Exploring Incidence of Potato Viruses in Major Growing Areas of Potato in Khyber Pakhtunkhwa, Pakistan
Research Article
Exploring Incidence of Potato Viruses in Major Growing Areas of Potato in Khyber Pakhtunkhwa, Pakistan
Zafrullah Khan1, Shah Alam Khan1, Tamana Bakht2, Luqman5*, Noor Muhammad1, Naeem Khan3, Iqbal Munir4, Ruqayya Qazi1 and Shazia2
1Department of Plant Protection, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Environmental Science, Department of Agriculture, Department of Forestry Shaheed Benazir Bhutto University, Sheringal Dir Upper, Pakistan; 3Department of Weed Science and Botany, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 4Institute of Biogenetics and Engineering, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 5Department of Agriculture Extension, Khyber Pakhtunkhwa, Pakistan.
Abstract | The Enzyme Linked Immunosorbant Assay (ELISA) test of potato leaves collected from different areas within three (3) selected divisions in Khyber Pakhtunkhwa, Pakistan was carried out in 2016. The region-wise results showed the over all incidence of potato virus Y (PVY) to be highest in all the three divisions on potato crop followed by the incidence of potato leaf roll virus (PLRV). The PVYand PLRV incidence in Malakand divisionwas noted by 62.7 and 44.6 %, respectively. In Peshawar division the incidence of PVY was 55.4% and that of the PLRV was 40.7% and the incidence of PVY and PLRV was 50.7 and 37.3%, respectively in Hazara. The field-wise results of Malakand region showed the highest incidence of PVY and PLRV in Kalam was found by 70 and 50%) followed by Miandam (62 and 46 %) and the lowest incidence was recorded in Malamjaba (56 and 38%). In Peshawar the highest incidence of PVY and PLRV wasnotedon potato crop in Warmarh (66 and 46%) followed by Regi (52 and 32%) and the lowest was noted in Pul Dheri Mehra (48 and 44%). In the Hazara potato crop in Kaghan had 62 and 54% PVY and PLRV incidence followed by Bagnotar (48 and 36%) and the lowest incidence was noted in Kalanderabad (42 and 22%), respectively.Results obtained by this study indicating that both PVY and PLRV are major viruses of potato crop in selected potato growing areas of KPK province which could pose serious threat to the potato crop, development of resistance cultivar against vector insect could be recommended.
Received | October 30, 2023; Accepted | May 26, 2024; Published | June 27, 2024
*Correspondence | Luqman, Department of Agriculture Extension, Khyber Pakhtunkhwa, Pakistan; Email: luqmanaup@yahoo.com
Citation | Khan, Z., S.A. Khan, T. Bakht, Luqman, N. Muhammad, N. Khan, I. Munir, R. Qazi and Shazia. 2024. Exploring incidence of potato viruses in major growing areas of potato in Khyber Pakhtunkhwa, Pakistan. Pakistan Journal of Weed Science Research, 30(2): 86-94.
DOI | https://dx.doi.org/10.17582/journal.PJWSR/2024/30.2.86.94
Keywords | Potato crop, Potato Aphid, Potato virus Y (PVY), ELISA kit
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Potato (Solanum tuberosum L.) being an important vegetable tuber crop in Pakistan can be attacked by various viral diseases such as the potato virus X (PVX), potato mop top virus (PMTV), potato virus S (PVS), potato virus A (PVA), alfalfa mosaic virus (AMV) and potato virus M (PVM) Myzus persicae being dual insect pest not only harm the potato crop by direct feeding but also play major role as vector of many viruses which can cause different viral diseases in potato (Valkonen, 2007). The most harmful viruses of potato in Pakistan are reported to be the potato virus Y (PVY), potato leaf roll virus (PLRV), (Qamar et al., 2015). The viral infection can be from no-to-100% in potato plants. Among the different vectors of disease transmission into the potato plants (e.g. potato psyllid (Bactericera cockerelli, Sulc)) M. persicae can also make major transmition of disease into the crop (Yao et al., 2016). Among the different viruses, the PVY and PLRV are the two significant viruses of potato crop in Pakistan (Mughal et al., 1988). These two viruses are widely spread and are major threats to the potato crop in Pakistan (Hameed et al., 2014). Virus infection in potato crop cause major reduction in the size and yield of potato tubers. Together viruses can cause up to 83 % yield losses to the potato crop (Mughal and Khalid, 1985) and c. 50 to 90 % yield losses can be caused only by the infection of PVY and PLRV (Qamar et al., 2015). The PVY of Potyviridae family and Potyvirus genus) and the PLRV of Luteoviridae family polerovirus genuscan both act as vectors of many important plant viruses.
The typical population of M. persicae on potato plant is c. 320 M. persicae per plant in Khyber Pakhtunkhwa. Such a large number of M. persicae on potato plants can be expected to transmit significant number of viruses such as the PVY and PLRV into the crop. The vectors can transmit the PVY and PLRV in a non-persistant and persistant way, respectivelly (Radcliffe and Ragsdale, 2002; Boquel et al., 2011). A good number of potato cultivars e.g. Sahiwal Red, Sarpo Mira and Rocco all have shown resistance to the M. persicae infestation (see tables of Antexenosis and Antibiosis in Resistance Chapter). The resistance potato cultivars may also play a role in reducing the incidence of viral diseases in potato crops growing areas. The incidence and distribution of virus infection could be varied on different potato cultivars in the field conditions. Thus it may be important to explore the incidence and distribution of the two widely spread viruses (PVY and PLRV) in potato cultivated fields in Khyber Pakhtunkhwa, Pakistan.
Visual symptomatic detection and confirmation of viral diseases in potato crop have been practiced since long time ago, however, visual detectionof symptoms of viral infection in potato crop cannot be scientific and accurate in all cases. For example, in case of some strains of PVX in latent infection, plants do not indicatedisease symptoms to detect and recognize the viral infection well on time and easily. Other conventional tests such as the neutralization, complement fixation and indirect immunofluorescence are all reliable however they posses a number of limitations. For example their practical complication, extensive hands on time, titration of reagents and specimens dilution and some timesinaccurate-positive or negative results.Serological test of detection and measurement of viral incidence in potato crop is a scientific, a more reliable and more accurate method. This method has been used widely by the scientific community around the world (Singh et al., 2017). The double antibodies sandiwich enzyme linked immunosorbent assay DAS-ELISA. In this present study serological method was used to explore the incidence of PVY and PLRV viruses in three major potato growing regions (Malakand, Peshawar and Hazara) of Khyber Pakhtunkhwa. This present study assessed the incidence and distribution of two major viruses of potato crop (PVY and PLRV) in three major potato growing areas in Khyber Pakhtunkhwa province of Pakistan in 2016.The main objective of this present study was to detect the incidence of PVY and PLRV viruses in potato fields in three major growing areas in Khyber Pakhtunkhwa, Pakistan and to record if there was resistance of potato cultivars to the virusesunder field condition.
Materials and Methods
Field surveys and samples collection
Surveys of potato fields in three different regions i.e., Peshawar, Malakand and Hazara divisions of Khyber Pakhtunkhwa province of Pakistan were conducted in 2016.The surveys of Malakand and Hazara divisions were undertaken during August 2016 and survey of Peshawar division was undertaken during November 2016. The three different regions for this study were selected on the basis of their major potato cultivation status. In each of the three divisions, threetypical farmer fields under potato cultivation were selected.In each selected potato field 50 samples of potato leaves (150 samples per region) were randomly collected from plants of potatos at c. 6 to 8 weeks growth stage. Potato samples showing paleness and upward rolling of young leaves, stunted growth and yellowing of leaf margins were detected serologically against PLRV. To make random collection ofleaf samples from within all the selected fields, the ‘Z’ pattern sampling methodpreviously used by many others (e.g. Gul et al., 2013) was used. In square shaped crops fields W or U shaped sampling patterns are usually used and in ractanguler filed Z or zig-zag pattern of sampling is commonly more reliable and effeicient. In this method we can sampled more number of plants easily and efficiently (Zehnder, 2010).The field sites selected in Peshawar division (Regi, Warmarh and Pul Dehri Mehra), Malakand division (Miandam valley, Kalam and Malamjaba) and Hazara (Kaghan, Bagnotar and Kalanderabad).The potato cultivars in selected and sampled farmer’s fields in Malakand division were Cardinal, Kurada and Sahiwal Red, in Peshawar division Desiree, Dura and Sarpo Mira and in Hazara division Rocco, Diamond and Desiree. One sample size consisted of three leaves per plant (manually cut from the top, middle and bottom) of potato plants. All the cut samples were separately placed in polythene zip-lock bags printed with regions, field sites, sample number and samples collection dates.The results of ELISA is more reliable and exhibited higher infection compared to visual inspection of potato crop. The results showed from the current experiment that potato virus Y and potato leafroll virus can effect the quality and production of potato in Khyber Pakhtunkhwa Pakistan. All collected samples were taken to the Virology Laboratory, Department of Plant Pathology, The University of Agriculture, Peshawar, Pakistan. As it was difficult to complete the serological test for all the samples immediately in a single day, samples were preserved in a refrigerator (4 ± 1oC) to avoid any possible viral infection lossfor further testing.
Serological detection test
All potato samples collected from the three different areas (Peshawar, Malakand and Hazara divisions) of Khyber Pakhtunkhwa province were tested for detection of PVY and PLRV infection by the DAS-ELISA methodology using antibodies (Bioreba Kit). The ELISAis one of the most reliable and a highly sensitive detectivemethod of a number of morphologically different viruses in purified preparations and in unclarified infected crop plants.Thismethod is capable to detect and quantify both the target antigen and molecules in study samples using a specific using an enzyme (peroxidase) reaction with its substrate.Monoclonal antibodies (IgG) were diluted in coating buffer (at 1μL:500μL) and 10plates of ELISA (each plate having 96 wells) were coated with diluted IgG (100μL/well) separately for each of the two viruses PVY and PLRV. The coated plates were air tight by aluminium foil and incubated for 3hours at room temperature. Potato leaves were ground using pestle and mortar in extraction buffer (Polyvinylprrolidone (PVP), albumin and Tween-20) to create sap, filtered through muslin cloth, sap was filled in well (each well being filled with 100 μl antigen sap) and a control (containing healthy control). The antigen coated plates were incubated over night c. 14 hours at 4°C while enzyme alkaline phosphatase (ALKP) conjugated IgG was diluted (1:500) in conjugate buffer and was added in each well. Each step of the test was completed at 3 minutes interval and buffer and plates were dried by using paper towels. Substrate buffer (150μL) containing p-nitro phenyl phosphate (1mgmL-1) was added to each well and all plates were incubated in dark for 1 hour and the color reaction was observed visually. A sample was considered and noted to be virus infected when the absorbance of 405 nm was at least three times greater than the healthy control. The incidence of potato viruses (PVY and PLRV) was calculated by the formula:
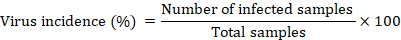
Statistical analysis
The recorded data, in all thethree regionsand within each of the selected potato grown field for survey on PVY and PLRV were tested for proportion difference by using Z-test. Before applying the Z-test, the proportion of positives and negatives for PVY and PLRV were computed, and it was considered that there is no significance difference between the proportion of positives of PVY and PLRV, in each of the selected surveyed region. All the results were described at 5% level of probability.
Results and Discussion
The results in Table 1 show the region-wise relative incidence and distribution of M. persicae borne viruses PVY and PLRV. The proportional difference between the PVY and PLRV in Malakand region was recorded to be 0.180, in Peshawar region 0.147 and in Hazara region 0.133 (Table 1). The Z-test values recorded for PVY and PLRV in the three selected regions were
Table 1: The proportional incidence and distribution of M. persicae borne viruses PVY and PLRV in three selected regions in Khyber Pakhtunkhwa, Pakistan.
|
Regions |
PVY |
PLRV |
Proportion difference |
S.E |
Z-test values |
P value |
||
|
Total samples tested |
Positive samples |
Total samples tested |
Positive samples |
|||||
|
Malakand |
150 |
94 |
150 |
67 |
0.180 |
0.058 |
3.01 |
0.0026 |
|
Peshawar |
150 |
83 |
150 |
61 |
0.147 |
0.058 |
2.43 |
0.0152 |
|
Hazara |
150 |
76 |
150 |
56 |
0.133 |
0.057 |
2.21 |
0.0271 |
3.01 2.43 and 2.21 for Malakan, Peshawar and Hazara, respectively. The P-value of proportional differences between PVY and PLRV in the three selected regions were Malakand - 0.0026, Peshawar-0.0152 and Hazara-0.0271 (Table 1). This experiment was undertakenwith main focus on detecting incidence of potato viruses including PVY and PLRV in potato in three majot growing areas in Malakand, Peshawar and Hazara, Khyber Pakhtunkhwa, Pakistan.The presence of the two major viruses PVY and PLRV in potato plants in Khyber Pakhtunkhwa, Pakistan can be found and as has been reported in many earlier studies (e.g. Abbas et al., 2016; Abbas and Amrao, 2017). In above Chapters (3, 4 and 5) a number of potato cultivars (e.g. Sahiwal Red, Sarpo Mira and Rocco) shown resistance against M. persicae under glasshouse and field conditions. Myzus persicae being an important vector of viruses, the resistant potato cultivars may have an impact on the transmission of viruses in potato crop in field conditions.
Table 2: The region-wise incidence and distribution of M. persicae transmitted Viruses PVY and PLRV in potato crop in Peshawar, Malakand and Hazara divisions of Khyber Pakhtunkhwa, Pakistan.
|
Region-wise PVY |
Total samples tested |
Total samples (+ve) |
Incidence (%) |
|
Malakand |
150 |
94 |
62 |
|
Peshawar |
150 |
83 |
55 |
|
Hazara |
150 |
76 |
50 |
|
Region-wise PLRV |
|||
|
Malakand |
150 |
67 |
44 |
|
Peshawar |
150 |
61 |
40 |
|
Hazara |
150 |
56 |
37 |
The Table 2 shows the incidence of PVY and PLRV in potato crop in survey fields inMalakand, Peshawar and Hazara regions. The results showed the incidence of PVY to be the highest in Malakand division (62%), followed by the Peshawar region (55%) and the lowest for Hazara region (50%). The incidence of PLRV ranged from 37 – 44 % among the three regions. The maximum incidence were recorded in Malakand region (44%), followed by Peshawar region (40%) whereas, the minimum incidence was recorded in Hazara region (37%) Table 2. The ELISA based results showed the incidence of both PVY and PLRV in potato crop in all the three surveyed regions, however, the incidence of PVY was widely spreadfollowed by the relatively scattered incidence of PLRV in potato grown fields in all the three regions (Tables 2 and 3). Interestingly the results showed low incidence of PVY and PLRV in fields cultivated with Sahiwal red and Rocco in sampled fields in all the three surveyed divisions while high incidence of the two viruses in fields cultivated with potato cultivars such as Desiree and cardinal (Tables 3 and 4).The diversed levels of incidenceof PVY and PLRV detectedin potato cultivated fields in the three regions may be due to the diverse resistance capabilities of the potato cultivars against the viruses under field conditions. A number of potato cultivars tested both under glasshouse and field conditions have shown resistance to M. persicae (Tables 1, 2 and 1-4 in Chapter 3 and 5). The lower incidence of PVY and PLRV detected in some of the potato cultivars e.g. Sarpo Mira and Rocco (48 and 44% and 42 and 22%, respectively) may have been due to the no-to-limited attack of M. persicae on plants of such potato cultivars and may have had restricted the transmition of the viruses in potato plants. In earlier studies (e.g. Nikan and Barker, 2012; Hameed et al., 2014) variedincidence of potato viruses was detected under field conditions due to either resistance of the potato cultivars to the viruses or due to the resistance of the potato cultivars to the M. persicae. The difference in the incidence of PVY and PLRV in potato cultivars could also be due to the agro-climatic conditions prevailing in the three surveyed regions when other abiotic and bioticaspects come into play. Agro-environmental conditions of an area have been reported to play important role in viruses incidence in potato (Roossinck, 2015; Bertschinger et al., 2017; Makarova et al., 2018).
Table 3: The field-wise incidence and distribution of M. persicae borne viruses PVY and PLRV in Malakand, Peshawar and Hazara regions of Khyber Pakhtunkhwa, Pakistan in 2016.
|
Fields selected |
Potato cultivar |
Samples tested |
PVY |
Incidence (%) |
PLRV |
Incidence (%) |
|
Malakand region |
||||||
|
Miandam valley |
Cardinal |
50 |
31 |
62 |
23 |
46 |
|
Kalam |
Kurada |
50 |
35 |
70 |
25 |
50 |
|
Malamjaba |
Sahiwal Red |
50 |
28 |
56 |
19 |
38 |
|
Peshawar region |
||||||
|
Regi |
Dura |
50 |
26 |
52 |
16 |
32 |
|
Warmarh |
Desiree |
50 |
33 |
66 |
23 |
46 |
|
Pul dehri Mehra |
Sarpo Mira |
50 |
24 |
48 |
22 |
44 |
|
Hazara region |
||||||
|
Kaghan |
Diamond |
50 |
31 |
62 |
27 |
54 |
|
Kalanderabad |
Rocco |
50 |
21 |
42 |
11 |
22 |
|
Bagnotar |
Desiree |
50 |
24 |
48 |
18 |
36 |
The results showed that the incidence and distribution of PVY and PLRV in Malakand regionto be ranged from 56-70 and 38-50%, respectively (Table 3). The highest incidence of PVY was noted in potato crop in Kalam (70 %) followed by the potato crop inMiandam (62%) and the lowest incidence of PVY in Malamjaba (56%) of Malakand region. Similarly, the incidence of PLRV was highest in Kalam (50%) followed by Miandam (46%) and lowest incidence was recorded in Malamjaba (38%; Table 3). The Peshawar region had the maximum PVY incidence in potato crop in Warmarh (66%) followed by the potato crop inRegi (52%) and the lowest incidence were found in Pul dehri Mehra (48%). Similarly, the incidence of PLRV were recorded maximum in Warmarh (46%) followed by Pul dehri Mehra (44%) and the minimum incidence of PLRV were recorded in Regi (32%) Table 3. The Hazara region had the highest PVY incidence in potato crop in Kaghan (62%) followed by the potato crop of Bagnotar (48 %) and the minimum incidence were noted in Kalanderabad (42%). Similarly, the incidence of PLRV were recorded maximum in Kaghan (54 %) followed by Bagnotar (36 %) and the minimum incidence of PLRV were recorded in potato crop of Kalanderabad (22%) Table 3. The serological testdetected the presence of PVY and PLRV in different surveyed regions of Khyber Pakhtunkhwa, Pakistan.Among the two viruses, PVY showed highest incidence in all the three regions as compared to the PLRV (Tables 2 and 3). The greater incidence of PVY in surveyed regions could be due to its more successful transmission by different vectors and or by different mechanical means. Earlier studies revealed that PVY had more infestations compared to other viruses such as PVS, PLRV and PVM (Gul et al., 2013). Results showed thatKalam valley in Malakand region, Kaghan valley in Hazara region and Warmarh in Peshawar regionto showgreaterincidence of PVY while Malamjabaregion in Malakand, Kalanderabad region in Hazara and Pul dheri Mehra region in Peshawar had the least incidence of the virus (Table 3). The diverse incidence of viruses in sampled locations within the three regions may be due to the different agro-climatic conditions as discussed in above lines.
The result shows the field wise proportional distribution of M. persicae borne viruses PLRV and PVY. The proportional difference between the two viruses (PLRV and PVY) in Malakand region were recorded in the three different fields (0.160) in Kalam, (0.200) in Miandam valley and in Malamjaba (0.180) Table 4. In Peshawar region (0.200) in Regi and Warmarh, while the (0.040) were recorded in Pul dehri Mehra, Table 4. The proportional difference between PVY and PLRV among three different fields of Hazara were recorded as (0.080) in Kaghan, (0.200) in Kalanderabadand (0.120) in Bagnotar, Table 4. The z test and p values were recorded for the two viruses and three different regions and different fields are (1.40, 0.1602) Kalam, (1.60, 0.0662) Miandam valley and (1.60, 0.1090) for Malamjaba were recorded for Malakand region, respectively. The z test and p values for Peshawar region were recorded for the two viruses and three different fields are (1.82, 0.0682) Regi, (1.81, 0.0698) Warmarh and (0.20, 0.8410) for Pul dehri Mehra were recorded, respectively Table 4. The z test and p values for Hazara region were recorded for the two viruses and three different fields are
Table 4: The proportional incidence and distribution of M. persicae borne viruses PVY and PLRV in three selected regions selected fields in Khyber Pakhtunkhwa, Pakistan.
|
Fields |
Potato cultivar |
Samples tested |
PVY |
PLRV |
Proportion difference |
S.E |
Z-test values |
P value |
|
Miandam valley |
Cardinal |
50 |
31 |
23 |
0.160 |
0.099 |
1.40 |
0.1602 |
|
Kalam |
Kurada |
50 |
35 |
25 |
0.200 |
0.098 |
1.84 |
0.0662 |
|
Malamjaba |
Sahiwal red |
50 |
28 |
19 |
0.180 |
0.099 |
1.60 |
0.1090 |
|
Regi |
Dura |
50 |
26 |
16 |
0.200 |
0.098 |
1.82 |
0.0682 |
|
Warmarh |
Desiree |
50 |
33 |
23 |
0.200 |
0.099 |
1.81 |
0.0698 |
|
Bridge dehri Mehra |
Sarpo Mira |
50 |
24 |
22 |
0.040 |
0.099 |
0.20 |
0.8410 |
|
Kaghan |
Diamond |
50 |
31 |
27 |
0.080 |
0.099 |
0.61 |
0.5433 |
|
Kalanderabad |
Rocco |
50 |
21 |
11 |
0.200 |
0.093 |
1.93 |
0.0537 |
|
Bagnotar |
Desiree |
50 |
24 |
18 |
0.120 |
0.099 |
1.01 |
0.3110 |
(0.61, 0.5433) Kaghan, (1.93, 0.0537) Kalanderabad and (1.01, 0.3110) for Bagnotar were recorded, respectively Table 4. In previous studies (Gul et al., 2013; Attaullah and Arif, 2017) different extent of PVY and PLRV incidences were reported in major potato growing areas in Khyber Pakhtunkhwa province of Pakistan. Potato virus Y and Potato leafroll virus reduce yields of the crop, in the case when the disease is caused through tuber seeds or transmitted via vector insects (Rykbost et al., 1999; Nolte et al., 2004). Taken as a whole the incidence of PVY was found to be higher than that of the PLRV in the potato in the all the three surveyed regions (Tables 2 and 3). The differential extent of PVY and PLRV may have been linked to a number of factors of which one may be the adaptability of the viruses to the agro-climatic conditions as discussed above and the second most important factor could be the degree of difference in resistance capability of the potato cultivars sown in the surveyed regions. Both these factors have been largrly reported to play role in restricting incidence of aphids and diseases (Batool et al., 2011; Valkonen, 2015).
Conclusions and Recommendations
The results obtained revealed the incidence of both PVY and PLRV but with different degree of their incidence in Malakand, Peshawar and Hazara divisions where PVY being the dominant virus in most of the surveyed fields of potato. The difference in the incidence of the PVY and PLRV could be due to a number of factors as has been discussed above. Neverthless incidence of these viruses of potato has been reported to hold an important position in reducing the potato yield in Khyber Pakhtunkhwa (Malko et al., 2019).
This present study did not measure the yield losses by the PVY and PLRV to the potato crop in the surveyed areas, future research could be useful to measure the exact yield losses as well as the tuber quality deterioration by the viruses.
Novelty Statement
Potatoes crop is very important crop worldwide. White aphid is serious pest to this crop. This study was conduct to screen out selected to screen out potatoes genotypes for their control of virus dictation through ELISA against potato aphid infestation under laboratory condition.
Author’ Contribution
Noor Muhammad: conception and design.
Shah Alam Khan and Naeem khan: drafting the article.
Iqbal Munir: analysis and interpretation of the data;
Tamana Bakht: revising it critically for important intellectual content and
Luqman and Zafrullah Khan: helping in the correction of whole text
Ruqayya Qazi and Shazia Khushdil: helped in data collection.
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript
Conflict of interest
The authors have declared no conflict of interest.
References
Abbas, A. and L. Amrao. 2017. Potato virus Y: An evolving pathogen of potato world wide. Pak. J. Phytopathol., 29(1): 187-191. https://doi.org/10.33866/phytopathol.029.01.0310
Abbas, A., M. Arif and M. Ali. 2016. A review paper on potato leaf roll virus (PLRV) of potato in pakistan. Asian.
Abbas, M.F. and S. Hameed. 2012. Identification of disease free potato germplasm against potato viruses and PCR amplification of potato virus X. Int. J. Biol. Biotech., 9(4): 335-339.
Agindotan, B., P.J. Shiel and P.H. Berger. 2007. Simultaneous detection of potato viruses, PLRV, PVA, PVX and PVY from dormant potato tubers by TaqMan real-time RT-PCR. J. Virol. Methods, 142: 1-9. https://doi.org/10.1016/j.jviromet.2006.12.012
Ahmad, W., D. Iman and H.U. Jan. 2003. Recent trend of potato virus prevailing in potato growing areas of Punjab. Pak. J. Phytopathol., 15: 21-24.
Ali, A., S. Hassan and A. Ali. 2002. Incidence of six potato viruses in spring, summer and autumn potato crops of the North West Frontier Province of Pakistan. Austral. Plant Pathol., 31: 143-146. https://doi.org/10.1071/AP02006
Attaullah and M. Arif. 2017. Prevalence of major aphid and soil borne viruses infecting potato crop in North Western Pakistan. J. Ent. Zool. Stud., 5(3): 221-226.
Barker, H. and M.F.B. Dale. 2006. Resistance to viruses in potato. In: (eds. G. Lobenstein and J.P. Carr) Natural resistance mechanisms of plants to viruses, springer, dordrecht, The Netherlands, pp. 341-366. https://doi.org/10.1007/1-4020-3780-5_15
Batool, A., M.A. Khan, J. Farooq, S.M. Mughal and Y. Iftikhar. 2011. ELISA-based screening of potato germplasm against potato leaf roll virus. J. Agric. Res., 49: 57-63.
Bertschinger, L., L. Bühler, B. Dupuis, B. Duffy, C. Gessler, G.A. Forbes and P.C. Struik. 2017. Incomplete infection of secondarily infected potato plants. An environment dependent underestimated mechanism in plant virology. Front. Plant Sci., 8: 74. https://doi.org/10.3389/fpls.2017.00074
Bhatti, F.J. and A.R. Bhutta. 2002. Seed-borne pathogens associated with certified seed lots of wheat in Pakistan. Quart. Sci., 8(1): 112-115.
Boquel, S., A. Ameline and P. Giordanengo. 2011. Assessing aphids potato virus Y-transmission efficiency: A new approach. J. Virol. Methods, 178(1-2): 63-67. https://doi.org/10.1016/j.jviromet.2011.08.013
Burrows, M.E. and T.A. Zitter. 2005. Virus problems of potatoes. Department of Plant Pathology, USDA-ARS, Cornell University, NY 14853 April, Ithaca.
Chung, B.Y-W., W.A. Miller, J.F. Atkins and A.E. Firth. 2008. An overlapping essen- tial gene in the Potyviridae. Proc. Natl. Acad. Sci. USA. 105: 5807-5902. https://doi.org/10.1073/pnas.0800468105
Crosslin, J.M., P.B. Hamm, K.C. Eastwell, R.E. Thornton, C.R. Brown, D. Corsini, P.J. Shiel and P.H. Berger. 2002. First report of the necrotic strain of potato virus Y (PVYN) on potatoes in the northwestern United States. Plant Dis., 86: 1177. https://doi.org/10.1094/PDIS.2002.86.10.1177C
David, N., I. Mallik and N.C. Gudmestad. 2010. First report of Tobacco rattle virus associated with corky ringspot in potatoes grown in North Dakota. Plant Dis., 94: 130. https://doi.org/10.1094/PDIS-94-1-0130B
Draper, M.D., J.S. Pasche and N.C. Gudmestad. 2002. Factors influencing PVY development and disease expression in three potato cultivars. Am. J. Potato Res., 79: 155-165. https://doi.org/10.1007/BF02871931
El-Araby, W.S., I.A. Ibrahim, A.A. Hemeida, A. Mahmoud, A.M. Soliman, A.K. El-Attar and H.M. Mazyad. 2009. Biological, serological and molecular diagnosis of three major potato viruses in Egypt. Int. J. Virol., 5(2): 77-88. https://doi.org/10.3923/ijv.2009.77.88
Gieck, S., N. David and P. Hamm. 2007. Delayed emergence, stem distortion, stun- ting, and foliar symptoms associated with Tobacco rattle virus and Paratri- chodorus allius in potatoes grown in the pacific northwest. Plant Health Progress. https://doi.org/10.1094/PHP-2007-0917-01-BR
Glais, L., M. Tribodet and C. Kerlan. 2002. Genomic variability in Potato potyvirus Y (PVY): Evidence that PVYNW and PVYNTN variants are single to multiple recombinants between PVYO and PVYN isolates. Arch. Virol., 147: 363-378. https://doi.org/10.1007/s705-002-8325-0
Gul, Z., A.A. Khan, A.U. Khan and Z.U. Khan. 2013. Incidence of potato viruses in different districts of Khyber Pakhtunkhawa, Pakistan. Int. J. Phytol., 2(1): 32-36. https://doi.org/10.33687/phytopath.002.01.0045
Hameed, A., Z. Iqbal, S. Asad and S. Mansoor. 2014. Detection of multiple potato viruses in the field suggests synergistic interactions among potato viruses in Pakistan. Plant Pathol. J., 30(4): 407. https://doi.org/10.5423/PPJ.OA.05.2014.0039
Karasev, A.V., T. Meacham, X. Hu, J. Whitworth, S.M. Gray, N. Olsen and P. Nolte. 2008. Identification of potato virus Y strains associated with tuber damage during a recent virus outbreak in potato in Idaho. Plant Dis., 92: 1371. https://doi.org/10.1094/PDIS-92-9-1371A
Makarova, S., A. Makhotenko, N. Spechenkova, A.J. Love, N.O. Kalinina and M. Taliansky. 2018. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front. Microbiol., 9: 2582. https://doi.org/10.3389/fmicb.2018.02582
Malko, A., P. Frantsuzov, M. Nikitin, N. Statsyuk, V. Dzhavakhiya and A. Golikov. 2019. Potato pathogens in Russia’s regions: An instrumental survey with the use of real time PCR/RT-PCR in matrix format. Pathogens, 8(1): 18. https://doi.org/10.3390/pathogens8010018
Mowry, T.M., 2005. Insecticidal reduction of Potato leafroll virus transmission by Myzus persicae. Ann. Appl. Biol., 146: 81-88. https://doi.org/10.1111/j.1744-7348.2005.03149.x
Mughal, S.M. and S. Khalid. 1985. Virus diseases in relation to potato production in Pakistan, Proc. Pak. Swiss Potato Dev. Proj. Islamabad, pp. 157-165.
Mughal, S.M., S. Khalid, T.S. Gillani and A. Devaux. 1988. Detection of potato viruses in Pakistan. Proc. 2nd Triennial Conf., Jim. 12-26, Kuming, China, pp. 189-190.
Nauen, R. and I. Denholm. 2005. Resistance of insect pests to neonicotinoid insec- ticides: Current status and future prospects. Arch. Insect Biochem. Phys., 58: 200-215. https://doi.org/10.1002/arch.20043
Nerway, Z.A. and N.A. Kassim. 2014. Potato virus Y (PVY) purification and antiserum preparation. J. Agric. Vet. Sci., 7(4): 9-12. https://doi.org/10.9790/2380-07430912
Nikan, J. and H. Barker. 2012. Study of resistance in potato clone G8107 (1) to potato leafroll virus infection. World Appl. Sci. J., 20(10): 1347-1353.
Nolte, P., J.L. Whitworth, M.K. Thornton and C.S. McIntosh. 2004. Effect of seedborne Potato virus Y on performance of Russet Burbank, Russet Norkotah, and Shepody potato. Plant Dis., 88: 248-252. https://doi.org/10.1094/PDIS.2004.88.3.248
Nolte, P., J.S. Miller, B.D. Geary and D.L. Corsini. 2003. Disease mangement. In: (eds. J.C. Stark, S.L. Love) potato production Systems, University of Idaho Extension, USA, pp. 153-183.
Parker, W.E., J.J. Howard, S.P. Foster and I. Denholm. 2006. The effect of insecti- cide application sequences on the control and insecticide resistance status of the peach-potato aphid, Myzus persicae (Hemiptera:Aphididae), on field crops of potato. Pest Manage. Sci., 62: 307-315. https://doi.org/10.1002/ps.1162
Pelletier, Y., X. Nie, M.A. Giguère, U. Nanayakkara, E. Maw and R. Foottit. 2012. A new approach for the identification of aphid vectors (Hemiptera: Aphididae) of Potato virus Y. J. Eco. Ent., 105(6): 1909-1914. https://doi.org/10.1603/EC12085
Qamar, M.I., Y. Iftikhar, Z. Iqbal, M. Mubeen and A. Haq. 2015. Screening of potato germplasm through ELISA against potato virus X (PVX). Univ. J. Plant Sci., 3(2): 21–24. https://doi.org/10.13189/ujps.2015.030202
Radcliffe, E.B. and D.W. Ragsdale. 2002. Aphid-transmitted potato viruses: The importance of understanding vector biology. Am. J. Potato Res., 79(5): 353-386. https://doi.org/10.1007/BF02870173
Robert, Y., J.A.T. Woodford, D.G. Ducray-Bourdin. 2000. Some epidemiological approaches to the control of aphid borne virus diseases in seed potato crops in northern Europe. Virus Res., 71: 33-47. https://doi.org/10.1016/S0168-1702(00)00186-6
Roossinck, M.J., 2015. Plants, viruses and the environment: Ecology and mutualism. Virology, 479: 271-277. https://doi.org/10.1016/j.virol.2015.03.041
Rykbost, K.A., D.C. Hane, P.B. Hamm, R. Voss and D. Kirby. 1999. Effects of seedborne potato virus Y on Russet Norkotah performance. Am. J. Potato Res., 76: 91-96. https://doi.org/10.1007/BF02855205
Shrestha, D., E.J. Wenninger, P.J. Hutchinson, J.L. Whitworth, S. Mondal, S.D. Eigenbrode and N.A. Bosque-Pérez. 2014. Interactions among potato genotypes, growth stages, virus strains, and inoculation methods in the Potato virus Y and green peach aphid pathosystem. Envirn. Ent., 43(3): 662-671. https://doi.org/10.1603/EN13323
Singh, R.P., D.L. McLaren, X. Nie and M. Singh. 2003. Possible escape of a recombinant isolate of Potato virus Y by serological indexing and methods of its detection. Plant Dis., 87: 679-685. https://doi.org/10.1094/PDIS.2003.87.6.679
Singh, R., D. Sharma, D. Choskit and S. Gupta. 2017. Serological detection of Potato leaf roll virus from subtropical zone of Jammu and Kashmir. Plant Dis. Res., 32(2): 211-215.
Urcuqui-Inchima, S., A.L. Haenni and F. Bernadi. 2001. Potyvirus proteins: A wealth of functions. Virus Res., 74: 157-175. https://doi.org/10.1016/S0168-1702(01)00220-9
Valkonen, J.P., 2015. Elucidation of virus-host interactions to enhance resistance breeding for control of virus diseases in potato. Breed. Sci., 65(1): 69-76. https://doi.org/10.1270/jsbbs.65.69
Valkonen, J.P.T., 2007. Viruses, economical losses and biotechnological potential. In: (ed. J. Vreugdenhil) Potato Biol. and Biotech. Elsevier, New York, pp. 619–641. https://doi.org/10.1016/B978-044451018-1/50070-1
Whitworth, J., P. Nolte, C. McIntosh and R. Davidson. 2006. Effect of potato virus Y on yield of three potato cultivars grown under different nitrogen levels. Plant Dis., 90: 73-76. https://doi.org/10.1094/PD-90-0073
Yao, J., P. Saenkham, J. Levy, F. Ibanez C. Noroy, A. Mendoza O. Huot, D.F. Meyer and C. Tamborindeguy. 2016. Interactions candidatus liberibacter solanacearum. Bactericera cockerelli: Haplotype effect on vector fitness and gene expression analyses front. Cell. Infect. Microbiol., 6: 62. https://doi.org/10.3389/fcimb.2016.00062
Zehnder, G., 2010. Overview of monitoring and identification techniques for insect pests. Clemson University. extension, Cooperative Extension System, USA.
To share on other social networks, click on any share button. What are these?





