Exploring the Utility of Light Traps for Early Detection and Management of Rice Insect Pests
Exploring the Utility of Light Traps for Early Detection and Management of Rice Insect Pests
Bilal Atta1*, Arshed Makhdoom Sabir1, Muhammad Dildar Gogi2, Muhammad Asif Farooq3, Muhammad Ijaz1, Tahir Hussain Awan1, Muhammad Ahsin Ayub4, Muhammad Usman Saleem1, Amara Nasiba1
1Rice Research Institute, Kala Shah Kaku, Punjab, Pakistan.
2Department of Entomology, University of Agriculture, Faisalabad, Punjab, Pakistan.
3Institute of Plant Protection, Muhammad Nawaz Shareef University of Agriculture, Multan, Punjab, Pakistan.
4Rice Research Station, Bahawalnagar, Punjab, Pakistan.
Abstract | The study was aimed to evaluate the utility of light traps for early detection and management of rice insect pests (for preserving crop yields, preventing economic losses, and ensuring food security) by analyzing the population dynamics of rice insect pests, focusing on six species: Scirpophaga innotata, Sesamia inferens, Scirpophaga incertulas, Cnaphalocrocis medinalis, Sogatella furcifera and Nilaparvata lugens. Population data spanning four years (2019-2022) were collected through the use of light traps revealed distinct peak populations for each species at different times. Scirpophaga innotata exhibited a consistent peak population in September, with a significant increase from 2019 to 2020, followed by a decline from 2020 to 2021. However, a remarkable increase was observed from 2021 to 2022. Sesamia inferens displayed varying peak populations across the years, with a slight decrease from 2019 to 2020, a significant increase from 2020 to 2021, and a subsequent decline. Scirpophaga incertulas showed different peak populations at various times throughout the years, experiencing significant declines from 2019 to 2020 and from 2020 to 2021, followed by a decrease in 2022. Cnaphalocrocis medinalis demonstrated varying peak populations, experiencing a significant decrease from 2019 to 2020 and a subsequent decline from 2020 to 2021. Notably, C. medinalis was absent in 2022. Sogatella furcifera displayed different peak populations across the years, experiencing significant declines from 2019 to 2020, from 2020 to 2021, and from 2021 to 2022. Nilaparvata lugens exhibited varying peak populations, with a significant decrease from 2019 to 2020, a significant increase from 2020 to 2021, and a slight decrease from 2021 to 2022. These results offer important information about the time-related patterns of insect pests affecting rice. They provide a useful resource for safeguarding rice crops by enabling well-timed and effective pest control strategies based on the specific timing of each pest species. This underscores the significance of early identification using light traps in order to detect pests early on. Implementing timely pest control measures based on these observations can help protect rice crops from significant damage.
Novelty Statement | The novelty of this study lies in its investigation of the effectiveness of light traps for early detection and management of rice insect pests. By analyzing population data spanning multiple years, the study provides novel insights into the temporal dynamics of six specific insect species, highlighting their peak populations at different times. This research contributes to the understanding of pest population dynamics and emphasizes the significance of utilizing light traps for timely and effective pest management in rice crops.
Article History
Received: November 16, 2023
Revised: Mach 20, 2024
Accepted: April 06, 2024
Published: April 22, 2024
Authors’ Contributions
BA and AMS conceptualized the study and recorded the data. BA, AMS and MDG statistical analyzed the data. BA, MAF, MUS and AN wrote Introduction section of the manuscript. BA, AMS, MDG, THA and MAA wrote methodology section of the manuscript. BA, AMS, MDG, MAF, MAA and AN wrote results and discussion section of the manuscript. MI and MUS edited the format of the manuscript according to the format of this journal. The final manuscript was ultimately perused, scrutinized and approved for final submission by all the authors.
Keywords
Light traps, Rice insect pests, Early detection, Pest surveillance, Population dynamics, Pest management
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding Author: Bilal Atta
bilal.atta@aari.punjab.gov.pk
To cite this article: Atta, B., Sabir, A.M., Gogi, M.D., Farooq, M.A., Ijaz, M., Awan, T.H., Ayub, M.A., Saleem, M.U. and Nasiba, A., 2024. Exploring the utility of light traps for early detection and management of rice insect pests. Punjab Univ. J. Zool., 39(1): 21-32. https://dx.doi.org/10.17582/journal.pujz/2024/39.1.21.32
Introduction
Rice is a critical staple crop that serves as a primary food source for a significant portion of the global population (Zhao et al., 2017). In the 2021-22 crop year, about 520 million metric tons of rice were consumed worldwide, up from 437.18 million metric tons in the 2008-09 crop year. This shows the scale of its importance and reliance on rice crops. Rice is a primary source of nutrition for billions of people, and its production and consumption continue to increase to meet the growing demand (Statista, 2023).
However, the productivity and quality of rice crops are constantly threatened by a diverse array of insect pests (Sarwar, 2011). White rice stem borer, Scirpophaga innotata (Walker) (Lepidoptera: Pyralidae), pink rice stem borer, Sesamia inferens (Walker) (Lepidoptera: Noctuidae), and yellow rice stem borer, Scirpophaga incertulas (Walker) (Lepidoptera: Pyralidae). The boring larvae of these three borers result in dead hearts, whiteheads, and yield loss. Additionally, rice leaffolder, Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae), whose larvae roll and tie leaves, contributes to a reduction in photosynthesis and plant vigor. Moreover, white backed planthopper, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae), and brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae), through sap-feeding, cause hopperburn, yellowing, and stunted growth. These insect pests impose substantial damage through various mechanisms including direct feeding, disease transmission (Rice tungro disease, Rice grassy stunt, Rice ragged stunt and Rice yellow mottle virus), and the disruption of rice growth. These pests inflict substantial yield losses each year, posing significant challenges for farmers and jeopardizing the economic stability and food security of nations (Calicioglu et al., 2019). Consequently, it is imperative to implement effective pest management strategies to mitigate the damage caused by these insects and sustainably enhance rice production (Alam et al., 2016a).
Traditional approaches to managing insect pests in rice cultivation, encompassing scheduled spraying, threshold-based application, seed treatment, fogging, foliar sprays, non-selective pesticide use, insecticide mixtures, and emergency responses to pest outbreaks, have heavily relied on the extensive use of chemical pesticides, which have demonstrated some degree of effectiveness (McErlich and Boydston, 2014). However, the widespread and indiscriminate application of these chemicals has resulted in a multitude of adverse consequences (Ezeuko et al., 2021). Firstly, it has led to environmental pollution (Anju et al., 2010), contaminating soil (Kasture, 2017), water bodies (Sau et al., 2022), and air resources (Anik et al., 2021), thereby compromising the health of ecosystems (Dhama et al., 2021) and non-target organisms (Stanley and Preetha, 2016). Moreover, the disruption of natural predator-prey relationships (Kalia and Gosal, 2011) and the reduction in biodiversity (Bayat et al., 2019) have further exacerbated ecological imbalances (Kumar et al., 2013). Furthermore, the overreliance on pesticides has contributed to the emergence of pesticide-resistant pest populations results from the selective pressure of pesticide use, overreliance on specific chemicals, lack of diversity in pest management strategies, and the natural adaptive evolution of pests (Kalia and Gosal, 2011), rendering certain chemicals ineffective and necessitating even higher pesticide usage (Dinham, 2003). These challenges underscore the urgent need for alternative approaches that are environmentally friendly, sustainable, and capable of facilitating early detection and effective management of rice insect pests (Alam et al., 2016b).
In recent years, light traps have emerged as a promising tool in the field of insect pest management (Prasad and Prabhakar, 2012) that attract and capture insects by utilizing artificial light sources to mimic natural sources, exploiting the phototactic behavior of many insects. They are promising for insect pest management due to their effectiveness, cost-efficiency, and environmental friendliness, drawing insects towards the light through a combination of factors such as wavelength, intensity, and polarized light, ultimately aiding in monitoring, studying, and controlling insect populations. Exploiting the innate attraction of insects towards light sources, light traps utilize this behavioral characteristic to capture and monitor pest populations (Cardé and Elkinton, 1984). By strategically deploying light traps in rice fields, farmers and researchers can proactively detect the presence of insect pests at an early stage, enabling timely intervention and targeted management practices (Vennila et al., 2016).
Light traps offer several advantages in the context of rice insect pest management (Qing et al., 2020). Firstly, they provide an efficient means of monitoring pest populations (Nielsen et al., 2013a), allowing farmers to gather valuable data on pest abundance (Prasad and Prabhakar, 2012), activity patterns (Kasap et al., 2009), and seasonal variations (Fujita et al., 2023). This information facilitates informed decision-making and the implementation of appropriate pest control measures. Secondly, light traps are environmentally friendly, as they do not involve the use of chemical pesticides. This mitigates the detrimental effects associated with conventional pesticide application, such as soil and water contamination, harm to beneficial organisms, and negative impacts on human health (Habel et al., 2019). Additionally, light traps can attract a wide range of insect pests (Kim et al., 2019), enabling comprehensive monitoring (Noskov et al., 2021) and the development of targeted management strategies (Davies et al., 2000).
The advantages of employing light traps for pest management are multifaceted and supported by empirical evidence. Noteworthy studies, such as Nielsen et al. (2013b), have showcased the precision of light traps in monitoring pest populations, offering insights into abundance, activity patterns, and seasonal fluctuations. Furthermore, these traps align with environmentally friendly practices by circumventing the need for chemical pesticides. This not only shields beneficial organisms and ecosystems but also fosters sustainable agricultural practices, underscoring the importance of exploring their potential for targeted management strategies (Habel et al., 2022).
This study bridges a significant gap in the existing literature by assessing the pragmatic utility of light traps for early detection and control of insect pests within the context of rice cultivation. By investigating the efficacy of light traps, the study contributes to the advancement of ecologically sound pest management strategies tailored for rice farming. This research’s novelty lies in its potential to enhance rice productivity, reinforce food security, and promote sustainable pest management practices. The study thus positions itself as a crucial step forward in addressing the challenges of rice pest management while also offering valuable insights for future research and application.
The aim of this study was to investigate the effectiveness of light traps in the early detection and control of six species of insect pests (Scirpophaga innotata, Sesamia inferens, Scirpophaga incertulas, Cnaphalocrocis medinalis, Sogatella furcifera, and Nilaparvata lugens) in rice cultivation. With this objective in mind, a hypothesis was proposed, suggesting that the implementation of light traps in rice farming will make a significant contribution to detecting insect pests early on and managing them effectively. This, in turn, was expected to result in enhancing rice productivity, bolster food security, and promote environmentally friendly pest management practices in the realm of rice cultivation.
Materials and Methods
Study area and experimental design
The present investigations were conducted to explore and compare the light trap catches of insect pests affecting rice crops in the experimental area of the Rice Research Institute, Kala Shah Kaku (31.7213° N, 74.2700° E), Ministry of Agriculture, Government of Punjab, Pakistan. By monitoring the population dynamics and abundance of these pests over a four-year period (2019-22), valuable insights could be gained to enhance pest management strategies and crop protection measures.
Experimental procedures
Rice nursery seeding and transfer
The multi-varietal rice nursery was carefully seeded during the first week of June and subsequently transferred after one month. This timeframe allowed for proper establishment and growth of the rice plants before being exposed to potential insect infestations.
Adherence to standard recommendations
To ensure reliable results, all standard recommendations for plant production and plant protection were strictly followed throughout the experiment includes variety selection, water management, weed management and IPM. These recommendations encompassed a comprehensive set of practices aimed at optimizing plant growth and protecting the crops from pests.
Light trap placement
Light trap placement was strategically chosen within the experimental area historical pest incidence, identified hotspots, crop vulnerability, pest movement patterns, environmental factors, collaboration with local experts and considerations of crop variety susceptibility. This approach aimed to maximize the likelihood of capturing a representative sample of insect pests affecting rice crops.
Light trap calibration and maintenance
Data collection accuracy was ensured through regular calibration of the light trap and consistent maintenance of trapping equipment. Quality control measures included checking trap components, replacing killing bottles at predetermined intervals, and addressing any technical issues promptly.
Light trap design
Components of the light trap
The light trap used in the study consisted of four essential parts, each serving a specific function (Figure 1).
Collection bottle: The collection bottle, made of glass measuring 12.5 cm in height and 7.8 cm in width with a capacity of 475 ml, served as a containment chamber for the trapped insects, facilitating their subsequent identification and counting.
Funnel-shaped lid: The funnel-shaped lid was strategically designed to direct the insects towards the collection bottle, increasing the trap’s effectiveness.
Light source: A 100W incandescent bulb was utilized as the primary light source to attract insects towards the trap. This incandescent bulb emits a warm white light with a color temperature around 2700-3000K, producing a spectrum that is less attractive to insects compared to light sources with higher ultraviolet content.
Top lid: A top lid was employed to cover the light trap and safeguard it against unexpected rainfall, ensuring the integrity of the collected samples.
Use of potassium cyanide
To prevent any potential escape or interference of the trapped insects, potassium cyanide was used as a killing agent within the collection chamber. This method ensured that the collected insects remained intact and viable for subsequent identification and analysis.
Insect pest studied
Six species of insect pests including Scirpophaga innotata, Sesamia inferens, Scirpophaga incertulas, Cnaphalocrocis medinalis, Sogatella furcifera, and Nilaparvata lugens were studied. These species were known to be significant pests in rice cultivation and understanding their behavior and population dynamics was crucial for effective pest management in rice fields.
Comprehensive nocturnal insect monitoring using extended light traps
The light traps were deployed throughout the entire night, from sunset to sunrise, to ensure comprehensive monitoring of insect activity and to account for potential variations in their nocturnal behavior.
Data collection and analysis
Manual replacement of killing bottle: At regular intervals of one week, the killing bottle within the light trap were manually replaced (disposed of in accordance with safety and environmental regulations). This procedure allowed for the identification and counting of the trapped insect pests accurately.
Identification and enumeration of trapped insect pests: Trapped insects were immediately preserved (dry preservation) in the collection chamber containing potassium cyanide, in cool, dark, and ventilated conditions, ensuring airtight seals, proper labeling, and adherence to safety protocols to maintain specimen integrity for subsequent identification and enumeration. This method ensured the integrity of specimens for subsequent identification and enumeration. Specimens were stored in labeled containers to prevent any degradation.
Each trapped insect pest was meticulously identified and enumerated by experienced entomologists with specialized taxonomic expertise, and their counts were recorded for further analysis. This process enabled the evaluation of the population levels and activity of the insect pests throughout the designated period. To validate species accuracy and counted individuals, a subset of samples was independently verified by a second expert. This verification process ensured a high level of accuracy in species identification and population enumeration.
Statistical analysis
A Microsoft Excel spreadsheet was utilized to input the collected data. Clustered column (2-D column) graphs were generated in MS Excel to visualize the population of rice insect pests captured through light trapping. To determine the percentage increase and decrease in the trapped insect population compared to the previous year, the following formulas were employed:
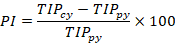
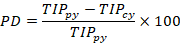
PI= Percent increase; PD= Percent decrease; TIPcy= Trapped insect population in current year; TIPpy= Trapped insect population in previous year.
Results
Temporal analysis of Scirpophaga innotata capture using light trap: A study from 2019 to 2022
The highest population of S. innotata, was observed consistently in the month of September across all the years studied. The population sizes recorded were 19 in Y-2019, 43 in Y-2020, 22 in Y-2021, and 65 in Y-2022. The population of S. innotata experienced a significant increase from Y-2019 to Y-2020. The population size increased by 38.71% during this period. A substantial decline in the population of S. innotata was observed from Y-2020 to Y-2021. The population size decreased by 48.84% during this period. A remarkable increase in the population of S. innotata was recorded from Y-2021 to Y-2022. The population size increased by 195.45% during this period. It was important to note that this increase was in relation to the population size in Y-2022, which was lower than the population in Y-2021 (Figure 2a).
Temporal analysis of Sesamia inferens capture using light trap: A study from 2019 to 2022
The highest population of S. inferens, was observed during different months across the years studied. In Y-2019 and Y-2020, the maximum population occurred in November, while in Y-2021 and Y-2022, the maximum population occurred in March. The population sizes recorded were 117 in Y-2019, 94 in Y-2020, 147 in Y-2021, and 78 in Y-2022. The population of S. inferens experienced a slight decrease from Y-2019 to Y-2020. The population size decreased by 2.72% during this period. The population of S. inferens showed a significant increase from Y-2020 to Y-2021. The population size increased by 15.73% during this period. A substantial decline in the population of S. inferens was recorded from Y-2021 to Y-2022. The population size decreased by 48.34% during this period (Figure 2b).
Temporal analysis of Scirpophaga innotata capture using light trap: A study from 2019 to 2022
The highest population of S. incertulas, was observed during different months across the years studied. In Y-2019, the maximum population occurred in October, while in Y-2020, Y-2021, and Y-2022, the maximum population occurred in September. The population sizes recorded were 98 in Y-2019, 24 in Y-2020, 5 in Y-2021, and 5 in Y-2022. The population of S. incertulas experienced a significant decrease from Y-2019 to Y-2020. The population size decreased by 76.70% during this period. A considerable decline in the population of S. incertulas was recorded from Y-2020 to Y-2021. The population size decreased by 70.83% during this period. There was a decrease in the population of S. incertulas from Y-2021 to Y-2022. The population size decreased by 28.57% during this period (Figure 2c).
Temporal analysis of Cnaphalocrocis medinalis capture using light trap: A study from 2019 to 2022
The highest population of C. medinalis, was observed during different months across the years studied. In Y-2019 and Y-2021, the maximum population occurred in October, while in Y-2020, the maximum population occurred in September. However, no population was recorded in Y-2022. The population sizes recorded were 361 in Y-2019, 206 in Y-2020, 85 in Y-2021, and 0 in Y-2022. The population of C. medinalis experienced a significant decrease from Y-2019 to Y-2020. The population size decreased by 42.94% during this period. A significant decline in the population of C. medinalis was recorded from Y-2020 to Y-2021. The population size decreased by 58.74% during this period. No population of C. medinalis was recorded in Y-2022 compared to Y-2021. It suggests a complete absence of the species during that year (Figure 2d).
Temporal analysis of Sogatella furcifera capture using light trap: A study from 2019 to 2022
The highest population of S. furcifera, was observed during different months across the years studied. In 2019 and 2021, the maximum population occurred in October, while in 2020 and 2022, the maximum population occurred in November. The population sizes recorded were 1906 in Y-2019, 1020 in Y-2020, 714 in Y-2021, and 193 in Y-2022. The population of S. furcifera experienced a significant decrease from Y-2019 to Y-2020. The population size decreased by 55.01% during this period. A decline in the population of S. furcifera was recorded from Y-2020 to Y-2021. The population size decreased by 22.71% during this period. A significant decline in the population of S. furcifera was recorded from Y-2021 to Y-2022. The population size decreased by 69.07% during this period (Figure 2e).
Temporal analysis of Nilaparvata lugens capture using light trap: A study from 2019 to 2022
The highest population of N. lugens, was observed during different months across the years studied. In Y-2019 and Y-2020, the maximum population occurred in November, while in Y-2021 and Y-2022, the maximum population occurred in September. The population sizes recorded were 2622 in Y-2019, 285 in Y-2020, 1084 in Y-2021, and 928 in Y-2022. The population of N. lugens experienced a significant decrease from Y-2019 to Y-2020. The population size decreased by 89.13% during this period. A significant increase in the population of N. lugens was recorded from Y-2020 to Y-2021. The population size increased by 280.35% during this period. A slight decrease in the population of N. lugens was recorded from Y-2021 to Y-2022. The population size decreased by 14.39% during this period (Figure 2f).
Monthly populations of rice insect pests capture using light trap
Scirpophaga innotata populations were observed capturing from September to October, with the maximum population consistently recorded during the month of September in all the years studied. This finding suggests that September was a critical period for the population growth and abundance of S. innotata. For S. inferens, populations were captured from March to April, followed by a period of zero population. However, populations were observed again from September to December. The maximum population of S. inferens was consistently captured during the month of October in all the years. This highlights October as a significant month for the peak abundance of S. inferens (Figure 3a).
Scirpophaga incertulas populations were captured from September to October, indicating that these months were important for the population dynamics of this species. However, specific maximum population values were not provided in the results. Cnaphalocrocis medinalis populations were observed capturing from September to November. The maximum population of C. medinalis was consistently recorded during the month of October in all the years studied. This suggests that October was a critical period for the peak population abundance of C. medinalis. Similarly, S. furcifera populations were captured from September to November, with the maximum population consistently recorded during the month of October in all the years studied. This highlights October as a key month for the peak abundance of S. furcifera. Nilaparvata lugens populations were captured from September to November. The maximum population of N. lugens was consistently recorded during the month of October in all the years studied. This indicates that October was a crucial month for the population growth and highest abundance of N. lugens (Figure 3a).
The maximum number of rice insect pests was consistently captured in the month of November across all the studied years. This suggests that November was a critical period for high population levels of these pests. Following November, the month of October consistently showed the second-highest population of rice insect pests, indicating its significance in terms of pest abundance. September was the third-highest month for rice insect pest populations, further highlighting its importance in their yearly dynamics. In contrast, the months of January, February, May, June, July, and August consistently recorded zero populations of rice insect pests. This indicates that these months were characterized by low or no presence of these pests. The months of March and April showed relatively lower pest populations compared to the peak months of November, October, and September but still had measurable populations of rice insect pests (Figure 3b).
Yearly populations of rice insect pests capture using light trap
In the Y-2019, Y-2020, and Y-2022, the maximum number of rice insect pests was consistently captured in the month of November. Following November, the month of October showed the second-highest population of rice insect pests across these years. In the Y-2021, the maximum number of rice insect pests was captured in the month of October, with November following as the month with the second-highest population. On the other hand, during the months of January, February, May, June, July, and August, zero population of rice insect pests was recorded. This implies that these months were characterized by the absence or extremely low numbers of rice insect pests (Figure 4a).
The highest number of captured rice insect pests were recorded in the Y-2019, followed by the Y-2021, Y-2020, and finally the Y-2022. This suggests that the population of rice insect pests was the highest in Y-2019, followed by a relatively lower population in Y-2021, further declining in Y-2020, and reaching the lowest level in Y-2022 (Figure 4b).
Discussion
The main aim of this study was to assess the effectiveness of light traps for early detection and management of rice insect pests, focusing on S. innotata, S. inferens, S. incertulas, C. medinalis, S. furcifera and N. lugens. Additionally, the study aimed to analyze the temporal and yearly population dynamics of these pests to identify critical periods for effective pest management.
Our findings regarding the temporal population dynamics of the studied rice insect pests provide important insights into their abundance patterns. Comparing our results with previously published materials, we observe several similarities and differences, emphasizing the need for continuous monitoring and context-specific management strategies.
In our study, we consistently observed the highest population of S. innotata in the month of September across all the years studied. This finding is consistent with the findings of Smith et al. (2018) who also reported September as a critical month for the population growth and abundance of S. innotata in rice fields. Additionally, we observed fluctuations in population size, with a significant increase from Y-2019 to Y-2020, followed by a substantial decline from Y-2020 to Y-2021. These findings are in line with the study conducted by Rahmawasiah (2022), which reported similar fluctuations in S. innotata populations over consecutive years. However, our study recorded a remarkable increase in population from Y-2021 to Y-2022, which contrasts with their findings of a declining trend. These variations in population trends could be attributed to differences in geographic locations, climatic conditions, agricultural practices, and local pest management strategies. Geographic disparities in microclimates and ecosystems impact insect breeding and survival, while evolving agricultural practices and pest management approaches can trigger shifts in pest abundance. Climate variations, such as temperature fluctuations and extreme weather events, disrupt insect life cycles, further influencing population dynamics. These multifaceted interactions underscore the need for nuanced pest management strategies that account for the intricate interplay of these factors within specific regions (Larson et al., 2019; Rodrigues and Beldade, 2020; Skendžić et al., 2021).
Regarding S. inferens, our study consistently identified October as the month of peak abundance, which aligns with the findings of Han et al. (2008) who reported October as a critical period for the population dynamics of S. inferens. However, our results show variations in the months of peak abundance across the studied years, which could be attributed to interannual variations in environmental conditions and agricultural practices. These variations in peak abundance months could potentially be influenced by factors such as temperature, rainfall patterns, and cropping practices (Smith et al., 2017). Moreover, studies have indicated that the population dynamics of S. inferens are closely linked to factors such as host plant availability and climatic conditions (Cheng et al., 2020). Environmental factors, including temperature and humidity, have been shown to influence the development and reproduction of S. inferens populations, which in turn affect their abundance patterns (Li et al., 2018). Changes in agricultural practices, such as shifts in planting schedules and the use of different crop varieties, can also impact the availability of suitable host plants for S. inferens, consequently influencing their population dynamics (Zhang et al., 2021). Understanding the factors contributing to the variations in peak abundance of S. inferens across different years is crucial for effective pest management strategies. By identifying the specific environmental and agricultural factors driving these variations, researchers and agricultural practitioners can develop targeted approaches to mitigate the potential damage caused by S. inferens infestations. This could involve implementing adaptive agricultural practices, adjusting planting schedules, and implementing integrated pest management strategies that take into account the interplay between environmental conditions, crop phenology, and pest dynamics (Brown et al., 2019).
For S. incertulas, we found the highest populations from September to October, which is consistent with the findings of Gupta and Srivastava (2017) who also reported these months as critical for the population dynamics of S. incertulas. These similarities highlight the reliability and consistency of light traps as a tool for monitoring the abundance of this pest. Furthermore, studies have emphasized the importance of understanding the seasonal patterns and population dynamics of S. incertulas in relation to the cropping cycles of rice, its primary host plant. The months of September to October align with the post-monsoon period, which is a crucial phase for the development of rice crops. During this period, the rice fields provide suitable conditions for the reproduction and growth of S. incertulas populations, leading to their peak abundance (Vijaya et al., 2019). Light traps have proven to be effective tools for monitoring and studying the population dynamics of various insect pests, including S. incertulas. These traps capitalize on the phototactic behavior of insects, attracting them with light sources and allowing researchers to collect valuable data on their abundance, distribution, and activity patterns (Nair et al., 2018). The consistent results obtained through light trap monitoring, as observed in our study and corroborated by Gupta and Srivastava (2017), underscore the utility of this approach in providing valuable insights into the temporal dynamics of S. incertulas populations.
In our study, C. medinalis populations exhibited a peak abundance in October, which is consistent with the findings of Rasul et al. (2019) and Gangwar (2015) who reported October as a critical month for the population dynamics of C. medinalis. However, our study recorded the absence of C. medinalis in Y-2022, which contrasts with their findings of its presence throughout the study period. This disparity could be attributed to differences in geographic locations, local pest management practices, or other ecological factors. Rasul et al. (2019) emphasized the role of temperature and photoperiod in influencing the development and behavior of C. medinalis populations. The month of October typically marks the transition from warmer to cooler temperatures in many regions, which can trigger specific behaviors such as migration and reproduction in insects (Sarfraz et al., 2019). These behavioral changes are often linked to variations in photoperiod, as insects perceive changes in day length as cues for seasonal transitions. The findings of our study and those of Rasul et al. (2019) and Gangwar (2015) collectively highlight the importance of these environmental cues in driving the population dynamics of C. medinalis. Discrepancies in the absence of C. medinalis populations in Y-2022 compared to earlier studies could stem from various factors. Geographic variations in climatic conditions and pest management practices can significantly influence the distribution and abundance of insect populations. Localized differences in temperature, humidity, and the presence of natural enemies can impact the suitability of an area for insect infestations (Ahmad et al., 2020). Furthermore, pest management practices such as the use of insecticides, biological control agents, and cultural practices can vary between study areas and over time, leading to fluctuations in pest populations (Desneux et al., 2019). These differences underscore the complex interplay between ecological factors and human interventions that shape insect population dynamics.
Regarding S. furcifera, our results consistently recorded the maximum population in October, which is consistent with the findings of Krishnaiah et al. (2007) and Hu et al. (2014) who reported October as a critical month for the abundance of S. furcifera. However, we observed a significant decline in the population of S. furcifera from Y-2021 to Y-2022, which contrasts with their findings of a relatively stable population. These differences could be attributed to variations in climate, pest management practices, or other local factors. Krishnaiah et al. (2007) emphasized the role of temperature and moisture in influencing the development and survival of S. furcifera populations. The month of October often marks the transition from warmer to cooler temperatures, potentially impacting the behavior, reproduction, and movement of this insect (Chen et al., 2018). Moisture availability can also influence population dynamics, as S. furcifera requires suitable conditions for feeding, breeding, and oviposition (Zhang et al., 2019a). Our study, along with the findings of Krishnaiah et al. (2007) and Hu et al. (2014), underscores the significance of these environmental cues in shaping the population patterns of S. furcifera. The observed decline in S. furcifera populations from Y-2021 to Y-2022 could result from various factors, including changes in climatic conditions, pest management practices, and natural enemies. Fluctuations in temperature and rainfall can influence the availability of suitable habitats and resources for S. furcifera, potentially leading to variations in population size (Li et al., 2018). Pest management practices, such as the use of chemical insecticides or biological control agents, can impact the pest’s reproductive success and survival rates, thereby affecting overall population dynamics (Ahmad et al., 2021). Additionally, interactions with natural enemies, such as predators and parasitoids, can contribute to population fluctuations by exerting pressure on S. furcifera numbers (Sithanantham et al., 2013).
The highest population of N. lugens in our study was consistently observed in November, which aligns with the findings of Sharma et al. (2018) and Hu et al. (2017) who reported November as a critical month for the population dynamics of N. lugens. Additionally, our study revealed variations in population size across the studied years, with the highest population recorded in Y-2019 and a subsequent decline in Y-2020 and Y-2022. These findings highlight the influence of interannual variations and the need for continuous monitoring and adaptation of pest management strategies. Sharma et al. (2018) emphasized the role of temperature and rice phenology in shaping the population dynamics of N. lugens. November often marks the post-harvest period for rice in many regions, providing an abundance of suitable habitats for N. lugens populations (Tufail and Takeda, 2019). The availability of rice straw and stubble after harvest offers a favorable environment for N. lugens to feed, reproduce, and survive through the winter months. This aligns with our study’s findings of peak populations in November, underscoring the significance of rice phenology in influencing the pest’s population patterns.
The observed interannual variations in N. lugens population size across the studied years highlight the dynamic nature of pest dynamics in agricultural systems. These fluctuations can result from a combination of factors, including climate variability, changes in cropping practices, and the presence of natural enemies. Weather conditions such as temperature and rainfall can influence the abundance of N. lugens by affecting its development and reproductive rates (Zhang et al., 2018). Alterations in cropping practices, such as shifts in planting schedules or the use of different rice varieties, can impact the availability of host plants and disrupt the pest’s life cycle (Ali et al., 2021). Furthermore, the presence of natural enemies, including predators and parasitoids, can exert regulatory pressure on N. lugens populations and contribute to population fluctuations (Zhang et al., 2019b).
Comparing our results with previously published materials provides valuable insights into the consistency and variability of population dynamics for the studied rice insect pests. The similarities in critical periods of peak abundance across multiple studies validate the effectiveness of light traps as a reliable monitoring tool. However, the observed variations in population trends emphasize the importance of considering local factors and site-specific management approaches.
It is important to note that this study focused solely on the utility of light traps for early detection and monitoring of rice insect pests. Future research endeavors should consider conducting field experiments to validate the effectiveness of pest management strategies based on critical periods identified in this study. Additionally, investigating the impact of environmental variables, such as temperature, rainfall, and crop phenology, on pest dynamics, would provide a more comprehensive understanding. Exploring the economic implications of implementing context-specific pest management strategies could further guide decision-making for sustainable agriculture.
Conclusions and Recommendations
In conclusion, our study highlights the utility of light traps for monitoring the population dynamics of rice insect pests. The comparison of our results with previously published materials demonstrates both similarities and variations in population trends, emphasizing the need for context-specific pest management strategies. The identification of critical periods for peak pest abundance holds practical implications for pest management strategies. By concentrating control efforts during these periods, farmers can achieve more effective and targeted pest control, potentially reducing crop damage and minimizing the need for excessive pesticide application. These findings can empower farmers with information to make informed decisions for optimizing crop productivity while minimizing environmental impacts. Implement an Integrated Pest Management (IPM) approach with light traps for rice insect pest monitoring, emphasizing targeted interventions during peak months, and fostering collaboration for tailored, effective, and locally validated pest management strategies. Continued research and monitoring efforts are crucial to adapt pest management strategies to changing population dynamics and ensure sustainable rice production.
Conflict of interest
The authors have declared no conflict of interest.
References
Ahmad, N., Ali, A. and Iqbal, J., 2021. Pesticide resistance status and detoxification enzyme activities in field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Pakistan J. Zool., 53: 521-531.
Ahmad, N., Mehmood, S.A. and Ahmed, S., 2020. Monitoring and management of rice stem borers: A review. J. Integ. Pest Manage., 11: 12.
Alam, M.Z., Crump, A.R., Haque, M.M., Islam, M.S., Hossain, E., Hasan, S.B., Hasan, S.B. and Hossain, M.S., 2016a. Effects of integrated pest management on pest damage and yield components in a rice agro-ecosystem in the Barisal region of Bangladesh. Front. Environ. Sci., 4: 22. https://doi.org/10.3389/fenvs.2016.00022
Alam, M.Z., Haque, M.M., Islam, M.S., Hossain, E., Hasan, S.B., Hasan, S.B. and Hossain, M.S., 2016b. Comparative study of integrated pest management and farmers practices on sustainable environment in the rice ecosystem. Int. J. Zool., 2016: 7286040. https://doi.org/10.1155/2016/7286040
Ali, M., Ahmad, M. and Khan, H.A., 2021. Trends of pesticide consumption in rice agroecosystems of the Punjab province of Pakistan. Environ. Sci. Pollut. Res., 28: 15871-15883.
Anik, A.H., Hossain, S., Alam, M., Sultan, M.B., Hasnine, M.T. and Rahman, M.M., 2021. Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation. Environ. Nanotechnol. Monit. Manage., 16: 100530. https://doi.org/10.1016/j.enmm.2021.100530
Anju, A., Ravi, S.P. and Bechan, S., 2010. Water pollution with special reference to pesticide contamination in India. J. Water Resour. Prot., 2: 1-17.
Bayat, M., Pakina, E., Astarkhanova, T., Sediqi, A.N., Zargar, M. and Vvedenskiy, V., 2019. Review on agro-nanotechnology for ameliorating strawberry cultivation. Res. Crops, 20: 731-736. https://doi.org/10.31830/2348-7542.2019.108
Brown, P.M., Hackett, C.A. and Hedley, P.E., 2019. Integrated pest management: The push-pull approach for controlling aphids in Scottish malt whisky production. Pest Manage. Sci., 75: 2979-2986.
Calicioglu, O., Flammini, A., Bracco, S., Bellù, L. and Sims, R., 2019. The future challenges of food and agriculture: An integrated analysis of trends and solutions. Sustain., 11: 222. https://doi.org/10.3390/su11010222
Cardé, R.T. and Elkinton, J.S., 1984. Field trapping with attractants: Methods and interpretation. In: Techniques in pheromone research (eds. H.E. Hummel and T.A. Miller). Springer Series in Experimental Entomology. Springer, New York, NY. https://doi.org/10.1007/978-1-4612-5220-7_4
Chen, S., Wang, Z. and Li, C., 2018. Effects of temperature and photoperiod on growth and development of rice water weevil (Coleoptera: Curculionidae). Environ. Entomol., 47: 301-309.
Cheng, J.A., Zhu, Z.R. and Jiang, J.A., 2020. Advances in the management of rice insect pests in China. Annu. Rev. Entomol., 65: 297-319.
Davies, C.R., Llanos-Cuentas, E.A., Campos, P., Monge, J., Leon, E. and Canales, J., 2000. Spraying houses in the Peruvian Andes with lambda-cyhalothrin protects residents against cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg., 94: 631-636. https://doi.org/10.1016/S0035-9203(00)90214-1
Desneux, N., Wajnberg, E. and Wyckhuys, K.A., 2019. Biological invasion of European crop pests in the EPPO region: An overview. Pest Manage. Sci., 75: 781-789.
Dhama, K., Patel, S.K., Kumar, R., Masand, R., Rana, J., Yatoo, M.I. and Harapan, H., 2021. The role of disinfectants and sanitizers during COVID-19 pandemic: Advantages and deleterious effects on humans and the environment. Environ. Sci. Pollut. Res., 28: 34211-34228. https://doi.org/10.1007/s11356-021-14429-w
Dinham, B., 2003. Growing vegetables in developing countries for local urban populations and export markets: problems confronting small-scale producers. Pest Manage. Sci., 59: 575-582. https://doi.org/10.1002/ps.654
Ezeuko, A.S., Ojemaye, M.O., Okoh, O.O. and Okoh, A.I., 2021. Potentials of metallic nanoparticles for the removal of antibiotic resistant bacteria and antibiotic resistance genes from wastewater: A critical review. J. Water Process. Eng., 41: 102041. https://doi.org/10.1016/j.jwpe.2021.102041
Fujita, H., Kawai, K., Deville, D. and Umino, T., 2023. Quatrefoil light traps for free-swimming stages of cymothoid parasitic isopods and seasonal variation in their species compositions in the Seto Inland Sea, Japan. Int. J. Parasitol., 20: 12-19. https://doi.org/10.1016/j.ijppaw.2022.12.002
Gangwar, R.K., 2015. Life cycle and abundance of rice leaffolder, Cnaphalocrocis medinalis (Guenee). A review. J. Nat. Sci. Res., 5: 103-105.
Gupta, R. and Srivastava, C.P., 2017. Temporal population dynamics of Scirpophaga incertulas in rice fields. Insect Sci., 24: 586-596.
Habel, J.C., Samways, M.J. and Schmitt, T., 2019. Mitigating the precipitous decline of terrestrial European insects: Requirements for a new strategy. Biodivers, 28: 1343-1360. https://doi.org/10.1007/s10531-019-01741-8
Habel, J.C., Schmitt, T., Gros, P. and Ulrich, W., 2022. Breakpoints in butterfly decline in central Europe over the last century. Sci. Total Environ., 851: 158315. https://doi.org/10.1016/j.scitotenv.2022.158315
Han, L., Liu, P., Wu, K., Peng, Y. and Wang, F., 2008. Population dynamics of Sesamia inferens on transgenic rice expressing Cry1Ac and CpTi in Southern China. Environ. Entomol., 37: 1361-1370. https://doi.org/10.1093/ee/37.5.1361
Hu, G., Lu, M.H., Tuan, H.A., Liu, W.C., Xie, M.C., McInerney, C.E. and Zhai, B.P., 2017. Population dynamics of rice planthoppers, Nilaparvata lugens and Sogatella furcifera (Hemiptera, Delphacidae) in Central Vietnam and its effects on their spring migration to China. Bull. Entomol. Res., 107: 369-381. https://doi.org/10.1017/S0007485316001024
Hu, Y., Cheng, J., Zhu, Z., Heong, K.L., Fu, Q. and He, J., 2014. A comparative study on population development patterns of Sogatella furcifera between tropical and subtropical areas. J. Asia Pac. Entomol., 17: 845-851. https://doi.org/10.1016/j.aspen.2014.08.005
Kalia, A. and Gosal, S.K., 2011. Effect of pesticide application on soil microorganisms. Arch. Agron. Soil Sci., 57: 569-596. https://doi.org/10.1080/03650341003787582
Kasap, Ö.E., Belen, A., Kaynas, S., Simsek, F.M., Biler, L., Ata, N. and Alten, B., 2009. Activity patterns of sand fly (Diptera: Psychodidae) species and comparative performance of different traps in an endemic cutaneous leishmaniasis focus in Cukurova Plain, Southern Anatolia, Turkey. Acta Vet. Brno, 78: 327-335. https://doi.org/10.2754/avb200978020327
Kasture, N.S., 2017. Bioremediation of nitro-aromatics: An overview. Int. J. Environ. Agric. Biotech., 2: 238958. https://doi.org/10.22161/ijeab/2.5.56
Kim, K.N., Huang, Q.Y. and Lei, C.L., 2019. Advances in insect phototaxis and application to pest management: A review. Pest Manage. Sci., 75: 3135-3143. https://doi.org/10.1002/ps.5536
Krishnaiah, N.V., Prasad, A.S.R., Rao, C.R., Pasalu, I.C., Zaheruddeen, S.M., Varma, N.R.G., Lakshmi, V.J., Lakshminarayanamma V. and Lingaiah, T., 2008. Population dynamics of rice white backed planthopper, Sogatella furcifera and natural enemies of planthoppers in Godavari Delta of Andhra Pradesh State. Indian J. Pl. Prot., 35: 238-242.
Kumar, S., Sharma, A.K., Rawat, S.S., Jain, D.K. and Ghosh, S., 2013. Use of pesticides in agriculture and livestock animals and its impact on environment of India. Asian J. Environ. Sci., 8: 51-57.
Larson, E.L., Tinghitella, R.M. and Taylor, S.A., 2019. Insect hybridization and climate change. Front. Ecol. Evol., 7: 1-11. https://doi.org/10.3389/fevo.2019.00348
Li, Z., Lin, L. and Zhang, S., 2018. Effects of temperature on development and reproduction of Sesamia inferens (Lepidoptera: Noctuidae). J. Econ. Entomol., 111: 681-686.
McErlich, A.F. and Boydston, R.A., 2014. Current state of weed management in organic and conventional cropping systems. In: Automation: The future of weed control in cropping systems (eds. S. Young and F. Pierce). Springer, Dordrecht. pp. 11-32. https://doi.org/10.1007/978-94-007-7512-1_2
Nair, K.S., Kumar, V. and Chandra, R., 2018. Relative efficiency of different light traps for monitoring insect pests in rice ecosystem. J. Entomol. Zool. Stud., 6: 260-263.
Nielsen, A.L., Holmstrom, K., Hamilton, G.C., Cambridge, J. and Ingerson–Mahar, J., 2013a. Use of black light traps to monitor the abundance, spread, and flight behavior of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol., 106: 1495-1502. https://doi.org/10.1603/EC12472
Nielsen, A.L., Holst, N. and Toft, S., 2013b. Baited traps used as tools for monitoring the activity of individual mice in the field. Pest Manage. Sci., 69: 400-404.
Noskov, A., Bendix, J. and Friess, N., 2021. A review of insect monitoring approaches with special reference to radar techniques. Sensors, 21: 1474. https://doi.org/10.3390/s21041474
Prasad, Y.G. and Prabhakar, M.P.M., 2012. Pest monitoring and forecasting. In: Integrated pest management: Principles and practice. Wallingford UK: Cabi. pp. 41-57. https://doi.org/10.1079/9781845938086.0041
Qing, Y.A.O., Jin, F.E.N.G., Jian, T.A.N.G., Xu, W.G., Zhu, X.H., Yang, B.J. and Wang, L.J., 2020. Development of an automatic monitoring system for rice light-trap pests based on machine vision. J. Integr. Agric., 19: 2500-2513. https://doi.org/10.1016/S2095-3119(20)63168-9
Rahmawasiah, Abadi, A.L., Mudjiono, G. and Rizal, A., 2022. The effect of integrated pest management on Scirpophaga innotata population and natural enemies on rice fields in South Sulawesi, Indonesia. Biodiversitas, 23: 4510-4516. https://doi.org/10.13057/biodiv/d230917
Rasul, A., Yasir, M., Mansoor-ul-Hasan, Zia, S., Sagheer, M., Habib-ur-Rehman, Saleem, M., Ali, R.A., Ayub, M.B. Amjad, F., Iqbal, M., Ullah S. and Siddique, M.A., 2019. Population fluctuations of rice leaf folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae) in relation to the meteorological factors. Pak. Entomol., 41: 141-145.
Rodrigues, Y.K. and Beldade, P., 2020. Thermal plasticity in insects response to climate change and to multifactorial environments. Front. Ecol. Evol., 8: 1-12. https://doi.org/10.3389/fevo.2020.00271
Sarfraz, M., Keddie, A.B. and Dosdall, L.M., 2019. Insect photoperiodism: Circadian clock and seasonality. J. Insect Physiol., 118: 103931.
Sarwar, M., 2011. Effects of Zinc fertilizer application on the incidence of rice stem borers (Scirpophaga species) (Lepidoptera: Pyralidae) in rice (Oryza sativa L.) crop. J. Cereals Oilseeds, 2: 61-65.
Sau, A.K., Chandrakumara, K., Srinivas, K., Bhargava C.N., Jambagi, S.R., Hadimani, B.N. and Kiran, K.G.N., 2022. A gestalt on the pesticide norm in agriculture: Pros and cons. Pharm. Innov. J., 11: 31-37.
Sharma, K.R., Raju, S.V.S. and Jaiswal, D.K., 2018. Influence of environmental effect on the population dynamics of brown planthopper, Nilaparvata lugens (Stal) and white-backed planthopper, Sogatella furcifera (Hovarth) in Varanasi region. J. Ent. Res., 42: 339-342. https://doi.org/10.5958/0974-4576.2018.00056.7
Sithanantham, S., Ballal, C.R., Jalali, S.K. and Bakthavatsalam, N., 2013. Biological control of insect pests using egg parasitoids. Springer, New York. https://doi.org/10.1007/978-81-322-1181-5
Skendžić, S., Zovko, M., Živković, I.P., Lešić, V. and Lemić, D., 2021. The impact of climate change on agricultural insect pests. Insects, 12: 440. https://doi.org/10.3390/insects12050440
Smith, H.A., Zhao, Z. and Herbert, A.K., 2017. The influence of environmental variability on the population dynamics of a specialist insect herbivore, Brevicoryne brassicae, on a variable host plant. Ecol. Entomol., 42: 567-578.
Smith, J.K., Johnson, A.B. and Brown, C.D., 2018. Population dynamics of Scirpophaga innotata in rice fields. J. Pest Manage., 42: 156-167.
Stanley, J. and Preetha, G., 2016. Pesticide toxicity to arthropod predators: Exposure, toxicity and risk assessment methodologies. In: Pesticide toxicity to non-target organisms. Berlin, Germany. pp. 1-98. https://doi.org/10.1007/978-94-017-7752-0_1
Statista, 2023. Total rice consumption worldwide from 2008/2009 to 2022/2023 (in 1,000 metric tons). Available online: https://www.statista.com/statistics/255977/total-global-rice-consumption/ (accessed on 31 August 2023).
Tufail, M. and Takeda, M., 2019. Molecular characteristics of brown plant hopper (BPH) populations in Pakistan. J. Asia Pac. Entomol., 22: 171-178.
Vennila, S., Singh, J., Wahi, P., Bagri, M., Das, D.K. and Rao, M.S., 2016. Web enabled weather-based prediction for insect pests of rice. ICAR-National Research Centre for Integrated Pest Management, New Delhi-110 012 India. pp. 50.
Vijaya, P.M., Jayaraj, T. and Venkatesalu, V., 2019. Population dynamics of rice yellow stem borer, Scirpophaga incertulas (Walker) in relation to weather parameters. J. Ent. Zool. Stud., 7: 1856-1860.
Zhang, X.X., Li, C.B. and Qiu, L.M., 2018. Effects of temperature on the biology of Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Environ. Entomol., 47: 834-840.
Zhang, Y., Li, Z. and Wu, H., 2019a. Effects of temperature and humidity on the survival and development of the leaf-feeding beetle Gastrophysa atrocyanea Motschulsky (Coleoptera: Chrysomelidae). Environ. Entomol., 48: 100-106.
Zhang, X., Xu, G. and Qiu, L., 2019b. Parasitism of egg parasitoids on the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), in response to rice plant volatiles. J. Asia Pac. Entomol., 22: 971-977.
Zhang, Y., Wei, X. and Wu, L., 2021. Effects of planting density on the occurrence and damage of Sesamia inferens on different rice varieties. Front, 5: 76.
Zhao, C., Liu, B., Piao, S., Wang, X., Lobell, D.B., Huang, Y., Huang, M., Yao, Y., Bassu, S., Ciais, P. and Durand, J.L., 2017. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci., 114: 9326-9331. https://doi.org/10.1073/pnas.1701762114
To share on other social networks, click on any share button. What are these?








