Field Efficacy of Selected Synthetic and Botanical Insecticides against Lepidopterous Borers, Earias vittella and Helicoverpa armigera (Lepidoptera: Noctuidae), on Okra (Abelmoschus esculentus (L.) Moench)
Field Efficacy of Selected Synthetic and Botanical Insecticides against Lepidopterous Borers, Earias vittella and Helicoverpa armigera (Lepidoptera: Noctuidae), on Okra (Abelmoschus esculentus (L.) Moench)
Mudassar Javed1, Muhammad Zeeshan Majeed1,*, Muhammad Sufyan1, Sajjad Ali2 and Muhammad Afzal1
1Department of Entomology, College of Agriculture, University of Sargodha, Sargodha
2Department of Entomology, The Islamia University of Bahawalpur, Bahawalpur
ABSTRACT
Okra (Abelmoschus esculentus (L.) Moench) is an important summer vegetable in many tropical and subtropical countries of Africa and Asia. However, okra production is hampered by the incidence of many insect pests. Particularly, okra shoot and fruit borer (Earias vittella) and okra fruit borer (Helicoverpa armigera) cause substantial quantitative and qualitative losses to okra crop. In this study, 18 pesticides including conventional and new-chemistry synthetic insecticides and different botanical extracts were screened against these pests on okra variety ‘Pusa Sawani’ under field conditions during 2015 and 2016 in a randomized complete block design. The average number of lepidopterous larvae in plots treated with the synthetic insecticides was 1.87 and 2.12 larvae/plant in 2015 and 2016, respectively, compared with 6.5 larvae/plant in untreated control plots. For the botanicals, an average of 2.57 and 2.92 larvae/plant was recorded in 2015 and 2016 respectively, compared with 4.12 in untreated control plots. Among the synthetic insecticides tested, emamectin benzoate and indoxacarb caused the most significant reduction of okra borers, followed by abamectin and lambda-cyhalothrin. While among the botanicals, Azadirachta indica, Citrullus colocynthis and Nicotiana tabacum caused the most significant reduction of okra borers in both years, followed by Curcuma longa and Corymbia citriodora. These synthetic insecticides and botanicals on average reduced okra fruit infestation by 20 to 56% and by 18 to 10%, respectively, and increased okra fruit yield up to 45%. We therefore recommend their integration in biorational IPM programmes against lepidopterous borers of okra and other vegetables.
Article Information
Received 14 April 2018
Revised 15 May 2018
Accepted 22 May 2018
Available online 31 August 2018
Authors’ Contribution
MZM and MJ conceived and designed the experimental protocols. MJ and MS performed experiments. MZM and MA provided technical assistance in experimentation. MZM, SA and MJ performed statistical analyses. MZM, MS and MJ prepared the manuscript.
Key words
Abelmoschus esculentus, Earias vittella, Field efficacy, Fruit infestation, Helicoverpa armigera, Plant extracts, Synthetic insecticides.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.6.2019.2028
* Corresponding author: shani2000_uaf@yahoo.com;
zeeshan.majeed@uos.edu.pk
0030-9923/2018/0006-2019 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
Okra (Abelmoschus esculentus (L.) Moench, Malvaceae) is endemic to Africa and is an important annual summer vegetable crop cultivated for its green tender pods in many Afro-Asian countries and in Indo-Pak region (Iqbal et al., 2012; Abang et al., 2014). Because of its high yield over investment, farming community prefers okra over other vegetable crops (Aziz et al., 2011). It is a good source of vitamin A, B and C and also rich in carbohydrates, protein and many minerals (Norman, 1992).
Several insect pests and diseases attack okra crop at its different growth stages (Mohammed et al., 2013; Munthali and Tshegofatso, 2014; Halder et al., 2016) but okra shoot and fruit borers (Earias vittella Fabricius and Helicoverpa armigera Hübner) are the most important pests (Aziz et al., 2011). Both of these lepidopterous pests damage okra, particularly young growing shoots and fruits (pods), and decrease okra fruit yield significantly both in terms of quality and quantity (Aziz et al., 2011). These pests cause about 35-40% of yield loss in okra crop, but under optimal environmental conditions for these pests, the level of damage can increase to 60-70% (Salim, 1999).
In developing countries in Africa and Asia, farmers rely on various conventional and traditional methods for the suppression or eradication of vegetable insect pests, mostly relying on synthetic and persistent agro-chemicals. Irrational use of these synthetic chemicals has created several problems such as development of insecticide resistance, eradication of the beneficial fauna, revival of minor and exotic insect pests, and environmental contamination (Edwards, 2013). Although many conventional insecticides are now ineffective, some such as chlorpyriphos and cypermethrin are still effective against many pests. Similarly, many new-chemistry insecticides, insect growth regulators and other 4th generation insecticides have demonstrated excellent potential for use against lepidopterous pests (Khan et al., 2007; Devi et al., 2015).
Keeping in view the ecological consequences of the extensive use of persistent synthetic insecticides on vegetables, there is a need to screen out the existing synthetic insecticides particularly the new-chemistry biorational insecticidal formulations and to investigate other alternative biorational approaches such as botanical insecticides for use against lepidopterous pests of okra and other vegetables. Hence, the objective of this study was to evaluate different selective synthetic insecticides and botanical extracts against okra shoot and fruit borers (E. vittella and H. armigera) under field conditions.
Materials and methods
The study was conducted in the research field of the College of Agriculture, University of Sargodha (32°07’58’’N; 72°41’32’’E) (Sargodha, Punjab, Pakistan). Firstly, available okra genotypes were screened out under indigenous agro-climatic conditions of Sargodha district in order to determine their relative tolerance or susceptibility against lepidopterous shoot and fruit borers. According to this preliminary screening, the most susceptible okra variety was found to be ‘Pusa Sawani’ which was then further used in the experiment in order to ensure sufficient pest infestation for insecticidal trials and to be able to visualise the effect of treatments more clearly. Total plot area was 760 m2 which was further divided into three blocks (replications) and in each block all 18 treatments were apportioned according to randomized complete block design. Plant to plant and row to row distance was kept at 15 and 75 cm, respectively. Moreover, a buffer zone of about 6 m was maintained around the trial area and in-between the blocks. Herbicide (Dual-Gold 960 EC; S-metolachlor) was used to suppress weeds at the start of experiment. Irrigation, fertilization and hoeing were carried out as per routine recommendations for okra crop.
Nine synthetic insecticides belonging to different modes of action (Table I) and nine aqueous extracts of indigenous plants (Table II) were evaluated in this study. Insecticides were procured from the authentic and authorised dealers from the local pesticide market of Sargodha district, while the botanical extracts were prepared according to previously described protocol (Onunkun, 2012). In brief, plant material was ground to get the homogenous powder and 100 g of this powder was mixed with 2 liter tap water and then was put on rotary shaker for 24 h. Later on, this extract was filtered and served as stock solution for making 5% aqueous extract of each botanical. Insecticide treatments were sprayed on okra crop according to the dose rates given in Table I using a manual back-mounted knapsack sprayer fitted with a hollow-cone spray nozzle at 50° angle.
Table I.- Synthetic insecticides evaluated under field conditions against lepidopterous borers (Earias spp. and H. armigera) on okra (Abelmoschus esculentus).
|
Chemical name |
Chemical class |
Mode of action |
Brand name |
Comp any |
Label dose/ Acre |
|
Abamectin |
Avermectins |
Glutamate-gated chloride channel(GluCl) allosteric modulator (Nerve and muscle action) |
Sure 1.8 EC |
Pan Paci fic® |
120 mL |
|
Emamectin benzoate |
Avermectins |
Glutamate-gated chloride channel(GluCl) allosteric modulator (Nerve and muscle action) |
Proclaim 1.9 EC |
Synge nta® |
100 mL |
|
Chlorpyrifos |
Organophosphate |
Acetylcholinesterase (AChE) inhibitor(Nerve action) |
Lorsban 40 EC |
Arysta LifeSc ience® |
500 mL |
|
Profenofos |
Organophosphate |
Acetylcholinesterase (AChE) inhibitor (Nerve action) |
Curacron 500 EC |
Synge nta® |
800 mL |
|
Indoxacarb |
Oxadiazines |
Voltage-dependent sodium channel blocker (Nerve action) |
Steward 150 SL |
Du Pont® |
100 mL |
|
Bifenthrin |
Pyrethroid |
Sodium channel modulator (Nerve action) |
Talstar |
FMC® |
330 mL |
|
Cypermethrin |
Pyrethroid |
Sodium channel modulator (Nerve action) |
Arrivo 10 EC |
FMC® |
330 mL |
|
Lambda-cyhalothrin |
Pyrethroid |
Sodium channel modulator (Nerve action) |
Karate 2.5 EC |
Synge nta® |
330 mL |
|
Spinosad |
Spinosyns |
Nicotinic acetylcholine receptor (nAChR) allosteric modulator (Nerve action) |
Tracer 240 SC |
Arysta LifeSc ience® |
50 mL |
Table II.- Botanical extracts evaluated under field conditions against lepidopterous borers (Earias vittella and Helicoverpa armigera) on okra (Abelmoschus esculentus).
|
Common name |
Botanical name |
Family |
Major insecticidal constituent |
Plant parts extracted |
|
Turmeric |
Curcuma longa |
Zingiberaceae |
Turmerone and ar-turmerone |
Rhizomes |
|
Tobacco |
Nicotiana tabacum |
Solanaceae |
Anabasine (neonicotine), nicotine and nornicotine |
Leaves |
|
Garlic |
Allium sativum |
Amaryllidaceae |
Salkyl-cysteine sulphoxides (allicins) |
Bulbs |
|
Bitter apple |
Citrullus colocynthis |
Cucurbitaceae |
Colocynthin, cucurbitacins and lepidine |
Fruits and leaves |
|
Sour orange |
Citrus aurantium |
Rutaceae |
Flavanones and limonenes |
Peels and seeds |
|
Onion |
Allium cepa |
Amaryllidaceae |
Flavonoids and dimethyl trisulfides |
Bulbs |
|
Lemon-eucalyptus |
Corymbia citriodora |
Myrtaceae |
Eucalyptol (1,8-cineole) and citronellal |
Leaves and seeds |
|
Ginger |
Zingiber officinale |
Zingiberaceae |
Monoterpenes (1,8-cineole, α -pinene, myrcene) and sesquiterpenes (zingiberene, zingiberol) |
Rhizomes |
|
Neem |
Azadirachta indica |
Meliaceae |
Azadirechtins and triterpenoids |
Leaves and seeds |
Treatments were applied on all okra plots collectively upon attainment of ETL of E. vittella and H. armigera (i.e. 5% of fruit infestation or appearance of one egg or larva or dead-heart (withered shoot/plant). Treatments were sprayed twice with an interval of 15 days i.e. first at 8-week crop when plant height was about 3 ft and 2nd at 10-week crop when plant height was approx. 4.5 ft. Care was taken to avoid the drift of insecticides being sprayed on the selective plots towards other non-target plots by hanging a 1H x 3L m polythene sheet against prevailing wind direction. The data regarding insect pest population (larval counts) and fruit and shoot infestation was recorded 1 day before treatment application, and again at 1, 3 and 7 days after treatment application. Percent fruit infestation of okra was calculated according to the following formula:
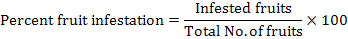
Data was statistically analysed using Statistix 8.1 V (Tallahassee, FL, USA) using two-way factorial analysis of variance to determine the effect of treatments (insecticides and botanical extracts) and different time intervals on the larval population and plant infestation by shoot and fruit borers. Comparisons of means were performed using Tukey’s honestly significance different (HSD) test at the 0.05 level of significance.
Results
Effect of insecticides on okra fruit infestation
The analysis showed that all tested synthetic insecticides significantly reduced the fruit infestation in 2015 (F9, 107= 11.28, P < 0.001) and in 2016 (F9, 107= 4.90, P < 0.000) (Supplementary Table I). Polynomial regression showed that as compared to control, all insecticidal treatments reduced the incidence of fruit infestation with R2 values mostly more than 0.9 (Tables III, IV). New-chemistry insecticides were generally more effective than conventional insecticides (synthetic pyrethroids and organophosphates). Emamectin benzoate and indoxacarb were significantly more effective and resulted in the maximum reduction of fruit infestation followed by lambda-cyhalothrin, abamectin and spinosad. However, in 2016, profenofos efficiency was similar to emamectin benzoate and indoxacarb (Table IV).
Average fruit infestation in control plots varied from 20.6 to 26.5% in 2015 and from 22.7 to 27.6% in 2016 (Tables III, IV). According to date-wise analyses (Supplementary Table II), there was no statistical difference in fruit infestation in all treatment plots including the control at one day before application of insecticides, as can be seen clearly in Table III (2015) and Table IV (2016). At one day after treatment application (1DAT), a slight difference was observed in fruit infestation only for 2015 trial. In this case, only avermectins (emamectin benzoate and abamectin) showed significant reduction (F9, 18 = 2.71, P < 0.034; Supplementary Table II) in fruit infestation as compared to control and other insecticides. At day 3 post treatment, emamectin benzoate, abamectin and indoxacarb were found more effective as compared to other treatments in 2015 trial (Table III) and emamectin benzoate, lambda-cyhalothrin and profenofos gave the maximum control of borer infestation in 2016 trial (Table IV). At 7th day post treatment application, similar trend was found for both year trials and all treatments significantly reduced fruit infestation at P ≤ 0.01. However, averagely emamectin benzoate, indoxacarb, spinosad and lambda-cyhalothrin caused significant reduction in fruit infestation till the last observation at day seven. Cypermethrin, chlorpyrifos and bifenthrin reduced fruit infestation till 3rd day post-application but not significantly. Later on, at 7th day, infestation again increased for these three insecticides in both year trials.
Table III.- Percent fruit infestation by lepidopterous borers (Earias vittella and Helicoverpa armigera) on okra (Abelmoschus esculentus) treated with different synthetic insecticides during 2015.
|
Treatments |
1 DBT |
1 DAT |
3 DAT |
7 DAT |
Trend(Polynomial Regression Fit) |
|
Emamectin benzoateD |
13.7 ± 3.9* a |
9.6 ± 1.9 b |
8.6 ± 1.1 c |
8.8 ± 1.0 d |
y = 1.06x2 - 6.8x + 19.41 (R² = 0.99) |
|
Cypermethrin B |
18.3 ± 5.8 a |
14.9 ± 4.7 ab |
13.9 ± 5.7 bc |
13.2 ± 0.5 b |
y = 0.67x2 - 4.98x + 22.50 (R² = 0.99) |
|
Chlorpyrifos BC |
19.1 ± 8.0 a |
13.9 ± 2.4 ab |
10.9 ± 1.9 bc |
12.1 ± 1.4 bc |
y = 1.62x2 - 10.48x + 28.04 (R² = 0.99) |
|
Lambda-cyhalothrinC |
13.9 ± 9.5 a |
14.3 ± 5.0 ab |
12.2 ± 3.1 bc |
11.8 ± 2.6 cd |
y = -0.68x2 + 3.14x + 9.79 (R² = 0.53) |
|
Abamectin CD |
14.9 ± 3.6 a |
11.8 ± 0.7 b |
8.9 ± 0.8 c |
9.3 ± 2.3 d |
y = 0.87x2 - 6.34x + 20.52 (R² = 0.98) |
|
Profenofos B |
18.5 ± 4.4 a |
15.2 ± 3.9 ab |
12.7 ± 0.3 bc |
13.1 ± 1.9 b |
y = 0.93x2 - 6.51x + 24.21(R² = 0.99) |
|
Spinosad CD |
17.8 ± 1.9 a |
14.9 ± 3.5 ab |
13.1 ± 3.5 bc |
11.3 ± 1.0 cd |
y = 0.27x2 - 3.50x + 20.96 (R² = 1.00) |
|
Bifenthrin B |
20.8 ± 8.2 a |
14.8 ± 4.6 ab |
15.0 ± 2.2 b |
12.2 ± 2.0 bc |
y = 0.81x2 - 6.61x + 26.17 (R² = 0.90) |
|
Indoxacarb D |
13.9 ± 5.9 a |
12.8 ± 2.4 ab |
8.9 ± 1.0 c |
10.5 ± 0.6 d |
y = 0.67x2 - 4.76x + 18.40 (R² = 0.77) |
|
Control A |
20.6 ± 4.9 a |
23.0 ± 5.1 a |
24.0 ± 5.7 a |
26.5 ± 3.7 a |
y = 0.02x2 + 1.73x + 18.99 (R² = 0.98) |
*values are means of three independent replications for each treatment ± standard deviation. Means within a column bearing different letters are significantly different at P ≤ 0.05. DBT/DAT = days before treatment / days after treatment.
Table IV.- Percent fruit infestation by lepidopterous borers (Earias vittella and Helicoverpa armigera) on okra (Abelmoschus esculentus) treated with different synthetic insecticides during 2016.
|
Treatments |
1 DBT |
1 DAT |
3 DAT |
7 DAT |
Trend(Polynomial Regression Fit) |
|
Emamectin benzoate D |
18.8 ± 1.0* a |
17.2 ± 9.4 a |
10.3 ± 2.4 c |
10.5 ± 1.1 c |
y = 0.45x2 - 5.45x + 24.40 (R² = 0.87) |
|
Cypermethrin BC |
17.9 ± 5.7 a |
16.0 ± 2.1 a |
15.0 ± 3.3 bc |
14.8 ± 3.3 bc |
y = 0.41x2 - 3.06x + 20.50 (R² = 1.00) |
|
Chlorpyrifos BC |
21.6 ± 7.3 a |
13.9 ± 4.2 a |
13.9 ± 0.7 bc |
13.5 ± 1.8 bc |
y = 1.85x2 - 11.69x + 31.05 (R² = 0.93) |
|
Lambda-cyhalothrin D |
15.1 ± 1.4 a |
13.0 ± 3.2 a |
13.5 ± 0.9 c |
11.6 ± 1.2 c |
y = 0.05x2 - 1.25x + 16.03 (R² = 0.80) |
|
Abamectin B |
21.2 ± 2.6 a |
18.9 ± 2.4 a |
15.6 ± 1.0 bc |
12.9 ± 2.4 bc |
y = -0.12x2 - 2.23x + 23.60 (R² = 0.99) |
|
Profenofos CD |
14.2 ± 4.1 a |
13.9 ± 2.5 a |
11.8 ± 2.6 c |
12.3 ± 1.3 bc |
y = 0.18x2 - 1.69x + 15.89 (R² = 0.75) |
|
Spinosad CD |
21.1 ± 11.3 a |
16.7 ± 3.8 a |
14.8 ± 5.3 bc |
13.9 ± 1.6 bc |
y = 0.88x2 - 6.76x + 26.93 (R² = 1.00) |
|
Bifenthrin B |
17.3 ± 10.1 a |
18.3 ± 6.2 a |
19.2 ± 5.3 ab |
20.9 ± 6.0 b |
y = 0.21x2 + 0.15x + 17.01 (R² = 0.99) |
|
Indoxacarb CD |
21.0 ± 6.6 a |
19.0 ± 6.0 a |
14.3 ± 3.9 bc |
12.5 ± 3.0 bc |
y = 0.02x2 - 3.09x + 24.32 (R² = 0.97) |
|
Control A |
22.7 ± 8.2 a |
22.6 ± 11.0 a |
25.7 ± 3.2 a |
27.6 ± 4.7 a |
y = 0.26x2 - 0.63x + 23.00 (R² = 0.96) |
*values are means of three independent replications for each treatment ± standard deviation. Means within a column bearing different letters are significantly different at P ≤ 0.05. DBT/DAT = days before treatment / days after treatment.
Table V.- Percent fruit infestation by lepidopterous borers (Earias vittella and Helicoverpa armigera) on okra (Abelmoschus esculentus) treated with different botanical extracts during 2015.
|
Treatments |
1 DBT |
1 DAT |
3 DAT |
7 DAT |
Trend(Polynomial Regression Fit) |
|
Curcuma longa CD |
15.4 ± 3.2 a |
14.8 ± 1.1ab |
9.7 ± 2.9 bc |
8.6 ± 3.0 b |
y = -0.13x2 - 1.88x + 17.80 (R² = 0.90) |
|
Nicotiana tabacum D |
16.7 ± 5.8 a |
13.6 ± 4.7 b |
9.3 ± 1.2 bc |
9.4 ± 1.2 b |
y = 0.68x2 - 5.04x + 18.63 (R² = 0.99) |
|
Allium sativum A |
20.6 ± 4.2 a |
17.3 ± 2.9 a |
16.9 ± 2.7 a |
17.9 ± 2.8 a |
y = 1.05x2 - 6.07x + 25.51 (R² = 0.99) |
|
Citrullus colocynthis D |
16.9 ± 3.9 a |
11.1 ± 0.6 b |
8.5 ± 0.9 bc |
7.5 ± 0.2 b |
y = 1.19x2 - 9.02x + 24.65 (R² = 1.00) |
|
Citrus aurantium AB |
18.6 ± 3.4 a |
16.1 ± 2.3 ab |
15.9 ± 1.4 a |
14.6 ± 1.2 a |
y = 0.31x2 - 2.74x + 20.88 (R² = 0.94) |
|
Allium cepa BC |
14.9 ± 5.9 a |
14.2 ± 2.1 ab |
13.5 ± 3.6 ab |
14.7 ± 2.6 a |
y = 0.48x2 - 2.55x + 17.08 (R² = 0.86) |
|
Corymbia citriodora AB |
18.3 ± 2.7 a |
15.6 ± 1.9 ab |
15.2 ± 3.3 a |
15.1 ± 3.5 a |
y = 0.65x2 - 4.22x + 21.71 (R² = 0.96) |
|
Zingiber officinale A |
20.4 ± 11.2 a |
18.0 ± 5.9 a |
16.9 ± 3.9 a |
16.7 ± 3.7 a |
y = 0.55x2 - 3.97x + 23.82 R² = 1.00) |
|
Azadirachta indica D |
15.2 ± 5.4 a |
11.4 ± 3.2 b |
6.5 ± 2.3 c |
6.5 ± 0.4 b |
y = 0.96x2 - 7.90x + 22.46 (R² = 0.97) |
|
Control A |
18.6 ±6.3 a |
21.0 ±5.8 a |
25.4 ±5.7 a |
27.3 ±4.1 a |
y = 0.27x2 + 2.54x + 19.87 (R² = 0.94) |
*values are means of three independent replications for each treatment ± standard deviation. Means within a column bearing different letters are significantly different at P ≤ 0.05. DBT/DAT = days before treatment / days after treatment.
Effect of botanicals on okra fruit infestation
The analysis of variance showed that overall all the tested botanical extracts significantly reduced fruit infestation in 2015 (F9, 107 = 10.93, P < 0.001) and in 2016 (F9, 107 = 22.94, P < 0.000) (Supplementary Table III). Polynomial regression showed that as compared to control, most of the botanical treatments reduced the incidence of fruit infestation with R2 value more than 0.9 (Tables V, VI). Among the botanical extracts evaluated, A. indica, N. tabacum and C. colocynthis were significantly the most effective in reducing fruit infestation in both years, while C. longa and C. citriodora were also significantly better than the control in 2015 and 2016, respectively.
Average fruit infestation in control plots varied from 18.6 to 27.3% in 2015 and from 23.5 to 28.3% in 2016 (Tables V, VI). According to date-wise analyses (Supplementary Table IV), there was no statistical difference in fruit infestation for all treatment plots including control at one day before application of botanical extracts (Tables V, VI). At day 1 after treatment application, a significant difference was observed in fruit infestation for both years, i.e. in 2015 (F9, 18 = 2.06, P = 0.05) and in 2016 (F9, 18 = 3.89, P < 0.05) (Supplementary Table IV). In both years, extracts of A. indica, N. tabacum and C. colocynthis showed significant reduction in fruit infestation as compared to control and other botanical extracts, while results of C. longa and C. citriodora were found to be intermediate between those of significant treatments and insignificant ones including control (Tables V, VI). At day three post treatment application, A. indica was found more effective as compared to other treatments in 2015 trial (Table V) reducing fruit infestation from 15.2 to 6.5%, and A. indica, N. tabacum and C. colocynthis gave maximum reduction of fruit infestation in 2016 trial (Table VI). At day seven post treatment application, similar trend was found for both year trials and all treatments significantly reduced fruit infestation at P ≤ 0.01. However, on average basis, A. indica, N. tabacum and C. colocynthis caused significant reduction in fruit infestation up to the last observation at day 7. Minimum reduction in okra fruit infestation was observed respectively for Z. officinale, A. cepa, A. sativum and C. aurantium for both years of experimentation.
Effect of insecticides on larval population of okra borers
Table VII presents the average larval numbers of okra fruit borers of both species, i.e. E. vittella and H. armigera observed from five randomly selected okra plants from each treatment plot. Again the trend was similar to that found in case of fruit infestation. Average number of lepidopterous larvae/plant in treated plots was found to be 1.87 in 2015 and 2.12 in 2016 season. According to Table VII, all insecticides reduced larval density on okra plants when observed at 3 and 7 days after treatment, while in control plots, larval numbers gradually increased from about 4.5 to 8.1 larvae/plant. However, the reduction of larvae in response to treatment was only statistically significant for emamectin benzoate, abamectin, indoxacarb, spinosad lambda-cyhalothrin and profenofos at P ≤ 0.05. Cypermethrin, chlorpyrifos and bifenthrin also caused larval reduction but not statistically significant.
Table VI.- Percent fruit infestation by lepidopterous borers (Earias vittella and Helicoverpa armigera) on okra (Abelmoschus esculentus) treated with different botanical extracts during 2016.
|
Treatments |
1 DBT |
1 DAT |
3 DAT |
7 DAT |
Trend(Polynomial Regression Fit) |
|
Curcuma longa A |
24.1 ± 2.3 a |
20.7 ± 1.3 ab |
19.2 ± 0.7 a |
23.1 ± 10.4 a |
y = 1.82x2 - 9.57x + 32.06 (R² = 0.96) |
|
Nicotiana tabacum C |
14.2 ± 2.1 c |
11.4 ± 2.0 c |
8.8 ± 1.2 b |
7.7 ± 0.8 c |
y = 0.44x2 - 4.39x + 18.19 (R² = 1.00) |
|
Allium sativum A |
21.9 ± 1.1 ab |
20.2 ± 1.2 ab |
20.1 ± 0.9 a |
18.8 ± 2.1 ab |
y = 0.11x2 - 1.47x + 23.07 (R² = 0.91) |
|
Citrullus colocynthis C |
16.9 ± 3.9 bc |
11.1 ± 0.6 c |
8.3 ± 1.1 b |
7.3 ± 0.4 c |
y = 1.20x2 - 9.13x + 24.77 (R² = 1.00) |
|
Citrus aurantium A |
22.5 ± 1.0 ab |
20.0 ± 3.1 ab |
19.4 ± 0.5 a |
18.9 ± 1.3 ab |
y = 0.51x2 - 3.73x + 25.65 (R² = 0.98) |
|
Allium cepa A |
22.5 ± 9.8 ab |
20.7 ± 13.1 ab |
22.6 ± 7.9 a |
20.8 ± 5.4 a |
y = 0.01x2 - 0.37x + 22.54 (R² = 0.16) |
|
Corymbia citriodora B |
18.3 ± 2.7 ab |
14.7 ± 1.9 bc |
12.1 ± 2.7 b |
12.2 ± 2.3 bc |
y = 0.91x2 - 6.61x + 24.04 (R² = 0.99) |
|
Zingiber officinale A |
20.6 ± 4.9 ab |
20.0 ± 2.1 ab |
18.5 ± 3.6 a |
20.4 ± 2.1 a |
y = 0.63x2 - 3.35x + 23.55 (R² = 0.64) |
|
Azadirachta indica C |
12.9 ± 1.3 c |
9.7 ± 0.5 c |
8.5 ± 2.3 b |
8.8 ± 0.3 c |
y = 0.87x2 - 5.69x + 17.65 (R² = 1.00) |
|
Control A |
23.5 ± 5.1 a |
25.9 ± 10.3 a |
26.7 ± 6.5 a |
28.3 ± 5.8 a |
y = 0.27x2 - 0.71x + 24.04 (R² = 0.97) |
*values are means of three independent replications for each treatment ± standard deviation. Means within a column bearing different letters are significantly different at p ≤ 0.05. DBT/DAT = days before treatment / days after treatment.
Table VII.- Average number of larvae of lepidopterous borers (Earias vittella and Helicoverpa armigera) per plant observed on okra (Abelmoschus esculentus) plots treated with different insecticides.
|
Treatments |
2015 |
2016 |
||||
|
1 DBT |
3 DAT |
7 DAT |
1 DBT |
3 DAT |
7 DAT |
|
|
Emamectin benzoate |
3.40 |
0.80* |
0.60* |
4.20 |
0.80* |
0.60* |
|
Cypermethrin |
3.20 |
1.20 |
2.00 |
3.80 |
1.20 |
2.20 |
|
Chlorpyrifos |
3.80 |
0.80 |
1.80 |
4.40 |
1.60 |
2.00 |
|
Lambda-cyhalothrin |
3.80 |
0.60* |
1.20 |
3.60 |
1.20 |
0.80* |
|
Abamectin |
4.40 |
1.00 |
1.00* |
3.60 |
1.40 |
1.20 |
|
Profenofos |
4.00 |
0.80 |
0.80* |
4.00 |
1.20 |
1.00* |
|
Spinosad |
3.40 |
1.00 |
0.80 |
3.80 |
1.00 |
0.80* |
|
Bifenthrin |
3.60 |
1.00 |
1.20 |
3.80 |
1.40 |
1.80 |
|
Indoxacarb |
3.20 |
0.80* |
0.60* |
4.20 |
0.80 |
0.80* |
|
Control |
4.20 |
5.40 |
7.80 |
4.00 |
5.80 |
8.20 |
Values are means of 5 random replications. *indicates significance reduction in larval numbers at P≤0.05.
Table VIII.- Average number of larvae of lepidopterous borers (Earias vittella and Helicoverpa armigera) per plant observed on okra (Abelmoschus esculentus) plots treated with different botanical extracts.
|
Treatments |
2015 |
2016 |
||||
|
1 DBT |
3 DAT |
7 DAT |
1 DBT |
3 DAT |
7 DAT |
|
|
Curcuma longa |
4.75 |
1.63* |
2.05 |
5.70 |
2.05 |
1.60 |
|
Nicotiana tabacum |
5.25 |
1.95* |
2.25* |
5.75 |
2.45* |
2.95* |
|
Allium sativum |
4.30 |
2.31 |
2.82 |
5.40 |
2.60 |
3.52 |
|
Citrullus colocynthis |
4.85 |
1.35* |
2.45 |
5.50 |
2.45* |
1.55* |
|
Citrus aurantium |
4.40 |
2.03 |
2.51 |
5.50 |
2.87 |
2.20 |
|
Allium cepa |
5.50 |
2.30 |
1.80 |
5.20 |
3.21 |
2.75 |
|
Corymbia citriodora |
4.65 |
1.75 |
1.55* |
5.05 |
1.75* |
1.55* |
|
Zingiber officinale |
4.60 |
3.01 |
2.72 |
4.70 |
3.00 |
3.83 |
|
Azadirachta indica |
4.70 |
1.34* |
1.64* |
5.20 |
1.81* |
1.32* |
|
Control |
4.50 |
5.90 |
8.30 |
5.50 |
6.35 |
8.67 |
Values are means of 5 random replications. *indicates significance reduction in larval numbers at p ≤ 0.05.
Effect of botanical extracts on larval population of okra lepidopterous borers
Table VIII presents the average larval numbers of okra fruit borers of both species, i.e. E. vittella and H. armigera observed from five randomly selected okra plants from each treatment plot. Again the trend was similar as in case of fruit infestation. Average number of larvae per plant was found 2.57 in 2015 and 2.92 in 2016 season. According to Table VIII, all botanical extracts reduced larval density on okra plants when observed at 3 and 7 days after treatment, while in control plots, larval number gradually increased from 4.5 to 8.8 larvae/plant in 2015 and from 5.5 to 9.2 larvae/plant in 2016. However, the reduction of larvae in response to treatment was only statistically significant for A. indica, N. tabacum and C. colocynthis and C. citriodora at P ≤ 0.05 (Table VIII). Other botanical extracts also caused larval reduction but not statistically significant.
Effect of insecticides on okra fruit yield
On per hectare basis, okra yield (marketable green fruits) was found minimum for control plots in both year trials, i.e. 5,421 kg/ha for 2015 and 6,645 kg/ha for 2016 (Table IX). In 2015 experiment, maximum okra fruit yield was found for plots treated with emamectin benzoate (i.e. 8,206 kg/ha) followed by indoxacarb (8,196 kg/ha), abamectin (7,752 kg/ha), spinosad (6,928 kg/ha) and lambda-cyhalothrin (6,549 kg/ha), while the minimum yield was recorded for cypermethrin (5,705 kg/ha), chlorpyrifos (5,637 kg/ha) and bifenthrin (5,812 kg/ha). For year 2016, maximum okra fruit yield was recorded for emamectin benzoate (9,706 kg/ha) followed by indoxacarb (9,588 kg/ha), spinosad (8,428 kg/ha) and lambda-cyhalothrin (8,001 kg/ha), while the minimum okra yield was recorded for chlorpyrifos (7,237 kg/ha) and bifenthrin (7,312 kg/ha). On average for both seasons, emamectin benzoate, indoxacarb, abamectin, spinosad and lambda-cyhalothrin increased marketable okra fruit yield by 45, 44, 38, 25 and 18%, respectively.
Table IX.- Fruit yield of okra (Abelmoschus esculentus) treated from different insecticides during 2015 and 2016.
|
Treatments |
Yield(Kg/hectare) |
% increase in yield over control |
||
|
2015 |
2016 |
2015 |
2016 |
|
|
Emamectin benzoate |
8206* |
9706 |
51 |
40 |
|
Cypermethrin |
5705 |
7205 |
5 |
4 |
|
Chlorpyrifos |
5637 |
7237 |
6 |
4 |
|
Lambda-cyhalothrin |
6549 |
8001 |
21 |
15 |
|
Abamectin |
7752 |
9252 |
43 |
33 |
|
Profenofos |
6387 |
7887 |
18 |
14 |
|
Spinosad |
6928 |
8428 |
28 |
21 |
|
Bifenthrin |
5812 |
7312 |
7 |
5 |
|
Indoxacarb |
8196 |
9588 |
51 |
38 |
|
Control |
5421 |
6645 |
- |
- |
*, yield is calculated for each treatment from data of five consecutive pickings of marketable green fruits post treatment application.
Table X.- Fruit yield of okra (A. esculentus) treated from different botanical extracts during 2015 and 2016.
|
Treatments |
Yield*(Kg/hectare) |
% increase in yield over control |
||
|
2015 |
2016 |
2015 |
2016 |
|
|
Curcuma longa |
4855 |
6297 |
11 |
9 |
|
Nicotiana tabacum |
7206 |
8633 |
65 |
50 |
|
Allium sativum |
4637 |
6220 |
6 |
8 |
|
Citrullus colocynthis |
6752 |
8238 |
54 |
43 |
|
Citrus aurantium |
5649 |
7028 |
29 |
22 |
|
Allium cepa |
5387 |
6870 |
23 |
19 |
|
Corymbia citriodora |
6188 |
7615 |
41 |
32 |
|
Zingiber officinale |
4812 |
6295 |
10 |
9 |
|
Azadirachta indica |
7419 |
8753 |
69 |
52 |
|
Control |
4378 |
5772 |
- |
- |
*, yield is calculated for each treatment from data of five consecutive pickings of marketable green fruits post treatment application.
Effect of botanical extracts on okra yield
On per hectare basis, okra yield was found minimum for control plots in both year trials, i.e. 4,378 kg/ha for 2015 and 5,772 kg/ha for 2016 (Table X). In 2015 experiment, maximum okra fruit yield was found for plots treated with A. indica extract (i.e. 7,419 kg/ha) followed by N. tabacum (7,206 kg/ha), C. colocynthis (6,752 kg/ha), C. citriodora (6,188 kg/ha) and C. aurantium (5,649 kg/ha), while the minimum yield was recorded for okra plots treated with A. sativum (4,637 kg/ha) and Z. officinale (4,812 kg/ha). For year 2016, maximum okra fruit yield was recorded for A. indica (8,753 kg/ha) followed by N. tabacum (8,633 kg/ha), C. colocynthis (8,238 kg/ha) and C. citriodora (7,615 kg/ha), while the minimum okra yield was recorded for A. sativum (6,220 kg/ha) and C. longa (6,297 kg/ha). Averagely for both years, A. indica, N. tabacum, C. colocynthis and C. citriodora increased marketable okra fruit yield by 61, 57, 47 and 36%, respectively.
Discussion
Infestation of okra crop by lepidopterous pests such as E. vittella and H. armigera has been one of the contemporary issues of farmers in African and Indo-Pak regions (Ahmed, 2000; Dabire-Binso et al., 2009). These economically important pests are being controlled by okra growers using hazardous synthetic insecticides without satisfactory control results (Aziz et al., 2011). Nevertheless, irrational and blind use of synthetic chemicals has been creating many environmental issues as described above. Therefore, this study was aimed to screen out under field conditions some existing conventional and new-chemistry synthetic insecticides and certain botanical extracts, with known anti-insect actions, against okra lepidopterous borers which can be integrated further in different biorational pest management programmes.
The experiments were conducted for two years in two different seasons. Eighteen treatments including nine selective synthetic insecticides and nine selective botanical formulations were compared and almost all treatments reduced okra fruit infestation as compared to unsprayed control plots. However, infestation by okra borers was higher in 2016 than in 2015 season. Main reason of this high infestation in seconded year trial would be the growing season because 2016 trial was performed in the rainy season, i.e. in July-August which created conditions more conducive to lepidopterous and other insect pests on okra crop (Sugonyaev and Monastyrskii, 2008; Aziz et al., 2011).
Among the insecticides, emamectin benzoate appeared to be the most effective and promising novel insecticide against E. vittella and H. armigera. These results are in accordance with those of Birah and Raghuraman (2011) who also evidenced that the emamectin benzoate formulations, both in EC or SWG form, effectively control E. vittella incidence on okra crop along with high fruit yield. Similarly, our results are in line with the findings of Shivalingaswamy et al. (2008) and Bengochea et al. (2014) who have demonstrated through field and laboratory experiments that emamectin benzoate is equally effective to control infestation of different lepidopterous pests of vegetables, i.e. brinjal shoot and fruit borer (Leucinodes orbonalis), okra shoot and fruit borer (E. vittella), beet armyworm (Spodoptera exigua) and cabbage diamondback moth (Plutella xylostella).
Second most effective insecticide in our experiments is found to be indoxacarb which reduced fruit infestation till 7th day post-treatment concurrently with an increased okra fruit yield. Umrao et al. (2013) revealed the same result that indoxacarb gave the maximum control of okra shoot and fruit borer, E. vittella. This insecticide has been proved to be effective against a number of lepidopterous insect pests including cabbage looper (Plutella xylostella) (Liu et al., 2002), gram pod borer (H. armigera) (Babariya et al., 2010), tomato fruit borer (Heliothis armigera) (Ravi et al., 2008), fall armyworm (Spodoptera frugiperda) (Wing et al., 2000) and codling moths (Cydia pomonella and C. molesta) (Loriatti et al., 2009).
Thirdly, abamectin and spinosad also showed significant reduction of both E. vittella and H. armigera but not as effectively as emamectin and indoxacarb. Same toxicity trend was found for lambda-cyhalothrin and profenofos while remaining insecticides (i.e. chlorpyrifos, bifenthrin and cypermethrin) did not cause much reduction of borers’ infestation and of larval population and were not statistically different from control plots. One of the reasons behind this may be the development of resistance in field populations of many lepidopterous pests, particularly of E. vittella and H. armigera because these conventional synthetic insecticides have been widely used in the region of study on cotton, gram, maize, tomato and other vegetable crops upon which these both insect species appear regularly (Ahmad and Arif, 2009; Qayyum et al., 2015).
Among botanical treatments, extract of A. indica appeared to be the most effective against E. vittella and H. armigera infestation and significantly increased the okra yield (almost by 60%). Similar findings were obtained by Ahmed (2000) who revealed a significant effect of aqueous neem extract on insect pest reduction and on yield increase in different vegetable crops including okra. Many studies have shown the potential efficacy of neem extract against different lepidopterous pests e.g. against brinjal fruit and shoot borer (Leucinodes orbonalis), okra fruit and shoot borer (E. vittella), beet armyworm (Spodoptera exigua), maize borer (Sesamia calamistis) and cabbage diamondback moth (P. xylostella) (Leskovar and Boales, 1996; Ukeh et al., 2007; Akhtar et al., 2008).
Second most effective botanical extract in this study appeared to be of N. tabacum, which reduced fruit infestation till 7th day post-treatment and increased okra fruit yield by about 57%. Many studies have demonstrated that aqueous tobacco extract could successfully control the insect pests of okra and other vegetables, and even at 1.25%, it can control external parasites of livestock (Olivo et al. 2009), at 2–6 % concentration, it is effective against stored grain insect pests (Kulat et al., 1997; Sarmamy et al., 2011).
Thirdly, the extracts of C. colocynthis and C. citriodora also showed significant reduction of okra fruit and shoot borers but not as effectively as A. indica and N. tabacum. Same toxicity trend was found for C. aurantium, while botanical extracts of remaining plants belonging to Amaryllidaceae and Zingiberaceae families (i.e. A. cepa, A. sativum, C. longa and Z. officinale) did not cause much reduction of fruit infestation and of larval population of okra borers and were not significantly different from control plots.
However, most of the botanicals have been found effective for a very short period of time as it is evident from Tables 6 and 8 that fruit infestation was reduced only at 3rd day post treatment and again flared up at 7th day post treatment for all treatments except for A. indica and N. tabacum extracts. One reason for this short-spanned effect of botanicals against insect pests would be the crude and raw nature of aqueous extracts rather than pure extractives such as essential oils as described by Isman (2006, 2008), Dubey et al. (2010); Sarmamy et al. (2011) and Gulzar et al. (2017).
Conclusions
Based on above mentioned results and discussion, avermectins (emamectin benzoate and abamectin) and oxadiazine (indoxacarb) insecticides and aqueous extracts of A. indica and N. tabacum are recommended for sustainable management of shoot and fruit borers on okra and other vegetable crops because these are not only effective against target lepidopterous pests but also exhibit least non-target effects to other organisms and environment. However, application or rotation of pesticides with different modes of action is one of the prerequisites for an effective and sustainable IPM programme. Hence, we recommend the integration of effective synthetic new-chemistry insecticides and botanical extracts in order to develop an efficient biorational IPM strategy against lepidopterous pests.
Acknowledgements
The authors are thankful to Muhammad Asam Riaz and Zhang Bo for their technical support in improving experimental protocols and Sami Ullah for assisting in statistical analysis of data. The authors are also grateful to the farm manager of College of Agriculture, University of Sargodha, Pakistan for his valuable support during the conduction of experiments in both years.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2018.50.6.2019.2028
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abang, A.F., Srinivasan, R., Kekeunou, S., Hanna, R., Chagomoka, T., Chang, J.C. and Bilong, C.B., 2014. Identification of okra (Abelmoschus spp.) accessions resistant to aphid (Aphis gossypii Glover) in Cameroon. Afri. Entomol., 22: 273-284. https://doi.org/10.4001/003.022.0201
Ahmad, M. and Arif, M.I., 2009. Resistance of Pakistani field populations of spotted bollworm Earias vittella (Lepidoptera: Noctuidae) to pyrethroid, organophosphorus and new chemical insecticides. Pest Manage. Sci., 65: 433-439. https://doi.org/10.1002/ps.1702
Ahmed, M.M.M., 2000. Studies on the control of insect pests in vegetables (okra, tomato and onion) in Sudan with special reference to neem-preparations. Ph. D. dissertation, University of Göttingen, Germany.
Akhtar, Y., Yeoung, Y.R. and isman, M.B., 2008. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev., 7: 77-88. https://doi.org/10.1007/s11101-006-9048-7
Aziz, M.A., Hasan, M. and Ali, A., 2011. Impact of abiotic factors on incidence of fruit and shoot damage of spotted bollworms Earias spp. on okra (Abelmoschus esculentus L.). Pakistan J. Zool., 43: 863-868.
Babariya, P.M., Kabaria, B.B., Patel, V.N. and Joshi, M.D., 2010. Chemical control of gram pod borer, Helicoverpa armigera Hubner infesting pigeonpea. Legume Res., 33: 224-226.
Bengochea, P., Sánchez-Samos, I., Saelices, R., Amor, F., del Estal, P., Viñuela, E. and Medina, P., 2014. Is emamectin benzoate effective against the different stages of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Irish J. Agric. Fd. Res., 2: 37-49.
Birah, A. and Raghuraman, M., 2011. Impact of emamectin benzoate on fruit and shoot borer, Earias vitella (Fabricius) in okra. Indian J. Ent., 73: 42-44.
Dabire-Binso, C.L., Ba, M.N., Somé, K. and Sanon, A., 2009. Preliminary studies on incidence of insect pest on okra, Abelmoschus esculentus (L.) Moench in central Burkina Faso. Afri. J. agric. Res., 4: 1488-1492.
Devi, L.L., Ghule, T.M., Chatterjee, M.L. and Senapati, A.K., 2015. Biorational management of shoot and fruit borer of okra (Earias vittella Fabricius) and their effect on insect predators. Environ. Ecol., 33: 1052-1054.
Dubey, N.K., Shukla, R., Kumar, A., Singh, P. and Prakash, B., 2010. Prospects of botanical pesticides in sustainable agriculture. Curr. Sci., 98: 479-480.
Edwards, C.A. (ed.), 2013. Environmental pollution by pesticides. Springer Science and Business Media, New York, U.S.A., pp. 542. eISBN: 978-1-4615-8942-6.
Gulzar, A., Maqsood, A., Ahmed, M., Tariq, M., Ali, M. and Qureshi, R., 2017. Toxicity, antifeedant and sub-lethal effects of Citrullus colocynthis extracts on cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Pakistan J. Zool., 49: 2019-2026. http://dx.doi.org/10.17582/journal.pjz/2017.49.6.2019.2026
Halder, J., Sanwal, S.K., Deb, D., Rai, A.B. and Singh, B., 2016. Mechanisms of physical and biochemical basis of resistance against leaf-hopper (Amrasca biguttula biguttula) in different okra (Abelmoschus esculentus) genotypes. Indian J. agric. Sci., 86: 480-481.
Iqbal, J., Sagheer, M. and Nadeem, M., 2012. Management of Amrasca biguttula biguttula (Ishida) on okra, Abelmoschus esculentus (L.) Monech. Pak. J. agric. Sci., 49: 179-184.
Isman, M.B., 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Ent., 51: 45-66. https://doi.org/10.1146/annurev.ento.51.110104.151146
Isman, M.B., 2008. Botanical insecticides: For richer, for poorer. Pest Manage. Sci., 64: 8-11. https://doi.org/10.1002/ps.1470
Khan, R.R., Ahmed, S., Saleem, M.W. and Nadeem, M., 2007. Field evaluation of different insecticides against spotted bollworms Earais spp. at district Sahiwal. Pak. Ent., 29: 129-133.
Kulat, S.S., Nimbalkar, S.A. and Hiwase, B.J., 1997. Relative efficacy of some plant extracts against Bemisia tabaci and Aphis gossypii Glover and Amrasca devastans of okra. PKV Res. J., 21: 146-148.
Leskovar, D.I. and Boales, A.K., 1996. Azadirachtin: potential use for controlling lepidopterous insects and increasing marketability of cabbage. HortScience, 31: 405-409.
Liu, T.X., Sparks, A.N., Chen, W., Liang, G.M. and Brister, C., 2002. Toxicity, persistence, and efficacy of indoxacarb on cabbage looper (Lepidoptera: Noctuidae) on cabbage. J. econ. Ent., 95: 360-367. https://doi.org/10.1603/0022-0493-95.2.360
Loriatti, C., Anfora, G., Angeli, G., Civolani, S., Schmidt, S. and Pasqualini, E., 2009. Toxicity of emamectin benzoate to Cydia pomonella (L.) and Cydia molesta (Busck) (Lepidoptera: Tortricidae): Laboratory and field tests. Pest Manage. Sci., 65: 306-312. https://doi.org/10.1002/ps.1689
Mohammed, I.G., Osipitan, A.A., Pitan, O.R. and Atayese, M. 2013. Evaluation of 15 varieties of okra (Abelmoschus esculentus (L.) Moench) to field infestation by flea beetles (Podagrica spp). Afri. Ent., 21: 70-78.
Munthali D.C. and Tshegofatso, A.B., 2014. Major insect pests attacking okra; Abelmoscus esculentos (L) Moench, in Sebele. Botswana J. Agric. appl. Sci., 9: 90-96.
Norman, J.C., 1992. Tropical vegetable crops. Arthur H. Stockwell Ltd., Ilfracombe, U.K., pp. 252.
Olivo, C.J., Heimerdinger, A., Ziech, M.F., Agnolin, C.A., Meinerz, G.R., Both, F. and Charão, P.S., 2009. Aqueous extract of rope tobacco for the control of cattle ticks. Ciênc. Rural, 39: 1131-1135. https://doi.org/10.1590/S0103-84782009000400026
Onunkun, O., 2012. Evaluation of aqueous extracts of five plants in the control of flea beetles on okra (Abelmoschus esculentus (L.) Moench). J. Biopestic., 5: 62-67.
Qayyum, M.A., Wakil, W., Arif, M.J., Sahi, S.T., Saeed, N.A. and Russell, D.A., 2015. Multiple resistances against formulated organophosphates, pyrethroids, and newer-chemistry insecticides in populations of Helicoverpa armigera (Lepidoptera: Noctuidae) from Pakistan. J. econ. Ent., 108: 286-293. https://doi.org/10.1093/jee/tou037
Ravi, M., Santharam, G. and Sathiah, N., 2008. Ecofriendly management of tomato fruit borer, Helicoverpa armigera (Hubner). J. Biopestic., 1: 134-137.
Salim, M., 1999. Diversity: Role in integrated pest management. Sci. Technol. Dev., 18: 26-31.
Sarmamy, A.G., Hashim, H. and Sulayman, A., 2011. Insecticidal effects of some aqueous plant extracts on the control of Khapra Trogoderma granarium Evert. In: Int. Conf. Chem., Biol. Environ. Sci. (ICCEBS’2011), Bangkok, pp. 288-292.
Shivalingaswamy, T.M., Kumar, A., Satpathy, S. and Rai, A.B., 2008. Efficacy of emamectin benzoate in the management of vegetable pests. Progr. Horti., 40: 193-197.
Sugonyaev, E.S. and Monastyrskii, A.L., 2008. Basic components of the program of ecological integrated management of lepidopterous rice pests (Lepidoptera, Pyralidae) in the Red River Delta in North Vietnam: V. General manual. Ent. Rev., 88: 525-542. https://doi.org/10.1134/S0013873808050023
Ukeh, D.A., Emosairue, S.O., Udo, I.A., Ofem, U.A., Du, H., Cui, M. and Dotun, O., 2007. Field evaluation of neem (Azadirachta indica A. Juss) products for the management of lepidopterous stem borers of maize (Zea mays L.) in Calabar, Nigeria. Res. J. appl. Sci., 2: 15-19.
Umrao, R.S., Singh, S., Kumar, J., Singh, D.R. and Singh, D.K., 2013. Efficacy of novel insecticides against shoot and fruit borer (Earias vittella Fabr.) in okra crop. HortFlora Res. Spectr., 2: 251-254.
Wing, K.D., Sacher, M., Kagaya, Y., Tsurubuchi, Y., Mulderig, L., Connair, M. and Schnee, M., 2000. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot., 19: 537-545. https://doi.org/10.1016/S0261-2194(00)00070-3
To share on other social networks, click on any share button. What are these?










