluble proteins, Water relations, Gas exchange attributes
Introduction
Wheat (Triticum aestivum L.) is an important and valuable cereal crop which is staple food of many countries of the world including Pakistan (Malik, 2006). However, it is now facing extreme water shortage in many parts of the world. Major causes of water scarcity are intensive use by growing human population and insufficient and irregular patterns of rainfalls which has raised levels of aridity and drought in many parts of the world (Giannakoula and Ilias, 2013). Drought stress is becoming a serious threat affecting the quality and yield of wheat (Kirigwi et al., 2007). Water resources are depleting which put emphasizes on cultivation of field crops under limited irrigation systems. Therefore, understanding the mechanisms of plant adaptations under drought stress has become the main emphasis of agriculturists.
Drought stress restricts plant growth and productivity and its effects can be accessed through physiological characters and grain yield. Crop yield is highly reduced even under the short spell of soil water deficit especially at the reproductive stage. Spike development phase of a crop is generally considered more sensitive to water as extreme water scarcity at this stage has negative effects on all yield parameters (Moayedi et al., 2010). According to an estimate, about 50% reductions in grain yield occur under extreme water scarcity worldwide while 17% wheat yield loss is reported in Pakistan (Ashraf and Harris, 2004).
Biochemistry of plants is also disturbed under the influence of soil moisture deficit. Many morphological and physiological responses occurred in plants when they encounter oxidative stress. Photosynthesis, enzymatic activities, gas exchange characteristics and chlorophyll fluorescence suppress under drought (Boagle et al., 2011) due to closing of stomata, denaturation of chloroplast and chlorophyll pigments (Waraich et al., 2011). Accumulation of osmolytes (such as sugars and amino acids) is considered as an adaptation to overcome water deficit (Yang et al., 2010). Literature cited that sucrose, fructose and glucose are the three main sugars which accumulate under water stress (Ackerson, 1981). Apart from in vitro biosynthesis, carbohydrates are the novel regulators of plant growth and development which controls gene expression in response to environmental stresses (Price et al., 2003). Glucose is particularly associated with primary development processes such as germination, expansion of cotyledon, leaf development, onset of flowering and senescence (Rolland et al., 2006). High doses of exogenous glucose may inhibit the germination process as well as seedling development (Gibson, 2005) while it represses the inhibitory effects of absisic acid on germination of seeds in lower concentrations (Finkelstein and Lynch, 2000). Meanwhile, higher concentrations of sugars could be used as marker trait for screening drought tolerant genotypes while Javid et al. (2011) documented that sugars (fructose, glucose, mannose etc.) are directly involved in osmo-protection and antioxidant responses in rice during salt stress.
It is an established fact that sucrose accumulation is less correlated with drought as compared to glucose concentrations. Glucose level decelerates in plants suffering from water shortage while its optimum quantity is retained after re-watering (Kameli and Loselo, 1993). Sucrose availability is much affected by drought and it reduces under water-limited environment. Duration of grain formation is shortened while protein contents are enhanced under drought (Prasad et al., 2011). Moreover, accumulation of carbohydrates increases the resistance of wheat against drought (Keyvan, 2010). Glucose activates drought responsive genes and the endogenous glucose levels raise proline contents in wheat under drought stress (Meng-Yun et al., 2009).
In order to overcome the adversities of water scarcity, many strategies could be adopted to maintain the crop yield. Exogenous supply of plant growth regulators is one of the techniques to minimize negative effects of drought stress. Plant growth enhancers may be supplied as seed priming, foliar spray or through rooting medium. Seed priming is a low cost, partial seed hydration process which minimizes the lag phase resulting in early germination and synchronize seedling stand establishment. It involves seed soaking in the solution of low osmotic potential for specific duration, which activates the metabolic processes required for germination of seed (Farooq et al., 2007). Many growth promoting substances as foliar spray can efficiently adjust the osmotic potential of plants under osmotic stress. Glucose acts as a signaling molecule which organizes plant development through gene expression. It could be found effective in reducing the adverse effects of climatic stresses in wheat by improving seed vigor, germination and antioxidant responses (Afzal et al., 2007; Singh et al., 2011).
Hence, present study is conducted to optimize the concentration and time of exogenously supplied glucose best suited for enhancing drought tolerance in wheat. Drought tolerance potentials were assessed through the studies of growth, physiology and yielding aspects of wheat crop.
Materials and Methods
The experiment was carried out in fields of Ayub Agriculture Research Institute (AARI) Faisalabad in year 2011-12 while physiological analyses were conducted in plant physiology laboratory, Botany department, Government College University, Faisalabad. Seeds of wheat variety AARI-11 were obtained from AARI-Faisalabad. Seeds were sown on 10th December, 2011. Experimental layout was comprised of two drought treatments, i.e. control (which was properly irrigated) and drought treatment (with no irrigation). Drought treatment plot was provided with shelter to avoid rainfall.
Seed priming treatments: Seeds were soaked in aerated solutions of various concentrations of glucose (0, 5, 10, 25 and 50 mM) for 12 h. After that, seeds were thoroughly rinsed with distilled water and dried up till the original weight of seeds was regained following the methodology of Farooq et al., 2006.
Foliar treatments of glucose: Glucose was sprayed in five concentrations (0, 5, 10, 25 and 50 mM) at vegetative and reproductive stages of wheat crop. Tween-20 (1 %) solution was used as surfactants.
Measurement of growth and yielding attributes: Number of tillers and plant fresh and dry biomass ratios were calculated at vegetative stage while yield related aspects such as plant height, number of spikes plant-1, grain yield pant-1 and 100-seeds weights were determined on crop maturity.
Physiological aspects: Various physiological parameters were determined in flag leaves at booting stage following the standard procedures. For determination of relative water contents (RWC), fresh weight (Wf) off lag leaves was measured at once after harvesting and dipped in water for 24 h. Turgid weights (Wt) were noted after 24 h and placed in oven at 60°C for 72 h. Dry weights (Wd) were measured and RWC were computed through following equation (Barr, Weatherley, 1962).
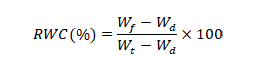
Leaf water potential (Ψw) was determined through pressure chamber (Boyer, 1966). Osmotic potential (Ψs) was assessed by using osmometer after extracting the cell sap of frozen flag leaves following the protocol described by Ludlow (1987). Ψs was measured by through osmometer.
Nitrate reductase enzymatic activity (NRA) was estimated in 0.5 g fresh leaves after extraction in 2 M sodium phosphate buffer (pH 7.5) containing KNO3 for 1 h in dark at 32ºC according to the method of Jordan (1984). For reaction, 1 mL solution was mixed with 0.5 mL 1 % sulphnailamide prepared in 3 N HCl and 0.02 % (1-Naphthyl)-ethylene diamine-dihydrochloride and incubated at 25°C for 20 min. and absorbance was noted at 542 nm. The values were represented as NO2 hr-1g-1 fresh weight. Total soluble proteins (TSP) were determined by using Bradford method (Bradford, 1995).
Gas exchange characteristics: Gas exchange characteristics including photosynthesis rate (Pn), transpiration rate and internal CO2 concentrations were determined in fully expanded leaves using portable IRGA (cl-340, CID, INC; USA). The IRGA was applied during 10:00 am to 12:00 pm to get favorable and accurate readings.
Results and Discussion
Growth attributes
Drought stress had exerted negative impacts on growth, yield and biochemistry of wheat crop (Akram, 2011), while exogenous glucose treatments as seed priming and foliar spray had slightly mitigated adversities of drought stress through modulations in growth and physiological patterns. Plants are usually autotrophic for carbon source but they may behave as carbon heterotrophs under environmental stresses. Carbohydrates are involved in metabolic processes, structural component and gene regulation for growth and development of plants (Koch, 1996). It has been reported that many sugars (such as sucrose, trehalose, sorbitol, mannose etc.) are produced in higher concentrations in plants facing drought (Mahajan and Tuteja, 2005). Many studies highlighted that lower concentrations of sugars are beneficent for plant development and productivity (Ackerson, 1981; Xiong et al., 2001). Exogenous application of higher glucose levels causes accumulation of ABA which delays germination and inhibits seedling development reducing the crop growth (Arenous-Huertero et al., 2000).
The statistics of data showed reduction in tillers and dry biomasses under drought. Plants under water deficit conditions showed 37% decrease in tillers per plant as compared to the plants under proper irrigation. Glucose seed treatments had little influence on enhancing the tillering capacity of wheat crop. However, 5 mM and 25 mM glucose treatments produced maximum tillers under irrigated (control) as well as drought stress, respectively (Figure 1A) while this trait had significant and positive correlation with grain yield (p<0.05) under drought. Interaction between glucose treatments and stress was found non-significant whereas interaction of growth stages and stress was highly significant statistically (Table 1).
Water deficiency also caused reduction in fresh and dry biomasses as well as fresh weight (F.W.) to dry weight (D.W.) ratios per plant. The plants under
Table 1: Analysis of variance data to check the effects of glucose treatments on growth and yielding attributes of wheat crop under control and drought conditions.
| Source | Df | Mean square | |||||
| NT | FW/DW | PH | NS | GYP | 100GW | ||
| Blocks | 2 |
0.6202ns |
0.082ns |
32.62ns |
5.59ns |
6.20ns |
0.063ns |
| Stress | 1 |
128.9*** |
1.41* |
4577.3*** |
1051.56*** |
1401.8*** |
1.50* |
| Chemical | 5 |
1.148ns |
0.62ns |
44.43* |
8.14ns |
11.08* |
0.28ns |
| Plant Stage | 2 |
199.1*** |
0.42ns |
75.22** |
10.67ns |
20.72** |
0.55ns |
| Stress*chemical | 5 |
5.24ns |
0.28ns |
17.47ns |
11.74* |
22.68*** |
2.11*** |
| Stress*stage | 2 |
101.3*** |
0.233ns |
20.53ns |
3.89ns |
2.02ns |
1.08* |
| Chemical*stage | 10 |
6.53** |
0.508ns |
21.68ns |
9.69* |
9.65* |
0.11ns |
| stress x chemical x plant stage | 10 |
5.59* |
0.527ns |
25.59ns |
11.14** |
13.32** |
0.275ns |
| Error | 70 | 2.42 | 0.328 | 14.81 | 3.92 | 3.89 | 0.2945 |
NT: Number of Tillers; FW/DW: Fresh and Dry Weight ratio; PH: Plant Height; NS: Number of Spikes; GYP: Grain yield per plant; GW: Grain Weight; * Significant at p ≤ 0.05, ** significant at p ≤ 0.01, *** significant at p ≤ 0.001, ns = non-significant (Duncan’s Test).
Table 2: Mean square values of physiological aspects of wheat under irrigated and drought conditions.
| Sources | Df | Mean square | |||||||
| RWC | OP | TP | NRA | Protein | TR |
CO2 |
Pn | ||
| Blocks | 2 |
35.59ns |
2.984ns |
0.242ns |
65123.4ns |
88831041.9ns |
0.192ns |
0.191ns |
3.23ns |
| Stress | 1 |
1311.6*** |
4.74*** |
0.0125ns |
71678.5ns |
79317395.3** |
0.026 |
0.026ns |
1.77ns |
| Chemical | 5 |
82.50ns |
6.087** |
0.477* |
41019.34ns |
187817351.9* |
2.14 |
2.14*** |
189.5*** |
| Plant Stage | 2 |
309.78** |
5.508*** |
13.53*** |
1124683.9*** |
1462722184.2*** |
3.08 |
3.08*** |
39.32*** |
| Stress*chemical | 5 |
64.59ns |
2.508ns |
0.052ns |
74928.39ns |
82603352.9ns |
0.48 |
0.48ns |
126.1*** |
| Stress*stage | 2 |
57.75ns |
5.384* |
0.427ns |
380851.62* |
168144790.2ns |
0.16 |
0.16ns |
32.05*** |
| Chemical*stage | 10 |
91.95* |
2.1269ns |
0.25ns |
76946.97ns |
141505172.4* |
0.427 |
0.427* |
11.93*** |
| Stress*chemical*plant stage | 10 |
54.08ns |
1.9113ns |
0.077ns |
47870.46ns |
25524573.4ns |
0.506 |
0.506* |
5.35** |
| Error | 70 | 41.50 | 1.023 | 0.154 | 82315.66 | 67708764.551 | 0.212 | 0.212 | 1.69 |
normal irrigation showed 4 % high values of fresh and dry weight ratios as compared to water stressed plants. All glucose treatments had increased plant biomass except 50 mM glucose treatment. Foliar spray of 5 mM glucose at reproductive stage executed overall greater fresh and dry biomass ratios under drought stress (Figure 1B).
Yielding attributes
Low soil moisture exerted negative effects on plant height and about 17 % decrease over control was measured. Glucose treatments had raised the length of plants as compared to the untreated control plants. Glucose treatments of 0, 5, 10, 25 and 50 mM increased the plant height by 4 %, 6 %, 5 %, 6 % and 5 %, respectively under normal irrigation while no significant differences were noted under drought. Foliar spray of glucose at vegetative exhibited best results compared to other two stages (Figure 1C). Effect of growth stages, stress and glucose application was found significant but their interactions remained non-significant (Table 1) while plant height was strongly correlated (p<0.01) with grain yield under terminal drought stress (Table 3).
Statistical analysis demonstrated that normal irrigated plants showed 59% more spikes as compared to the plants under no irrigation. Among glucose treatments, foliar spray of 25 mM and 50 mM concentrations at vegetative stage as well as 50 mM spray at reproductive stage contained more spikes per plant as compared to other treatments (Figure 2A). Glucose treatments remained ineffective in enhancing the productivity of crop under drought stress.
Statistical manipulation of data revealed that water stress caused much reduction in grain yield and the irrigated crop gave 64 % higher yields as compared to the drought. The application of 25 mM and 50 mM glucose as foliar spray at vegetative stage maximally raised the grain yield. Amelioration in grain yield occurred when 50 mM glucose was applied as foliar spray at reproductive stage. Glucose spray treatments at vegetative stage showed high yield per plant (Figure 2B). Earlier studies also documented reduction in yielding parameters under low soil moisture (Mirbahar et al., 2009; Killic and Yagbasanlar, 2010).
Contrasting results of glucose applications were obtained in terms of 100-seeds weight under irrigated and non-irrigated conditions. These treatments executed incremental effects on crop under drought while reducing effects under normal irrigation. All carbohydrate treatments showed decrease in 100-seeds weight except 0 mM (with 2 % increase over control) where as other carbohydrate treatments had reducing effects under irrigated conditions. Foliar spray of glucose at reproductive stage proved best with maximum grain weight under both irrigated and non-irrigated conditions as compared to seed priming and vegetative stage spray (Figure 2C).
Physiological characteristics
Soil water deficit executed diminishing effects on water relations of plants. Some researchers documented least water contents under limited available soil water contents in previous studies (Killic and Yagbasanlar, 2010; Bogale et al., 2011). The irrigated plants contained 7.72% more RWC than the plants subjected to drought while exogenously applied glucose had improved RWC under drought stress. The application of glucose at three stages showed that highest water contents were retained through foliar spray at reproductive stage (Figure 3A).
Water potential showed reduction under water deficit conditions (Siddique et al., 1999; Akram, 2011) and plants had 19.84% high value under normal irrigations than those plants subjected to drought stress. Diminished water potentials of leaves had also exerted negative effects on photosynthesis (Lawlor and Cornic, 2002). Carbohydrate seed treatments maintained high water potentials as compared to foliar treatments (Figure 3B). The effect of growth stages, glucose applications and stress were found significant for water potential, but their interactions remained trivial (Table 2). The 50 mM glucose treatment had maximally enhanced the osmotic potential under water scarcity. Highest osmotic potentials were recorded in reproductive phase foliar treatment while least OP was analyzed in seed priming treatments drought stress (Figure 3C). It was reported that significant correlation exists among plant water relations and compatible osmolytes accumulation under dehydrated soil conditions (Farooq et al., 2009).
Nitrate reductase is an enzyme involved in nitrate assimilation process which catalyses the conversion of NO3–1 to NO2–1 in N-cycle. Main plant regulatory processes are linked with both carbon source and nitrogen metabolism. Plants exhibited increase in NR-activity under the influence of exogenously applied glucose under drought stress. The 5 mM and 10 mM glucose applications as seed priming gave highest NR-activity. Moreover, 50 mM foliar spray of glucose at vegetative stage had also shown better enzymatic activity under normal irrigation (Figure 4A). Increased NR-activity by exogenous glucose supplement is an adaptive mechanism of plants to survive under stress; as more nitrates are assimilated which are involved in multiple metabolic processes. These results were supported by Shaik and Mehar (2016) study in which NR-activity was ameliorated by the application of natural growth enhancers in rice seedlings.
Drought stress had pronounced effects in protein degradation and all chemical treatments were found highly significant in protecting protein structures (Table 2). Protein contents were degraded under water stress (Praba et al., 2009); however, glucose treatments improved protein contents under drought stress. Reduction in total soluble proteins was the result of decreased rubisco enzyme as water deficiency causes proteolytic degradation of this enzyme (Cheng et al., 1998). Glucose seed treatments and spray at vegetative stage showed higher protein contents than those plants sprayed at reproductive stage. Among glucose treatments, 5 mM solution as seed priming and foliar spray at vegetative stage were found more effective in increasing the amount of protein under drought stress (Figure 4B).
It is a convincing fact that sugars are involved in regulating metabolic mechanisms in plants. These include expression of many genes of photosynthesis and
Table 3: Pearson correlation among various growth and physiological parameters under control (normal irrigation) and drought stress.
NT: Number of Tillers; FDR: Plant Fresh and Dry weight Ratio; RWC: Relative Water Contents; OP: Osmotic Potential; TP: Turgur Potential; NRA: Nitrate Reductase Activity; Prot: Protein; TR: Transpiration Rate; PR: Photosynthesis Rate; PH: Plant Height; NS: Number of Spikes per plant; GYP: Grain Yield Plant-1; TGW: 1000 Grain Weight; * Significant at p: 0.05, ** significant at p: 0.01
respiration which is controlled by sugars (sucores, hexoses etc.) (Winters et al., 1995; Felitti and Gonsales, 1998). During water stress, efficacy of photosynthesis was declined which depleted the concentration of carbohydrates in sink tissues. Reduction in the rate of photosynthesis (Pn) and transpiration (E) under water scarcity was also reported in previous studies (Chen et al., 2007; Boagle et al., 2011). Drought stress had decreased photosynthesis and transpiration rates of wheat crop. Plants under drought showed 1.35% decrease in TR as compared to control ones in non-treated. Glucose treatments of 10 mM and 25 mM applied at various growth stages had increased transpiration rates under proper irrigated and non-irrigated conditions. Foliar spray with various glucose levels at vegetative and reproductive stages showed better results as compared to seed priming (Figure 5A). Exogenous application of carbohydrates imposed inhibitory effects on the photosynthetic rates, as pn were reduced significantly with the increase in concentration of glucose. Only the hydro-priming and plants sprayed with 0 mM solutions had better photosynthesis as compared to all other treatments (Figure 5C). The depression in Pn might be the result of inactivity of rubisco enzyme (Salvucci and Crafts, 2004).
The internal CO2 concentration was raised under drought which had down regulated the rate of photosynthesis (Farrar and Gunn et al., 1996; Siddique et al., 1999). Different glucose applications had varying effects on concentration of internal CO2. The CO2 concentration was raised more in seed primed plants as compared to foliar glucose treatments. Highest CO2 was detected under the influence of 50 mM glucose treatment as seed priming and foliar spray at reproductive phase under drought stress (Figure 5 B).
Conclusions
Different concentrations of glucose applied exogenously in wheat proved effective in enhancing the growth and yield under terminal drought. Improved yield was linked with effective regulation of physiological aspects of crop which induces dehydration tolerance. Hence, exogenous glucose can be used as an effective treatment to enhance the productivity of arid lands.
Author’s Contribution
Muhammad Zahid: Data collection, data analysis
Naeem Iqbal: Conceived the idea
Sohaib Muhamamd: Overall management of the article
Summiya Faisal: Wrote introduction, results and discussion
Wajid Mahboob: Wrote abstract and methodology
Makhdoom Hussain: Technical inputs at every step
Zaheer-ud-Din Khan: wrote Conclusion and references.
References
Ackerson, R.C. Osmoregulation in cotton plants in response to water stress. II. Leaf carbohydrate status in relation to osmotic adjustment. Plant Physiol. 1981; 67: 489-493. https://doi.org/10.1104/pp.67.3.489
Afzal, I., Basra, S.M.A., Ahmad, N., Lodhi, T.E. 2007. Counteraction of salinity stress on wheat plants by pre-sowing seed treatments. Pak. J. Agric. Sci. 44(1): 23-29.
Akram, M. 2011. Growth and yield components of wheat under water stress of different growth stages. Bangladesh J. Agric. Res. 36(3): 455-468. https://doi.org/10.3329/bjar.v36i3.9264
Arenas-Huertero, F., Arroyo, A., Zhou, L., Sheen, J., Leon, P. 2000. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 14: 2086-2096.
Ashraf, M., Harris, J.C. 2004. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166: 3-16. https://doi.org/10.1016/j.plantsci.2003.10.024
Barr, H.D., Weatherley, P.E. 1962. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 15: 413-428. https://doi.org/10.1071/BI9620413
Bogale, A., Tesfaye, K., Geleto, T. 2011. Morphological and physiological attributes associated to drought tolerance of Ethiopian durum wheat genotypes under water deficit condition. J. Biodivers. Environ. Sci. 1(2): 22-36.
Boyer, J.S. 1966. Leaf water potentials measured with a pressure chamber. Plant Physiol. 42: 133-137. https://doi.org/10.1104/pp.42.1.133
Bradford, K.J. 1995. A water relationship and seed germination rate. Plant Physiol. 94(2): 840-849. https://doi.org/10.1104/pp.94.2.840
Chen, Z.H., Zhou, M.X., Newman, I.A., Mendham, N.J., Zhang, G.P., Shabala, S. 2007. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 34: 150-162. https://doi.org/10.1071/FP06237
Cheng, S.H., Moore, B., Seemann, J.R. 1998. Effects of short- and long term elevated CO2 on the expression of ribulose-1, 5-bisphosphatecarboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 2: 715-723. https://doi.org/10.1104/pp.116.2.715
Farooq, M., Basra, S.M.A., Hafeez, K. 2006. Seed vigoration by osmohardening in coarse and fine rice. Seed Sci. Technol. 34: 181-189. https://doi.org/10.15258/sst.2006.34.1.19
Farooq, M., Basra, S.M.A., Khan, M.B. 2007. Seed priming improves growth of nursery seedling and yield of transplanted rice. Arch. Agron. Soil Sci. 53: 311-322. https://doi.org/10.1080/03650340701226166
Farooq, M., Basra, S.M.A., Wahid, A., Ahmad, N., Saleem, B.A. 2009. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 195: 237-246. https://doi.org/10.1111/j.1439-037X.2009.00367.x
Farrar, J.F., Gunn, S. 1996. Effects of temperature and atmospheric carbon dioxide on source-sink relations in the context of climate change. Volume 1: Assimilate distrib. plants crops. pp. 389-406. Dekker, New York.
Felitti, S.A., Gonsales, D.H. 1998. Carbohydrates modulate the expression of the sunflower cytochrome c gene at the mRNA level. Planta. 206: 410-415. https://doi.org/10.1007/s004250050416
Finkelstein, R.R., Lynch, T.J. 2000. Abscisic acid inhibition of radical emergence but not seedling growth is suppressed by sugars. Plant Physiol. 122: 1179-1186. https://doi.org/10.1104/pp.122.4.1179
Gibson, S.I. 2005. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 8: 93-102. https://doi.org/10.1016/j.pbi.2004.11.003
Giannakoula, A.E., Ilias, I.F. 2013. The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon esculentum Mill.). Arch. Biol. Sci. Belgrade. 65(2): 611-620. https://doi.org/10.2298/ABS1302611G
Javid, M.G., Sorooshzadeh, A., Sanavy, S.A.M.M., Allahdadi, I., Moradi, F. 2011. Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul. 65: 305-313. https://doi.org/10.1007/s10725-011-9602-1
Kameli, A., Loselo, D.M. 1993. Carbohydrates and water status in wheat plants under water stress. New Phytologist. 125(3): 609-614. https://doi.org/10.1111/j.1469-8137.1993.tb03910.x
Keyvan, S. 2010. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 8(3): 1051-1060.
Kiliç, H., Yagbasanlar, T. 2010. The effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum ssp. durum) Cultivars. Notulae Botanicae Horticultura Agrobotanici Cluj-Napoca. 38(1): 164-170.
Kirigwi, F.M., Van Ginke, M., Brown-Guedira, G., Gil, B.S., Paulsen, G.M., Fritz, A.K. 2007. Markers associated with a QTL for grain yield in wheat under drought. Mol. Breeding. 20: 401-413. https://doi.org/10.1007/s11032-007-9100-3
Koch, K.E. 1996. Carbohydrate-modulated gene expression in plants. Annual Review: Plant Physiol. Plant Mol. Biol. 47: 509-540. https://doi.org/10.1146/annurev.arplant.47.1.509
Lawlor, D.W., Cornic, G. 2002. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 25: 275-294. https://doi.org/10.1046/j.0016-8025.2001.00814.x
Ludlow, M.M. 1987. Defining root water status in the most meaningful way to relate to physiological processes. Measurement of soil and plant water status. – Logan, UT, USA: Utah Agric. Exp. Stat. pp. 47-53.
Mahajan, S., Tuteja, N. 2005. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 444: 139-158. https://doi.org/10.1016/j.abb.2005.10.018
Malik, M.A., Irfan, M., Ahmed, Z.I., Zahoor, F. 2006. Residual effect of summer grain legumes on yield and yield components of wheat (Triticum aestivum L.). Pak. J. Agric. 22(1): 9-11.
Meng-Yun, H., Hui, L., Ying-Jun, Z., Qian, L. 2009. Photosynthesis and related physiological characteristics affected by exogenous glucose in wheat seedlings under water stress. Acta Agron. Sinica. 35(4): 724-732. https://doi.org/10.3724/SP.J.1006.2009.00724
Mirbahar, A.A., Markhand, G.S., Abro, M.S.A., Kanhar, N.A. 2009. Effect of water stress on yield and yield components of wheat (Triticum aestivum L.) varieties. Pak. J. Bot. 41(3): 1303-1310.
Moayedi, A.A., Boyce, A.N., Barakbah, S.S. 2010. Spike traits and characteristics of durum and bread wheat genotypes at different growth and developmental stages under water deficit conditions. Aust. J. Basic Appl. Sci. 4(2): 144-150.
Praba, M.L., Cairns, J.E., Babu, R.C., Lafitte, H.R. 2009. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J. Agron. Crop Sci. 195: 30-46. https://doi.org/10.1111/j.1439-037X.2008.00341.x
Prasad, P.V.V., Pisipati, S.R., MomcIlovic, I., Ristic, Z. 2011. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci. 197: 430-441. https://doi.org/10.1111/j.1439-037X.2011.00477.x
Price, J., Li, T.C., Kang, S.G., Na, J.K., Jang, J.C. 2003. Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol. 132: 1424-1438. https://doi.org/10.1104/pp.103.020347
Rolland, F., Baena-Gonzalez, E., Sheen, J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann. Rev. Plant Biol. 57: 675-709. https://doi.org/10.1146/annurev.arplant.57.032905.105441
Salvucci, M.E., Crafts-Brandner, S.J. 2004. Inhibition of photosynthesis by heat stress: the activation state of rubisco as a limiting factor in photosynthesis. Physiol. Plant. 120: 179-186. https://doi.org/10.1111/j.0031-9317.2004.0173.x
Shaik, M. and Mehar, S. K. 2015. Evaluating the allelopathic influence of mesquite (Prosopis juliflora DC.) aqueous leaf extract on the germination of rice (Oryza sativa L.) seeds using different germination indices. International Journal of Pharma and Bio Sciences,6(2): 280-287.
Siddique, M.R.B., Hamid, A., Islam, M.S. 1999. Drought stress effects on photosynthetic rate and leaf gas exchange of wheat. Bot. Bull. Acad. Sinica. 40: 141-145.
Singh, A., Singh, D., Gill, B.S. 2011. Planting time, methods, and practices to reduce the deleterious effects of high temperature on wheat. International conference on preparing agriculture for climate change, Punjab Agric. Univ. Ludhiana. pp. 338-339.
Waraich, E.A., Rashid, A., Saifullah, Ashraf, M.Y., Ehsanullah. 2011. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Agric. Crop Sci. 5(6): 764-777.
Winters, A.L., Gallagher, J., Pollock, C., Farrar, J.F. 1995. Isolation of a gene expressed during sucrose accumulation in leaves of Lolium temulentum L. J. Exper. Bot. 46: 1345-1350. https://doi.org/10.1093/jxb/46.special_issue.1345
Xiong, L., Ishitani, M., Lee, H., Zhu, J.K. 2001. The Arabidopsis los5/aba3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress and osmotic stress-responsive gene expression. Plant Cell. 13: 2063–2083. https://doi.org/10.1105/tpc.13.9.2063
Yang, S., Vanderbeld, B., Wan, J., Huang, Y. 2010. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant. 3: 469-490. https://doi.org/10.1093/mp/ssq016
To share on other social networks, click on any share button. What are these?







