Improvement in Nutrient Digestibility and Growth Performance of Catla catla Fingerlings Using Phytase in Moringa oleifera Leaf Meal Based Diet
Improvement in Nutrient Digestibility and Growth Performance of Catla catla Fingerlings Using Phytase in Moringa oleifera Leaf Meal Based Diet
Muhammad Mudassar Shahzad1*, Syed Makhdoom Hussain2, Afia Muhammad Akram1, Arshad Javid3, Majid Hussain5, Syed Zakir Hussain Shah4 and Asma Chaudhary1
1Department of Zoology, Division of Science and Technology, University of Education, Lahore, Pakistan
2Fish Nutrition Lab, Department of Zoology, Government College University, Faisalabad
3Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore
4Department of Zoology, University of Gujrat, Gujrat, Pakistan
5Department of Zoology, University of Okara, Okara, Pakistan
ABSTRACT
The present research work was conducted to evaluate the effect of phytase supplementation on growth and nutrient digestibility of the Catla catla fingerlings fed Moringa oleifera leaf meal (MOLM) based diet. The phytic acid’s presence in plant by-products decreases the bioavailability of nutrients to fish, resulting in poor fish growth and low nutrient digestibility in the body. Experimental diet was divided into six groups and were supplemented with graded levels (0, 300, 600, 900, 1200 and 1500 FTU kg-1) of phytase. Cr2O3 was incorporated in all diets at the rate of 1% as a non-digestible marker. The fingerlings were fed at the rate of 4% of live wet weight twice a day and faeces were collected from each tank. On the basis of results it was noted that phytase supplementation showed significant (p<0.05) improvement in growth indices (WG%, FCR, SGR) and digestibility of nutrients (i.e. CP, EE and GE) when C. catla fingerlings were fed at 900 FTU kg-1 level in MOLM based diet. It was further noted that phytase supplementation decreased the discharge of nutrients through faeces resulting in reduced eco-pollution. On the basis of results it was concluded that phytase supplementation at 900 FTU kg-1 level was helpful to develop a cost-effective as well as eco-friendly fish feed by using MOLM based diet.
Article Information
Received 01 June 2018
Revised 10 March 2019
Accepted 06 May 2019
Available online 18 October 2019
Authors’ Contribution
MMS conducted feeding trial, collected data and prepared manuscript. SMH planned and supervised the research and provided all materials for the research. AMA compiled the results. AJ helped in statistical analysis. AC and MH helped in manuscript preparation. SZHS helped in chemical analysis.
Key words
MOLM, Catla catla, Growth performance, Nutrient digestibility, Phytase
DOI: https://dx.doi.org/10.17582/journal.pjz/2020.52.1.157.168
* Corresponding author: drmudassarshahzad@gmail.com
0030-9923/2020/0157-0001 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
World population is continuously growing, thus great pressure is being imposed on availability of resources including food supply. Food scarcity is a serious problem these days, especially in the form of protein (Khan et al., 2015). The world has incredibly relied on fish as a fundamental supplier of nutrients, particularly protein on account of its cost (Agbo et al., 2014). So, higher production of fish is required to meet ever increasing needs of growing population. Feed, a major input in aquaculture production, is a fundamental challenge facing the development and growth of aquaculture (Gabriel et al., 2007). Catla catla commonly known as Thaila, is being cultured in Pakistan with other fish species and are surface feeders (Aslam et al., 2016). The reported production of this fish has increased during the first decade of 21st century and in 2012 was about 2.8 million tons per annum (FAO, 2015). Aquaculture feed mainly relies upon the utilization of fishmeal (FM) because of its high palatability and nutritious esteem (NRC, 2011). Whereas, unstable supply, higher demand and increasing cost of the FM made it necessary to search for alternative sources of protein (Rana et al., 2009; Hardy, 2010). These sources should be of low cost with higher protein contents (Lim et al., 2011). Use of different plant protein sources instead of FM was suggested by the researchers (Barnes et al., 2012; Dedeke et al., 2013; Hussain et al., 2016, 2019; Shahzad et al., 2016). Researchers have found positive effects on overall fish performance when different fish species were fed plant by-products based diets (Hussain et al., 2014, 2016; Chu et al., 2015; Muin et al., 2015; Liu et al., 2015; Shahzad et al., 2017). One of the better and cost effective plant protein sources is M. oleifera also known as miracle tree. Moringa belongs to the Moringaceae family. It is fast growing plant with several economically important feed supplements and medicinal uses. Moringa is a promising protein source when it was included in fish diets at low levels (Chiseva, 2006). High crude protein contents are present in moringa leaves which vary from 25% (Makkar and Becker, 1996) to 32% (Soliva et al., 2005). Leaves of moringa are important source of high amount of nutrients (Bosh, 2004; Grubben and Denton, 2004) and rich source of essential vitamins (Soliva et al., 2005).
But moringa leaves contain several anti-nutritional factors such as phytate that cannot be removed by soaking or heating (Liener, 1994). Growth, nutrient retention, absorption of minerals especially cation minerals, protein and phosphorus (P) are adversely affected by higher phytate or phytic acid concentrations in plant by-products or oilseeds meal based diets (Cao et al., 2007; Gatlin et al., 2007). It is estimated in plant by-products based diets, 60-80% of total P is present in chelated form that is known as phytic acid (Lei et al., 2013). This chelated phosphorous cannot be utilized by mono-gastric and a-gastric fishes, resulting in nutrient discharge into water media causing aquatic pollution (NRC, 1993). It is also capable of binding with proteins and starch (Jondreville et al., 2005; Noureddini and Dang, 2009). Furthermore, phytate binds with trypsin and other amino acids, decreases protein digestibility in mono-gastric and a-gastric fishes (Singh and Krikorian, 1982; Spinelli et al., 1983). Phytate complex only can be broken down by some enzymatic reactions because it is a stable compound (Vielma et al., 2000). Phytase that is known as myo inositol hexa-kisphosphatephospho-hydrolase, can breakdown the phytate complex enzymatically. Mono-gastric fishes cannot hydrolyze the phytate because their body cannot produce phytase enzyme. Supplemental dietary phytase is very effective to improve the nutrient digestibility and higher growth performance of fish. It also decreases water pollution by proper digestion and absorption of phosphorus in the fish body (Hussain et al., 2011; Liu et al., 2013; Hussain et al., 2016). It was noted that phytase improved nutrient digestibility and growth of C. catla and C. mrigala fed plant by-products based diets (Hussain et al., 2011, 2016; Shahzad et al., 2017). Therefore, the present research was focused on to find out the best and least cost protein sources for commercially important fish specie i.e. C. catla to enhance its production and to overcome the problems of expensive fish meal using phytase supplemented MOLM based diet.
MATERIALS AND METHODS
The present research work was carried out to evaluate the effects of phytase supplementation in MOLM based diet on nutrient digestibility and growth performance of C. catla fingerlings. The trial was performed in Fish Nutrition Lab, Department of Zoology, Government College University, Faisalabad. C. catla fingerlings were procured from the Government Fish Seed Hatchery, satiana Road, Faisalabad. Fingerlings were stocked in specially designed V-shaped water tanks (70 L water capacity) for two weeks and were fed on basal diet (Allan and Rowland, 1992). Parameters related to water quality were monitored using specific apparatus i.e. EC meter (HANNA: HI. 8633), pH meter (Jenway 3510) and DO meter (Jenway 970). Through capillary system, aeration was provided round the clock to all the experimental tanks. Before the start of trial with fingerlings of C. catla, all the pathogens were killed by 0.5 % NaCl saline solution treatment (Rowland and Ingram, 1991).
Experimental design
Moringa by-product such as M. oleifera leaf meal (MOLM) was used as test ingredient to formulate experimental diets by the supplementation of phytase with graded levels (0, 300, 600, 900, 1200 and 1500 FTU kg-1). One control diet and five phytase supplemented MOLM based test diets were fed to six fish groups stocked in water tanks. Triplicate tanks were used for each treatment and in each replicate 15 fingerlings were stocked. The fingerlings were fed at the rate of 4% of live wet body weight. The duration of the experiment was 90-days. Test diets supplemented with phytase were compared with control diet to determine growth performance parameters and nutrient digestibility by using completely randomized design (CRD).
Processing of M. oleifera leaves
M. oleifera fresh leaves were collected from a local garden and washed to remove the dirt and dust particles. The leaves were drained appropriately and dried under a shady place for six days to avoid the damage of vitamins by photo-dynamic oxidation or damage. Dried leaves of moringa were separated from the stalks to decrease crude fiber contents in the diet (Madalla et al., 2013). Processed moringa seeds and leaves were ground to make a powder.
Formation of feed pellets
The feed ingredients were procured from a commercial feed mill and were analysed for chemical composition (Table I) following AOAC (1995) prior to the formulation of the experimental diet. The feed ingredients were finely ground to pass through 0.3mm sieve size. All ingredients were mixed at appropriate concentration (Table II) in an electric mixer for 10 minutes and fish oil was gradually added. During mixing of ingredients 10–15% water was also added to prepare suitable texture dough and processed through pelleting machine to formulate pellets (Lovell, 1989). One control and five phytase supplemented test diets were prepared using each moringa by-product by spraying graded levels (0, 300, 600, 900, 1200 and 1500 FTU kg-1) of phytase as shown in Table III. The required concentrations of phytase enzyme (Phyzyme® XP 10000 FTU g−1; Danisco Animal Nutrition, Fin-65101 Vaasa, Finland) were prepared in 50 mL distilled water and sprayed on one kg of each test diet (Robinson et al., 2002). Control diet (0 FTU kg-1 level) was sprayed with a similar amount of distilled water to maintain the equivalent amount of moisture. All the sprayed diets were dried and stored at 4°C until use.
Table I. Chemical composition (%) of feed ingredients (dry matter basis).
|
Ingredients |
MOLM |
FM |
Rice polish |
Wheat flour |
SBM |
|
Dry matter (%) |
91.83 |
91.67 |
94.06 |
92.4 |
92.98 |
|
Crude protein (%) |
28.95 |
49.17 |
12.38 |
10.15 |
40.72 |
|
Crude fat (%) |
2.83 |
7.12 |
11.46 |
2.35 |
4.53 |
|
Crude fiber (%) |
19.95 |
1.12 |
12.74 |
2.67 |
8.67 |
|
Ash (%) |
10.87 |
24.66 |
15.17 |
2.96 |
12.16 |
|
Gross energy (kcal/g) |
3.84 |
2.65 |
3.18 |
2.95 |
3.91 |
|
Carbohydrates |
37.4 |
17.93 |
48.25 |
81.87 |
33.92 |
MOLM, M. oleifera leaf meal; FM, fish meal; SBM, Soybean meal.
Feeding protocol and sample collection
C. catla fingerlings were fed at the rate of 4% of live wet body weight on their prescribed diet twice daily. After the feeding session of two hours, the left over diet was drained out from each tank by opening the valves of the tanks. The tanks were washed completely to remove the feed particles and refilled with water. Faeces were collected by the opening valve-I and valve-II subsequently from the fecal collecting tube of each replicated tank. Fecal material was collected carefully to avoid the breakage of faeces for minimizing the leaching of nutrients in water. Faeces were dried in an oven at 65oC for 3-4 h and stored for further chemical analysis.
Growth study
Fifteen fingerlings of average weight (8.07±0.041g fish-1) were stocked in each replicate. The fish were bulk weighed in each tank on fortnightly basis throughout the whole experimental period to evaluate the growth performance of C. catla fingerlings. Growth parameters such as weight gain (WG), FCR (feed conversion ratio), SGR (specific growth rate) and weight gain (%) of fingerlings were calculated by using standard formulae (NRC, 1993).
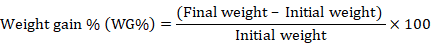

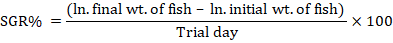
Table II. Ingredients composition (%) of experimental diet (dry matter basis).
|
Ingredients |
Test diet composition |
|
MOLM |
35 |
|
FM |
15 |
|
Soybean meal |
15 |
|
Rice polish |
8 |
|
Wheat flour* |
17 |
|
Fish oil |
6 |
|
Vitamin Premix** |
1.0 |
|
Chromic oxide |
1.0 |
|
Ascorbic acid |
1.0 |
|
Mineral mixture *** |
1.0 |
MOLM, M. oleifera leaf meal; * Phytase enzyme was used at the expense of wheat flour;
** Vitamin premix/kg, Vitamin D3, 3000000 IU; Vitamin A, 15000000 IU; Vitamin E, 30000 IU; Vitamin B1, 3000 mg; Vitamin B6, 4000 mg, Vitamin B12, 40 mg; Vitamin B2, 7000 mg; Vitamin C, 15000 mg; Vitamin K3, 8000 mg; Folic acid, 1500 mg; Calcium pantothenate, 12,000 mg; Nicotinic acid, 60000 mg.
*** Mineral premix/kg: Mn (Manganese), 2000 mg; Ca (Calcium), 155 g; Zn (Zinc), 3000 mg; Cu, (Copper), 600 mg; Co, (Cobalt), 40 mg; I (Iodine), 40 mg; P (Phosphorous), 135 g; Fe (Iron), 1000 mg; Mg (Magnesium), 55 g; Se (Selenium), 3 mg; Na (Sodium), 45 g.
Chemical analysis of feed and faeces
After 90 days of feeding trial, moisture contents of test diets and faeces were calculated after oven-drying of homogenised samples at 105oC for 12 h. Micro Kjeldahl Apparatus (InKjel M behr Labor Technik GmbH D-40599 Dusseldorf) was used to determine the crude protein (CP) contents (N × 6.25) whereas Soxhlet system (Soxhlet Extraction Heating Mantels, 250 ml 53868601) was used to analyse the crude fat by the help of petroleum ether extraction (EE) method. Crude fiber (CF) contents were calculated as loss on ignition of dried lipid-free residues after digestion with 1.25% H2SO4 and 1.25% NaOH whereas ash was determined by ignition at 650oC for 12 h. in electric furnace (Naberthern B170)
Table III. Effect of MOLM based phytase supplemented test diets on the compositions of nutrients in diets and faeces of C. catla fingerlings.
|
Experimental diets |
Phytase (FTU kg-1) |
CP (%) |
EE (%) |
GE (kcal g-1) |
|
Diet |
||||
|
Test diet –I (Control diet) |
0 |
28.28±0.15 |
6.91±0.09 |
3.00±0.08 |
|
Test diet –II |
300 |
28.31±0.09 |
6.91±0.02 |
3.01±0.06 |
|
Test diet –III |
600 |
28.35±0.11 |
6.92±0.04 |
2.98±0.05 |
|
Test diet –IV |
900 |
28.39±0.27 |
6.92±0.06 |
3.01±0.06 |
|
Test diet –V |
1200 |
28.36±0.30 |
6.93±0.06 |
3.01±0.07 |
|
Test diet –VI |
1500 |
28.41±0.02 |
6.92±0.04 |
2.96±0.06 |
|
Faeces |
||||
|
Test diet –I (Control diet) |
0 |
17.69±0.31e |
4.08±0.07a |
1.73±0.06e |
|
Test diet –II |
300 |
15.61±0.21d |
3.57±0.06b |
1.55±0.10d |
|
Test diet –III |
600 |
14.28±0.13c |
3.15±0.07c |
1.33±0.04c |
|
Test diet –IV |
900 |
9.53±0.13a |
2.53±0.11d |
0.93±0.04a |
|
Test diet –V |
1200 |
11.64±0.07b |
2.01±0.07e |
1.13±0.05b |
|
Test diet –VI |
1500 |
13.68±0.19c |
3.28±0.07c |
1.46±0.02cd |
Data are means of three replicates (± shows Standard Deviations); CP, Crude protein; EE, Ether Extract (crude fat); GE, Gross energy; a-f Means within column having different superscripts are significantly different at p<0.05; Data are means of three replicates (± shows Standard Deviations).
to constant weight. Total carbohydrates (N-free extract) were calculated by using standard formula i.e. Total carbohydrates (%) =100- (EE % + CP % + Ash % + CF %). Oxygen bomb calorimeter was used to estimate the gross energy (GE) of samples. Chromic oxide as an inert marker was added in test diets to determine nutrient digestibility. Chromic oxide content after oxidation of the ash samples of feces and experimental diets with perchloric reagent was estimated by acid digestion method (Divakaran, Leonard, and Lan, 2002) through UV-VIS 2001 spectrophotometer at 350nm.
Apparent nutrient digestibility coefficients (%) of test diets were calculated by the standard formula (NRC, 1993).
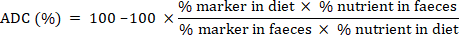
Statistical analysis
Finally, data of ADC% of nutrients (CP, EE and GE) and growth parameters (WG, WG%, SGR and FCR) were subjected to one-way Analysis of Variance (Steel et al., 1996). The differences among treatments were compared by Tukey’s Honesty Significant Difference Test and considered significant at p<0.05 (Snedecor and Cochran, 1991). The CoStat Computer Package (Version 6.303, PMB 320, Monterey, CA, 93940 USA) was used for statistical analysis.
RESULTS
Results showed that, there was an equal amount of nutrients in all the MOLM based diets including the control diet (Table III). Whereas, this table also shows maximum nutrients discharged in water through faeces when fish was fed on the control diet (0 FTU kg-1 level based diet) and minimum nutrients (CP and GE) were discharged at 900 FTU kg-1 level followed by 1200 FTU kg-1 level based diet. This reduction in nutrients excretion through faeces indicated that phytate complex was being broken-down by phytase addition. It was observed that further increase in phytase supplementation (1500 FTU kg-1), increased the nutrient excretion. Primary data confirms that highest ADC% was observed at 900 FTU kg-1 level when we talk about CP and GE. Maximum values of CP (69%) and GE (72%) digestibility were found in fish fed at 900 FTU kg-1 level of phytase supplemented MOLM based diet followed (CP 62% and GE 66%) by 1200 FTU kg-1 level based diet. Whereas lowest ADC% of CP (42%) and GE (47%) were recorded in fish fed on the control diet as shown in Table IV. Results of CP and GE were significantly (p<0.05) different from ADC% values observed for control and each other phytase supplemented test diet. On the contrary, minimum apparent EE contents were discharge through faeces at 1,200 FTU kg-1 followed by 900 FTU kg-1 diet (Fig. 1). Similarly, the maximum ADC% for EE (74%) was observed at 1,200 FTU kg-1 level followed by (67%) when fingerlings were fed on test diet IV supplemented with 900 FTU kg-1 level. These values were significantly (p<0.05) different from all the phytase supplemented test and control diets. Lowest (45%) ADC% was observed for fish fed on control diet (0 FTU kg-1 level based diet).
Table IV. Effect of MOLM based diets on the apparent nutrient digestibility of C. catla fingerlings.
|
Experimental diets |
Phytase levels (FTU kg-1) |
CP (%) |
EE (%) |
GE (%) |
|
Test diet –I (Control diet) |
0 |
42.22±0.10f |
45.43±0.98e |
46.57±0.86e |
|
Test diet –II |
300 |
48.51±0.46e |
51.67±0.65d |
52.73±0.62d |
|
Test diet –III |
600 |
53.38±0.35d |
57.64±0.80c |
58.54±0.92c |
|
Test diet –IV |
900 |
69.33±0.39a |
66.58±0.96b |
71.67±0.97a |
|
Test diet –V |
1200 |
62.50±0.11b |
73.54±0.96a |
65.63±0.72b |
|
Test diet –VI |
1500 |
55.15±0.33c |
56.19±0.93c |
53.63±0.97d |
a-f Means within column having different superscripts are significantly different at P<0.05; Data are means of three replicates (± shows Standard Deviations).
Table V. Effect of graded levels of phytase supplemented MOLM based diets on the growth performance of C. catla fingerlings .
|
Growth parameters |
Test Diet –I (Control diet) |
Test Diet –II |
Test Diet –III |
Test Diet –IV |
Test Diet –V |
Test Diet –VI |
|
Phytase levels (FTU kg-1) |
||||||
|
0 |
300 |
600 |
900 |
1200 |
1500 |
|
|
IW (g) |
8.13±0.03 |
8.1±0.04 |
8.09±0.03 |
8.08±0.05 |
8.09±0.03 |
8.06± 0.02 |
|
FW (g) |
20.42±0.11e |
20.93±0.03d |
21.85±0.10c |
24.22±0.14a |
23.32±0.1b |
21.12± 0.11d |
|
WG (g) |
12.29±0.13e |
12.84±0.06d |
13.76±0.13c |
16.14±0.17a |
15.23±0.11b |
13.06± 0.12d |
|
WG (%) |
151.13±1.95e |
158.55±1.46d |
170.02±2.1c |
199.80±2.93a |
188.14±1.71b |
162.10± 1.54d |
|
FI |
0.239±0.002 |
0.24±0.002 |
0.241±0.002 |
0.254±0.002 |
0.252±0.002 |
0.236± 0.003 |
|
WG (fish-1 day-1)g |
0.14±0.001e |
0.14±0.001d |
0.15±0.001c |
0.18±0.002a |
0.17±0.001b |
0.15± 0.001d |
|
FCR |
1.75±0.004a |
1.69±0.01b |
1.58±0.001d |
1.42±0.005f |
1.49±0.002e |
1.63± 0.01c |
|
SGR |
1.02±0.01e |
1.06±0.01d |
1.09±0.01c |
1.22±0.01a |
1.18±0.01b |
1.07± 0.01cd |
a-d Means within rows having different superscripts are significantly different at p<0.05; Data are means of three replicates (± shows Standard Deviations).
IW, Initial Weight; FW, Final Weight; WG, Weight gain; FI, Feed Intake; SGR, Specific Growth Rate; FCR, Feed Conversion Ratio.
Mean overall growth performance (WG, WG%, FCR and SGR) of C. catla fingerlings fed on MOLM based test diets is presented in Table V. These results clearly indicate that phytase supplemented MOLM based diet enhanced the growth parameters of C. catla fingerlings as compared to the control diet (without phytase supplementation) yet the initial weight of fish was statistically (p<0.05) similar in all the diets. From the current findings it was noted that all the parameters related to growth performance of C. catla fingerlings fed on MOLM based diet started to increase from 300 FTU kg-1 diet to its maximum at 900 FTU kg-1 level based diet. It was also found that MOLM based test diets having further higher levels (1,200 and 1,500 FTU kg-1 levels) of phytase supplementation resulted in decreased growth performance. Results showed that C. catla fingerlings fed MOLM based diet supplemented at 900 FTU kg-1 level significantly increased (p<0.05) growth performance when compared to the control (without phytase) and other phytase supplemented test diets.
Figure 2 shows that the highest WG (16g) and WG% (200%) were recorded in fingerlings fed MOLM based phytase supplemented (900 FTU kg-1 diet) followed (15g and 188%) by 1,200 FTU kg-1 diet. These values were significantly higher (p<0.05) than the control (12g and 151%) and remaining phytase supplemented test diets. Lowest FCR value (1.42) was noted in fish fed on test diet IV (900 FTU kg-1 level) followed by (1.49) at 1200 FTU kg-1 level based diet. These values were significantly (p<0.05) different from the values observed for fish that fed on the control and remaining phytase supplemented test diets. However highest FCR value (1.75) was found in fish that fed on the control diet (without phytase supplementation). Lowest FCR showed highest feed conversion into fish flesh. Similarly, C. catla fingerlings showed maximum SGR (1.22) at 900 FTU kg-1 level based diet followed by (1.18) when the fish fed at 1200 FTU kg-1 level and was significantly (p<0.05) higher from other test diets as well as control diet. Results showed that SGR of fish fed at 600 FTU kg-1 level based diet was statistically (p<0.05) at par with the SGR value of fish that fed at 300 FTU kg-1 level and 1500 FTU kg-1 based diet. Whereas minimum SGR (1.02) was found for fish fed on the control diet (0 FTU kg-1 level based diet).
From these findings it was clear that supplementation of phytase in MOLM based diet significantly improved ADC% of nutrients as well as growth performance for fingerlings fed 900 FTU kg-1 as well as 1200 FTU kg-1 diets (p<0.05). These results indicate that ADC% of CP and GE and growth parameters increased from 300 FTU kg-1 diet and reached its maximum at 900 FTU kg-1 diet. Further phytase supplementation (1,200 and 1,500 FTU kg-1) did not improve the ADC% of CP and GE and growth. Whereas ADC% of EE started to improve from 300 FTU kg-1 diet and reached its maximum at 1,200 FTU kg-1 diet, additional phytase supplementation to 1,500 FTU kg-1 diet decreased percent ADC. It was concluded that the 900 FTU kg-1 diet supplemented had maximum nutrient digestibility, growth and reduced nutrient’s discharge in water through faeces resulting in decreased aquatic pollution.
DISCUSSION
M. oleifera leaf meal (MOLM) based diet is virtuous sources of protein, fat as well as other nutrients. But in MOLM based diets, an anti-nutritional factor i.e. phytate or phytic acid is present (Gopalakrishnan et al., 2016; Worku, 2016). The presence of phytate in plant based diets can cause adverse effects on nutrient digestibility in fish (Hussain et al., 2016; Shahzad et al., 2018). According to results of the current study, it was found that addition of phytase in MOLM based diet was useful for the improvement in apparent digestibility coefficient (ADC %) of nutrients for C. catla fingerlings when compared with the control diet (without phytase supplementation). Highest values for ADC% of CP (69%) and GE (72%) were observed when fish were fed at the 900 FTU kg-1 of phytase supplemented MOLM based diet. Similarly, Ashraf and Goda (2007) observed that optimal level of phytase enzyme for highest CP and GE digestibility was 1000 FTU kg-1 level. They also found that ADC% of CP and GE could not be further improved on higher supplementation levels of enzyme. Hussain et al. (2017) and Shahzad et al. (2018) also found similar results. They found maximum CP and GE digestibility in C. catla when fingerlings were fed at 900 FTU kg-1 level based diet. Nearly similar to our results, Hussain et al. (2016) found higher CP and GE digestibility in Cirrhinus mrigala fingerlings when fed soybean meal based test diet supplemented with phytase at 1000 FTU kg-1 diet. Whereas, maximum CP and GE digestibility was observed in Labeo rohita fingerlings fed phytase supplemented soybean meal based diet (Baruah et al., 2007a) and soybean meal based diets (Hussain et al., 2014) at 750 FTU kg-1 level. In contrast to these findings, non-significant (p>0.05) values of protein digestibility were reported after phytase supplementation in plant meal based diets (Cheng and Hardy, 2002; Yan and Reigh, 2002; Dalsgaard et al., 2009). Whereas, in another study decreased CP digestibility was found in Psetta maxima (turbot) fed phytase supplemented rapeseed protein concentrate as compared to control diet (Danwitz et al., 2016). This variation in results is difficult to explain but the possible reasons may be due to the different fish species, feed ingredients, different experimental conditions, different sources of phytase enzyme, pH of the stomach, feed processing methods or feed drying methods (Liu et al., 2013).
Present results indicated that maximum ADC% of EE i.e. 73% was observed in fish fed on MOLM based phytase supplemented (1200 FTU kg-1 level) diet followed by (67%) when fingerlings were fed at 900 FTU kg-1 level based diet. Nearly similar to present findings, Hussain et al. (2014) found maximum EE digestibility at 1000 FTU kg-1 level when C. mrigala fingerlings fed soybean meal based diet. They also concluded that further phytase addition (1500 and 2000 FTU kg-1) leads to a decline in ADC% of EE. In contrary to these, 750 FTU kg-1 diet was suggested as the most suitable level for higher fat digestibility in L. rohita fingerlings (Baruah et al., 2007b). Whereas, higher EE digestibility was found in Nile tilapia when it fed at 1000 and 2000 FTU kg-1 level based test diets (Portz and Liebert, 2004). Reasons for these variations may be type or source of phytase and amount of phytate in plant ingredients. From these findings, it was clear that different levels of phytase supplementation showed maximum EE digestibility because of the different fish species, different protein source and experimental conditions. On contrary, Dalsgaard et al. (2009) observed non-significant (p>0.05) results of fat digestibility in Oncorhynchus mykiss (rainbow trout) fed phytase supplemented soybean meal based diets. Whereas, decreased EE digestibility was found in O. mykiss (rainbow trout) fed phytase supplemented soybean meal based diet (Wang et al., 2009). Cao et al. (2007) explained that the 250-1,500 FTU kg-1 levels of phytase supplementation are sufficient to release the crude lipid from phytate complex for higher fat digestibility.
The presence of phytate in feed results reduced fish growth performance in terms of weight gain and FCR (Spinelli et al., 1983). Maximum WG (16g) and WG% (200%) were observed in fish fed on 900 FTU kg-1 diet in MOLM based diets. These values of WG and WG% were much higher as compared to other phytase supplemented test diets and control diet (0 FTU kg-1 diet). Nearly similar results were found in terms of WG and WG%, when common carp fingerlings fed at 800 FTU kg-1 supplemented plant-meal based diet (Bai et al., 2003). Similar findings were discussed by Shahzad et al. (2017, 2018) in which they found maximum growth performance in the C. catla fingerlings fed 900 FTU kg-1 levels based diets as compared to control and other test diets. Nwanna et al. (2007) also found a significant (p<0.05) increase in overall Cyprinus carpio WG and WG% at 750 and 1,000 FTU kg-1 levels based diets. Nearly similar results were observed in a study conducted by Hussain et al. (2014), they found maximum weight gain and weight gain % of C. mrigala fingerlings fed soybean meal based diet supplemented with phytase at 1,000 FTU kg-1 level. Similarly, Yu and Wang (2000) also found the same results when fish was fed at 1,000 FTU kg-1 level based diet. In contrast, non-significant (p<0.05) effect of phytase supplementation was reported in case of O. mykiss growth when fingerlings fed phytase supplemented plant meal based diets (Vielma et al., 2000). In contradiction to the present findings many other researchers such as Robinson et al. (2002), Baruah et al. (2007a), Lim and Lee (2009) and Wang et al. (2009) did not find any significant effect (p>0.05) of phytase supplementation on fish growth performance, when these fish species were fed with or without phytase supplemented plant meal based diets. It was obvious from the previous literature that maximum growth performance increment in different fish species was observed when fed plant meal based diets supplemented with microbial phytase ranging between 250 to 1500 FTU kg-1 diet (Cao et al., 2007). This controversy in results for growth indices may be linked with many factors such as types of feed ingredients used, varying levels of phytase, processing methods of feed, stomach pH and methods used for feed drying (Wang et al., 2009).
The current study showed lowest FCR (1.42) and highest SGR (1.22) values of C. catla fingerlings fed at 900 FTU kg-1 level based diet in MOLM based diet. Similar to our results Riche and Garling (2004), Ashraf and Goda (2007), Cao et al. (2008) observed maximum SGR and minimum FCR values when O. niloticus (Nile tilapia) fed plant meal based diets with phytase supplementation at 1,000 FTU kg-1 level. Hussain et al. (2017) also observed highest SGR (1.37) and lowest FCR (1.15) in C. catla fingerlings when they were fed on M. oleifera seed meal based diet with phytase supplementation at 900 FTU kg-1 level based diet. Nwanna and Schwarz (2008) found improvement in growth parameters when C. carpio (common carp) fingerlings fed phytase supplemented plant meal based diet at 750 and 1000 FTU kg-1 levels. On the other hand, Hussain et al. (2011) observed maximum improvement in FCR of L. rohita fingerlings when they fed 750 FTU kg-1 level based diet that was close to the optimum level (900 FTU kg-1diet) found in present study. In contrast to current findings, higher FCR values of Monorone saxatilix (stripped seabass) were observed when fed with a little higher dose (at 1300 FTU kg-1 level) of phytase supplementation in plant meal based diet (Hughes and Soares, 1998). Similar results were found when C. catla fingerlings were fed on phytase supplemented moringa by-products based diets at 900 FTU kg-1 level based diet (Shahzad et al., 2018). They found minimum FCR (1.30) and maximum SGR (1.32) in C. catla fingerlings fed on moringa by-products based diet supplemented with 900 FTU kg-1 level. Another close result was also recorded in a study, when C. mrigala fingerlings fed phytase supplemented soybean meal based diets (Hussain et al., 2014). They found that fingerlings showed maximum growth performance in terms of WG (11g), WG% (155%) and FCR (1.43) when fish fed at 1,000 FTU kg-1 level based diet as compared to control and other levels of phytase supplemented test diets. It was noted that phytase supplementation improves growth performance but on varying levels. Activity of phytase supplementation depends on the quantity of phytate present in plant meal based diets, environmental conditions, experimental fish species and source of phytase enzyme (Kumar et al., 2011). In contrary, phytase supplementation cannot enhance the overall growth performance of O. mykiss fed phytase supplemented canola meal based diets (Vielma et al., 2000). Statistically, no significant (p<0.05) difference was observed in term of growth performance when Korean rock fish (Yoo et al., 2005), parrot fish (Lim and Lee, 2009) Japanese flounder (Masumoto et al., 2001), channel catfish (Yan and Reigh, 2002) and Atlantic salmon (Sajjadi and Carter, 2004) fed on different plant meal based diets supplemented with phytase at different levels. Researchers argued that these variations in results conducted by different researchers (on different fish species, different time interval and in different geographical areas) may be due to the methodology of feed preparation, type of phytase, species of fish and ingredients used in feed preparation (Baruah et al., 2007b; Cao et al., 2007).
Literature showed that there was not a single study conducted on supplementation of phytase in MOLM based diet. But, many researchers performed their respective trials to replace FM with leaf meal and found positive effects on nutrient digestibility in fish (Madalla et al., 2013; Bello and Nzeh, 2013; Ganzon-Naret, 2014; Abo-State et al., 2014; Ncha et al., 2015; Mehdi et al., 2016). Nwanna et al. (2008) performed a study to investigate effects of phytase supplemented Brazil nut and Leucaena leaf meal mixture based diet on nutrient digestibility in Amazon tambaqui which resulted in non-significant fish performance. The findings of present study showed that the supplementation of phytase in MOLM based diets resulted in reduced excretion of nutrients through faeces into water, which in turn enhanced nutrient digestibility as compared to fish fed at 0 FTU kg-1 diet. The utilization of nutrients in fish body and less discharge in water is very useful in improving growth performance and reducing the pollution in aquatic environment (Hussain et al., 2016). From these findings, it was concluded that phytase supplementation at 900 FTU kg-1level in MOLM based diet is essential for improving fish growth performance by higher nutrient digestibility as compared to control diet (without phytase supplementation).
Statement of conflict of interest
Authors have declared no conflict of interest.
REFERENCES
Abo-State, H., Hammouda, Y., El-Nadi, A. and Abo-Zaid, H., 2014. Evaluation of feeding raw moringa (Moringa oleifera Lam.) leaves meal in Nile tilapia fingerlings (Oreochromis niloticus) diets. Glob. Vet., 13: 105-111.
Agbo, N.W., Amisah, S., Tettey, E. and Frimpong, E A., 2014. Effects of dietary protein levels on growth performance of claroteid catfish, Chrysichthys nigrodigitatus fingerlings. Annls biol. Res., 5: 17-22.
Allan, G.L. and Rowland, S.J., 1992. Development of an experimental diet for silver perch (Bidynus bidyanus). Austasia Aqua., 6: 39-40.
AOAC (Association of Official Analytical Chemists). 1995. Official methods of analysis. 15th Ed. Association of Official Analytical chemists, Washington, D.C. USA., p. 1094.
Ashraf, M. and Goda, A.S., 2007. Effect of dietary soybean meal and phytase levels on growth, feed utilization and phosphorus discharge for Nile tilapia (Oreochromis niloticus L.). J. Fish. Aquacult. Sci., 2: 248-263. https://doi.org/10.3923/jfas.2007.248.263
Aslam, S., Abbas, S., Kalhoro, M.A. and Shoaib, A., 2016. Anchor worms (lernaeid parasites), Lernaea polymorpha yü and Lernaea cyprinacea (copépode: lernaeidae) on major carps at different fish farms in Punjab, Pakistan. Sci. Int., 28: 295-298.
Bai, D.Q., Qiao, X.T., Wei, D., Guo, L. and Qi, H.L., 2003. Effects of phytase on utilization ratio of nutrient composition (calcium, phosphorus etc.) of Carp (Cyprinus carpio L.). J. Tianj. Agric. Coll., 10: 6-11.
Barnes, M.E., Brown, M.L. and Rosentrator, K.A., 2012. Juveniles rainbow trout responses to diets containing distillers dried grain with soluble, phytase and amino acid supplements. Open J. Anim. Sci., 2: 69-77. https://doi.org/10.4236/ojas.2012.22011
Baruah, K., Pal, K.A.K., Narottam, P.S. and Debnath, D., 2007a. Microbial Phytase supplementation in rohu, Labeo rohita, diets enhances growth performance and nutrient digestibility. J. World aquaul. Soc., 38: 129-137. https://doi.org/10.1111/j.1749-7345.2006.00081.x
Baruah, K., Sahu, P.N., Pal, K.A., Jain, K.K., Debnath, D. and Mukherjee, C.S., 2007b. Dietary microbial phytase and citric acid synergistically enhances nutrient digestibility and growth performance of Labeo rohita (Hamilton) juveniles at sub-optimal protein level. Aquacul. Res., 38: 109-120. https://doi.org/10.1111/j.1365-2109.2006.01624.x
Bello, O.N. and Nzeh, G.C., 2013. Effects of varying levels of Moringa oleifera leaf meal diet on growth performance, hematological indices and biochemical enzymes of African catfish Clarias gariepinus (Burchell 1822). Elixir Aquacul., 57: 14459-14466.
Bosh, C.H., 2004. USDA National Nutrient Database for standard reference. In: Plant resources of tropical Africa (eds. G.J.H. Grubben and O.A. Denton). PROTOA Foundation, Wageningen Netherlands. pp. 392- 393.
Cao, L., Wang, W., Yang, C., Yang, Y., Diana, J., Yakupitiyage, A., Luo, Z. and Li, D., 2007. Application of microbial phytase in fish feed. J. Enzym. Microb. Tech., 40: 497-507. https://doi.org/10.1016/j.enzmictec.2007.01.007
Cao, L., Yang, Y., Wang, W.M., Yakupitiyage, A., Yuan, D.R. and Diana, J.S., 2008. Effect of pre-treatment with microbial phytase on phosphorus utilization and growth performance of Nile Tilapia (Oreochromis niloticus). Aquacul. Nutr., 14: 99-109. https://doi.org/10.1111/j.1365-2095.2007.00508.x
Cheng, Z.J. and Hardy, R.W., 2002. Effect of microbial phytase on apparent nutrient digestibility of barley, canola meal, wheat and wheat middlings, measured in vivo using rainbow trout (Oncorhynchus mykiss). Aquacul. Nutr., 8: 271-277. https://doi.org/10.1046/j.1365-2095.2002.00219.x
Chiseva, S., 2006. The growth rates and feed conversion ratios of fry fed conventional fry diets and Moringa oleifera supplemented diets. B.Sc. Dissertation, Bindura University of Science Education, Zimbabwe.
Chu, Z.J., Yu, D.H., Dong, G.F. and Gong, S.Y., 2015. Partial replacement of fish meal by soybean meal with or without methionine and phytase supplement in the diets for juvenile Chinese sucker. Aquacul. Nutr., 22: 989-996. https://doi.org/10.1111/anu.12318
Dalsgaard, J., Ekmann, K.S., Pedersen, P.B. and Verlhac, V., 2009. Effect of supplemented fungal phytase on performance and phosphorus availability by phosphorus depleted juvenile rainbow trout (Oncorhynchus mykiss) and on the magnitude and composition of phosphorus waste output. Aquaculture, 286: 105-112. https://doi.org/10.1016/j.aquaculture.2008.09.007
Danwitz, A.V., Bussel, C.G.J.V., Klatt, S.F. and Schulz, C., 2016. Dietary phytase supplementation in rapeseed protein based diets influences growth performance, digestibility and nutrient utilisation in turbot (Psetta maxima). Aquaculture, 450: 405–411. https://doi.org/10.1016/j.aquaculture.2015.07.026
Dedeke, G.A., Owa, S.O., Olurin, K.B., Akinfe, A.O. and Awotedu, O.O., 2013. Partial replacement of fish meal by earthworm meal (Libyodrilus violaceus) in diets for African catfish, Clarias gariepinus. Int. J. Fish. Aquacul., 5: 229-233.
Divakaran, S., Leonard, G.O. and Lan, P.F., 2002. Note on the methods for determination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
FAO., 2015. Fisheries and Aquaculture Department, Catla catla (Hamilton, 1822) Cultured Aquatic Species Information Programme.
Gabriel, U.U., Akinrotimi, O.A., Anyanwu, P.E., Bekibele, D.O. and Onunkwo, D.N., 2007. The role of dietry phytase in formulation of least cost and less polluting fish feed for sustainable aquaculture development in Nigeria. Afri. J. agric. Res., 2: 279-286.
Ganzon-Naret, E.S., 2014. Utilization of Moringa oleifera leaf meals as plant protein sources at different inclusion levels in fish meal based diets fed to Lates calcarifer. ABAH Bioflux, 6: 158-167.
Gatlin III, D.M., Barrows, F.T., Brown, P., Dabrowski, K., Gaylord, T.G., Hardy, R.W., Herman, E., Hu, G., Krogdahl, A., Nelson, R., Overturf, K., Rust, M., Sealey, W., Skonberg, D., Souza, E.J., Stone, D., Wilson, R. and Wurtele, E., 2007. Expanding the utilization of sustainable plant products in aqua feeds: a review. J. aquacul. Res., 38: 551-579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
Gopalakrishnan, L., Doriya, K. and Kumar, D.S., 2016. Moringa oleifera: A review on nutritive importance and its medicinal application. Fd. Sci. Human Welln., 5: 49-56. https://doi.org/10.1016/j.fshw.2016.04.001
Grubben, G.J.H. and Denton, O.A., 2004. Plant resources of tropical Africa. PROTA Foundation, Wageningen, Netherlands Netherlands. pp. 650.
Hardy, R.W., 2010. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. J. aquacul. Res., 41: 770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
Hughes, K.P. and Soares, J.H., 1998. Efficacy of phytase on phosphorus utilization in practical diets fed to striped bass, Morone saxatilis. aquacul. Nutri., 4: 133-140. https://doi.org/10.1046/j.1365-2095.1998.00057.x
Hussain, S.M., Afzal, M., Rana, S.A., Javed, A. and Iqbal, M., 2011. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal-based diets. Int. J. Biosci., 13: 916-922.
Hussain, S.M., Shahzad, M.M., Jabeen, F., Nasir, S., Afzal, M., Javid, A., Ahmad, S., Arsalan, M. Z.H., Riaz, D., Khichi, T.A.A., Ahmad, A.W. and Furqan, M., 2014. Growth performance and nutrient digestibility of Cirrhinus mrigala fingerlings fed on soybean meal-based diet supplemented by phytase. Int. J. Biosci., 5: 212-221. https://doi.org/10.12692/ijb/5.12.212-221
Hussain, S.M., Hameed, T., Afzal, M., Jabeen, F., Javid, A., Mustafa, I., Hussain, M., Ahmad, S. and Arsalan, M.Z., 2017. Growth performance and nutrient digestibility of Cirrhinus mrigala fingerlings fed on sunflower meal based diet supplemented with phytase. Pakistan J. Zool., 49: 1713-1724. https://doi.org/10.17582/journal.pjz/2017.49.5.1713.1724
Hussain, S.M., Shahzad, M.M., Aslam, N., Javid, A., Hussain, A.I., Hussain, M. and Arsalan, M.Z.H., 2016. Use of phytase at graded levels for improving nutrient digestibility, growth and hematology of Catla catla fingerlings fed MOSM based diet. Ind. J. Fish., 64: 48-57. https://doi.org/10.21077/ijf.2017.64.2.62418-08
Hussain, S.M., Ahmad, N., Rasul, A., Shahzad, M.M., Latif, M., Arsalan, M.Z.H., Umair, M. and Shafqat, H.N., 2019. Efficacy of nano-Cr particles supplemented sunflower meal based diets on growth performance, digestibility and hematology of Catla catla fingerlings. Pakistan J. Zool., 51: 1943-1952, 2019.
Jondreville, C., Hayler, R. and Feuerstein, D., 2005. Replacement of zinc sulphate by microbial phytase for piglets given a maize-soya-bean meal diet. J. Anim. Sci., 81: 77–83. https://doi.org/10.1079/ASC41440077
Khan, K.J., Khan, N., Rasool, F., Ullah, S. and Hassan, S., 2015. Apparent digestibility of selected plant based ingredients and their impacts on body composition of mori, Cirrhinus mrigala (Hamilton, 1882), Glob. Vet., 15: 536-540.
Kumar, V., Sinha, A.K., Makkar, H.P.S., De Boeck, G. and Becker, K., 2011. Phytate and phytase in fish nutrition. J. Anim. Physiol. Anim. Nutri., 96: 335–364. https://doi.org/10.1111/j.1439-0396.2011.01169.x
Lei, X.G., Weaver, J.D., Mullaney, E., Ullah, A.H. and Azain, M.J., 2013. Phytase a new life for an old enzyme. Annu. Rev. Anim. Biosci., 1: 283-309. https://doi.org/10.1146/annurev-animal-031412-103717
Liener, I.E., 1994. Implications of antinutritional components in soybean foods. Crit. Rev. Fd. Sci. Nutri., 34: 31–67. https://doi.org/10.1080/10408399409527649
Lim, S.J. and Lee, K.J., 2009. Partial replacement of fish meal by cottonseed meal and soybean meal with iron and phytase supplementation for parrot fish Oplegnathus fasciatus. Aquaculture, 290: 283-289. https://doi.org/10.1016/j.aquaculture.2009.02.018
Lim, S.J., Kim, S., Ko, G., Song, J., Oh, D., Kim, J. and Lee, K., 2011. Fish meal replacement by soybean meal in diets for tiger puffer, Takifugurubripes. Aquaculture, 313: 165-170. https://doi.org/10.1016/j.aquaculture.2011.01.007
Liu, H., Jin, J., Zhu, X., Han, D., Yang, Y. and Xie, S., 2015. Effect of substitution of dietary fish meal by soybean meal on different sizes of gibel carp (Carassius auratus gibelio): digestive enzyme gene expressions and activities, and intestinal and hepatic histology. Aquacul. Nutri., 23: 129-147. https://doi.org/10.1111/anu.12375
Liu, L.W., Su, J.M., Zhang, T., Liang X.Z. and Luo Y.L., 2013. Apparent digestibility of nutrients in grass carp diet supplemented with graded levels of phytase using pre-treatment and spraying methods. Aquacul. Nutri., 19: 91–99. https://doi.org/10.1111/j.1365-2095.2012.00942.x
Lovell, R.T., 1989. Nutrition and feeding of fish. Van Nostrand-Reinhold, New York, pp. 260. https://doi.org/10.1007/978-1-4757-1174-5
Madalla, N., Agbo, N.W. and Jauncey, K., 2013. Evaluation of aqueous extracted moringa leaf meal as a protein source for Nile Tilapia Juveniles. Tanzania J. agric. Sci., 12: 53-64.
Makkar, H.P.S., and Becker, K., 1996. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim. Feed Sci. Technol., 63: 211-228. https://doi.org/10.1016/S0377-8401(96)01023-1
Masumoto, T., Tamura, B. and Shimeno, S., 2001. Effects of phytase on bioavailability of phosphorus in soybean meal-based diets for Japanese flounder Paralichthys olivaceus. Fish. Sci., 67: 1075-1080. https://doi.org/10.1046/j.1444-2906.2001.00363.x
Mehdi, H., Khan, N., Iqbal, J.K., Rasool, F., Chaudhry, M.S. and Khan, K.J., 2016. Effect of Moringa oleifera meal on the growth, body composition and nutrient digestibility of Labeo rohita. Int. J. Biosci., 4: 11-17. https://doi.org/10.12692/ijb/8.4.11-17
Muin, H., Taufek, N.M., Abiodunr, A., Yusof, H.M. and Razak, S.A., 2015. Effect of partial and complete replacement of fishmeal with mushroom stalk meal and soy bean meal on growth performance of Nile tilapia, Oreochromis niloticus fingerlings. Sains Malay., 44: 511–516. https://doi.org/10.17576/jsm-2015-4404-05
NRC (National Research Council). 2011. Nutrient requirements of fish, 114.Washington, DC, National Academy Press.
Ncha, O.S., Michael, P.B., Nnabuchi, U.O. and Alex, E., 2015. Effect of diets with moringa leaf meal on growth, carcass composition and haematology of Clarias gariepinus. Int. J. Fish. aquat. Stud., 3: 397-401.
Noureddini, H. and Dang, J., 2009. Degradation of phytate in distillers’ grains and corn gluten feed by Aspergillus niger phytase. Appl. Biochem. Biotechnol., 159: 11-23. https://doi.org/10.1007/s12010-008-8365-2
Nwanna, L.C. and Schwarz, F.J., 2008. Effect of supplemental phytase on growth, phosphorus digestibility and bone mineralization of common carp (Cyprinus carpio L). Aquacul. Res., 38: 1037-1044. https://doi.org/10.1111/j.1365-2109.2007.01752.x
Nwanna, L.C., Eisenreich, R. and Schwarz, F.J., 2007. Effect of wet-incubation of dietary plant feedstuffs with phytases on growth and mineral digestibility by common carp Cyprinus carpio L. Aquaculture, 271: 461-468. https://doi.org/10.1016/j.aquaculture.2007.04.020
Nwannaa, L., Oishi, C. and Pereira-Filho, M., 2008. Use of phytase to improve the digestibility of alternative feed ingredients by Amazon tambaqui, Colossoma macropomum. J. Sci. Asia, 34: 353-360.
Portz, L. and Liebert, F., 2004. Growth, nutrient utilization and parameters of mineral metabolism in Nile tilapia Oreochromis niloticus (Linnaeus, 1758) fed plant based diets with graded levels of microbial phytase. J. Anim. Physiol. Anim. Nutri., 88: 311-320. https://doi.org/10.1111/j.1439-0396.2004.00486.x
Rana, K.J., Siriwardena, S. and Hasan, M.R., 2009. Impact of rising feed ingredient prices on aquafeeds and aquaculture production (No. 541). Food and Agriculture Organization of the United Nations, Paper No. 541FAO, Rome. (pp. 63).
Riche, M. and Garling, D.L., 2004. Effect of phytic acid on growth and nitrogen retention in tilapia Oreochromis niloticus L. Aquacul. Nutri., 10: 389-400. https://doi.org/10.1111/j.1365-2095.2004.00314.x
Robinson, E.H., Li, M.H. and Manning, B.B., 2002. Comparison of microbial phytase and dicalciumphosphate or growth and bone mineralization of pond raised channel catfish, Ictalurus punctatus. J. appl. Aquacul., 12: 81-88. https://doi.org/10.1300/J028v12n03_08
Rowland, S.J. and Ingram, B.A., 1991. Diseases of Australian native fishes. In: Fisheries bulletin 4 NSW Fisheries, Sydney, NSW, Australia, 21-23.
Sajjadi, M. and Carter, C.G., 2004. Effect of phytic acid and phytase on feed intake, growth digestibility and trypsin activity in Atlantic salmon (Salmo salar L.). Aquacul. Nutri., 10: 135-142. https://doi.org/10.1111/j.1365-2095.2003.00290.x
Shahzad, M.M., Hussain, S.M., Jabeen, F., Hussain, A.I., Arsalan, M.Z.H., Ahmad, N., Rehan, M.M.H. and Riaz, D., 2016. Carcass composition and hematological study of Catla catla fingerlings fed on phytase supplemented Moringa oleifera leaf meal (MOLM) based diet. J. Biodiv. environ. Sci., 9: 57-68.
Shahzad, M.M., Hussain, S.M., Javid, A. and Hussain, M., 2018. Role of phytase supplementation in improving growth parameters and mineral digestibility of Catla catla fingerlings fed moringa by-products based test diet. Turkish J. Fish. aquat. Sci., 18: 557-566.
Shahzad, M.M., Hussain, S.M., Jabeen, F., Hussain, A.I., Ahmad, S., Ashraf, A. and Arsalan, M.Z.H., 2017. Effect of phytase supplementation on mineral digestibility to Catla catla fingerlings fed Moringa oleifera leaf meal based test diets. Punjab Univ. J. Zool. 32: 065-073.
Singh, M. and Krikorian, A.D., 1982. Inhibition of trypsin activity in vitro by phytate. J. Agric. Fd. Chem., 30: 799–800. https://doi.org/10.1021/jf00112a049
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods. 8th Ed. Iowa State University. Press, Ames. USA, p. 503.
Soliva, C.R., Kreuzer, M., Foidl, N., Foidl, G., Machmüller, A. and Hess, H.D., 2005. Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim. Feed Sci. Technol., 118: 47-62. https://doi.org/10.1016/j.anifeedsci.2004.10.005
Spinelli, J., Houle, C.R. and Wekell, J.C., 1983. The effects of phytates on the growth of rainbow trout (Salmo gairdneri) fed purified diets containing varying quantities of calcium and magnesium. Aquaculture, 30: 71–83. https://doi.org/10.1016/0044-8486(83)90153-9
Steel, R.G.D., Torrie, J.H. and Dinkkey, D.A., 1996. Principles and procedures of statistics. 2nd Ed., McGraw Hill Book Co., Singapore.
Vielma, J., Makinen, T., Ekholm, P. and Koskela, J., 2000. Checked the Influence of dietary soy and phytase levels on performance and body composition of large rainbow trout _Oncorhynchus mykiss and algal availability of phosphorus load. Aquaculture, 183: 349–362. https://doi.org/10.1016/S0044-8486(99)00299-9
Wang, F., Yang, Y.H., Han, Z.Z., Dong, H.W., Yang, C.H. and Zou, Z.Y., 2009. Effects of phytase pretreatment of soybean meal and phytase-sprayed in diets on growth, apparent digestibility coefficient and nutrient excretion of rainbow trout (Oncorhynchus mykiss Walbaum). Aquacul. Int., 17: 143-157. https://doi.org/10.1007/s10499-008-9187-5
Worku, A., 2016. Moringa oleifera as a potential feed for livestock and aquaculture industry. Afri. J. agric. Sci. Tech., 4: 666-676.
Yan, W. and Reigh, R.C., 2002. Effects of fungal phytase on utilization of dietary protein and minerals, and dephosphorylation of phytic acid in the alimentary tract of channel catfish Ictalurus punctatus fed an all-plant protein diet. J. World aquacul. Soc., 33: 10–22. https://doi.org/10.1111/j.1749-7345.2002.tb00473.x
Yoo, G.Y., Wang, X., Choi, S., Han, K., Kang, J.C. and Bai, S.C., 2005. Dietary microbial phytase increased the phosphorus digestibility in juviniles Korean Rockfish Sebastes schlegeli fed diets containing soybean meal. Aquaculture, 243: 315-322. https://doi.org/10.1016/j.aquaculture.2004.10.025
Yu, F.N. and Wang, D.Z., 2000. The effects of supplemental phytase on growth and the utilization of phosphorus by crucian carp Carassius carassius. J. Fish. Sci. China, 7: 106–109.
To share on other social networks, click on any share button. What are these?










