Histopathological and Molecular Analyses of Leptospira borgpetersenii and Leptospira interrogans in Bovine Kidneys in Kota Bharu, Kelantan, Malaysia
Histopathological and Molecular Analyses of Leptospira borgpetersenii and Leptospira interrogans in Bovine Kidneys in Kota Bharu, Kelantan, Malaysia
Intan Noor Aina Kamaruzaman1*, Siew Zee Ong2, Iman Natasha Sofea Jafri2, Dayangku Umi Aqilah Pengiran-Isa2, Mohamad Sabri Abdul-Rahman3, Mohd Farhan Hanif Reduan2, Thilini Nisansala1, Soon Heng Goh1, Siti Nur Haslina Mamat2, C.W. Salma C.W. Zalati3, Sazaly AbuBakar4 and Shih Keng Loong4*
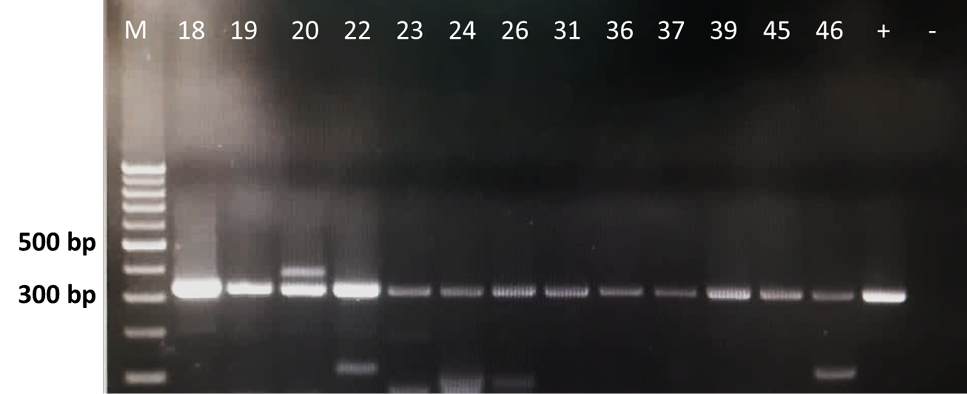
Leptospira 16S rRNA PCR amplification of bovine kidney samples. M: 1 kb marker, +/− (positive/negative) controls and the Leptospira PCR-positive samples were placed in between M and the controls (Samples no. 18, 19, 20, 22, 23, 24, 26, 31, 36, 37, 39, 45 and 46). L. interrogans serovar Canicola was used as positive control in this study.
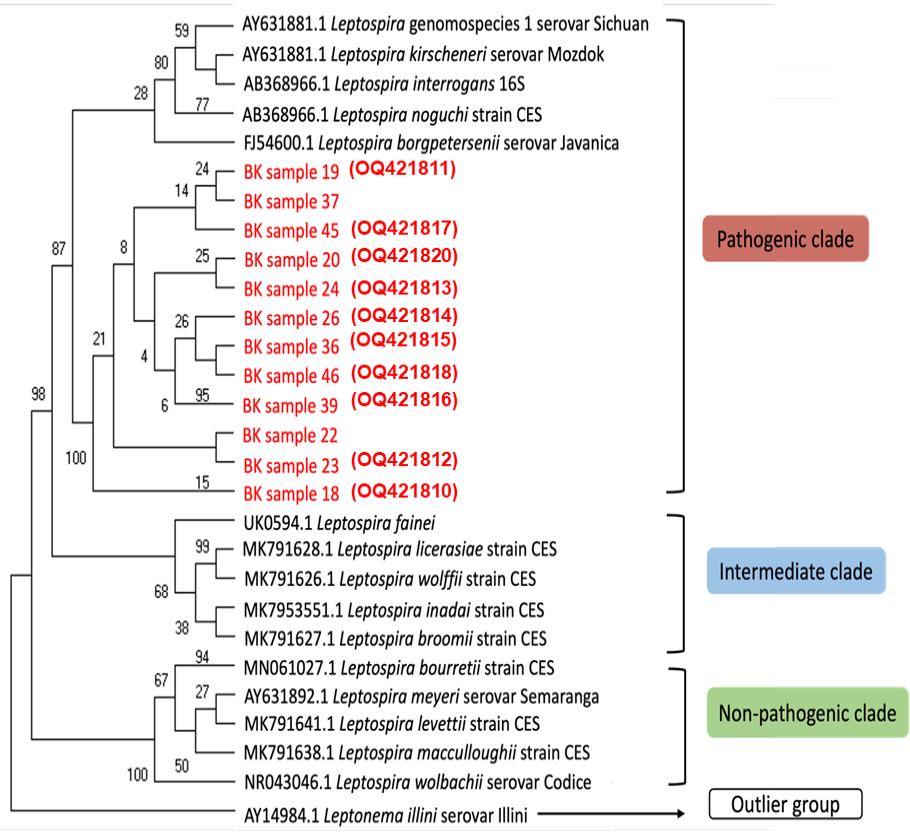
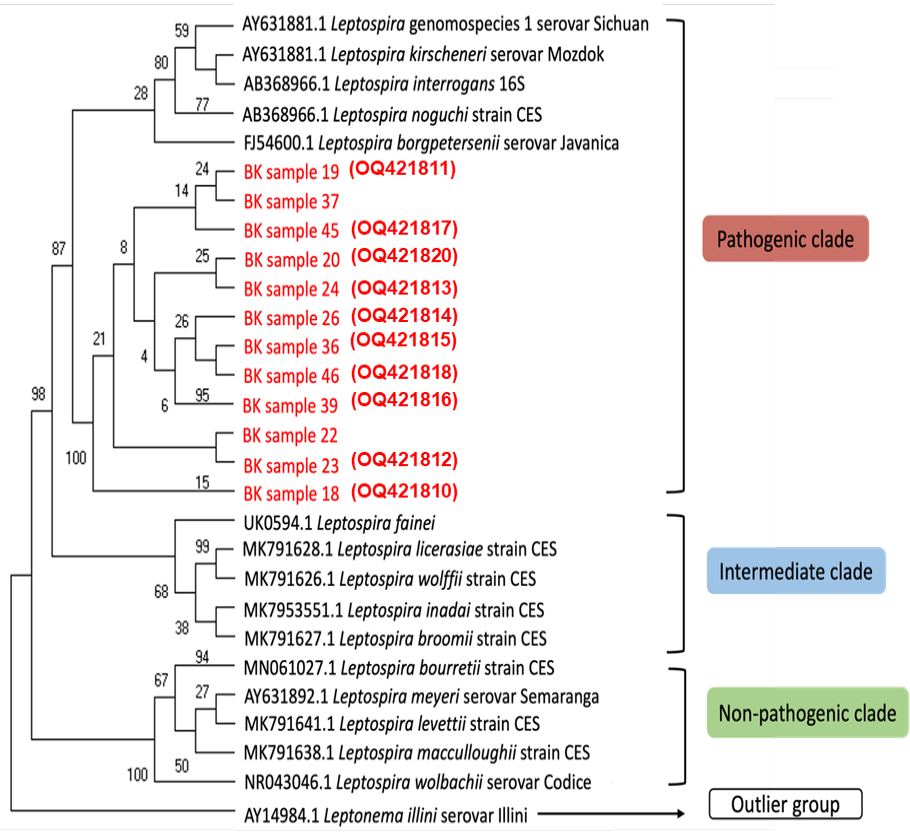
The phylogenetic analysis of 16S rRNA nucleotide sequences across various Leptospira group using several genomospecies as reference and one outlier group by maximum likelihood method constructed using MEGA11 [15]. Bootstrapping was performed 1,000 times, and all positions containing gaps and missing data were removed. Sequences obtained as part of this study are highlighted in red and the accession numbers generated from this study are in red parentheses.
Histopathological changes in the bovine kidney tissue samples; A, glomerular atrophy with slight deposition of homogenous hyaline droplets (tailed-arrow). B, deposition of pinkish homogenous hyaline cast within the glomerulus (arrow). C, infiltration of inflammatory cells (neutrophils and lymphocytes) in the renal interstitial (tailed-arrow). D, eosinophilic homogenous cytoplasmic hyaline droplets in the tubular epithelial cells (tailed-arrow), renal tubular necrosis (thin arrow) and margination of inflammatory cells in the blood vessel lumen (thick arrow). E, renal tubular necrosis (thin arrow) was prominent with infiltration of inflammatory cells in the renal interstitial (tailed-arrow). Eosinophilic homogenous cytoplasmic hyaline droplets were observed in the tubular epithelial cells (thick arrow). F, deposition of pinkish homogenous hyaline cast within the glomerulus (thick arrow), renal tubular necrosis (thin arrow) with inflammatory cells in the renal interstitial (tailed-arrow). Images A-D were PCR-positive (Sample no. 22), whilst images E and F were PCR-negative (Sample no. 32). (H and E stain; magnification of 400x).