In Vitro Antagonistic Activity for Selected Fungal Species Against Wilt Causing Phytopathogens
Research Article
In Vitro Antagonistic Activity for Selected Fungal Species Against Wilt Causing Phytopathogens
Sana Irshad Khan1*, Zafar Iqbal2, Hamid Ullah Shah2 and Shaukat Hussain3
1Soil and Water Testing Laboratory Charsadda, Directorate of Soil Plant Nutrition, ARI Tarnab, Peshawar Pakistan; 2Department of Agricultural Chemistry,Faculty of Nutrition Sciences, The University of Agriculture, Peshawar, Pakistan; 3Department of Plant Pathology, Faculty of Crop Protection, The University of Agriculture, Peshawar, Pakistan.
Abstract | The present study was carried out with the aim to assist screening of various fungal species for antimicrobial natural products. During this study five different fungi, including Alternaria alternata, Acremonium sp., Alternaria brassicicola, Pythium sp. and Aspergillus flavus were screened against Wilt causing pathogens using the dual culture assay. Among them, Aspergillus flavus was found active fungus against the selected vascular Wilt causing fungus and bacteria, i.e. Fusarium oxysporum (87.50 ± 0.11% inhibition), Xanthomonas campestris (67.00 ± 0.46% inhibition) and Clavibacter michiganensis (72.00 ± 1.02% inhibition). The A. flavus was grown on two different media, i.e. Potato Dextrose Broth (PDB) and Glucose Nutrient Broth (GNB) media for active secondary metabolites. Ethyl acetate (EtOAc) extract of PDB medium gave significant results against selected phytopathogens compared to GNB medium and selected for further study. Crude (1 µg.mL-1 ) obtained from PDB medium showed 2.50 ± 0.64 mm and 1.97 ± 0.21 mm inhibition zone against X. campestris and C. michiganensis respectively compared to positive reference (streptomycin) and negative refrence (EtOAc) using disc diffusion method. However, the same concentration of crude (1 µg.mL-1) showed 62.20 ± 2.51% growth inhibitions against Fusarium oxysporum using agar well diffusion method. Further investigation, including various chromatographic and spectroscopic analyses on the separation and isolation of potent metabolites is suggested to get a useful lead structure for development of novel antibiotics to combat resistant pathogens.
Received | Januray 04, 2017; Accepted | February 09, 2017; Published | March 10, 2017
*Correspondence | Sana Irshad Khan, Soil and Water Testing Laboratory Charsadda, Directorate of Soil Plant Nutrition, ARI Tarnab, Peshawar Pakistan; E mail: [email protected]
Citation | Khan S.I., Z. Iqbal H. Ullah Shah and S. Hussain. 2017. In Vitro Antagonistic Activity of Selected Fungal Species Against Wilt Causing Phytopathogens. Sarhad Journal of Agriculture. 33(1): 144-150.
DOI | http://dx.doi.org/10.17582/journal.sja/2017.33.1.144.150
Keywords | Phytopathogens, A. flavus, Antimicrobial assays, Ethyl acetate, Culture media
Introduction
A huge range of microorganisms has been known to cause plant diseases that result in significant food losses (Prieto et al., 2013). Among these phytopathogens, fungi and bacteria have been considered more potent in causing diseases (Sabat et al., 2009). Due to these diseases losses in productivity, cost of disease management and more interest of economic penalty in the growing of less profitable crops are the key factors that affect agriculture budget severally (Chakraborty et al., 2000). Each year 12-15% losses occur due to phytopathogens (Sabat et al., 2009).
Presently agriculture practices focus on the control of these diseases and various management strategies have been developed (Prieto et al., 2013; Kalyebara et al., 2006). However, these control measures have been proven ineffective and/or less effective due to one or other limitation. For example, in the absence of the dominant gene, resistant variety development is difficult as it takes more time to show results and sometime host resistant might be overcome by the occurrence of new races of pathogens (Shanmugam et al., 2011; Cachinero et al., 2002). Similarly, Lack of consistency and low efficacy under commercial conditions are limiting factor of biological control (Tripathi et al., 2004). Today chemical control of phytopathogens has been considered comparatively more profitable. Moreoever, in addition to its benefits, utilization of these pesticides may also have various side effects like drug resistant and its effects on non-target organisms in the environment (Sharma et al., 2009). Therefore, the need for the development of alternative control method, which are easily available, less expensive, comparatively more effective and having no and/or less adverse effects have been felt (Minz et al., 2012)
Natural product research is enjoying renewed attention with the outstanding developments in the areas of spectroscopic techniques, separation science, and microplate-based in vitro assays, for providing novel and interesting chemical scaffolds (Sarker et al., 2005). At present moment the presence and usage of about 23,000 biologically potent metabolites have been recorded in the literature (Sinha et al., 2014). Reports showed that since 1981 to 2005, more than 40% natural products have been obtained from microorganism (Qadri et al., 2013). Among them the usage of fungi and bacteria for human significant bioactive secondary metabolites production is more prominent (Moustafa, 2011).
For novel secondary metabolites, scientists mostly focus on fungi as small cell factory, since the discovery of penicillin in 1928 (Konakovsky et al., 2012). To keep this crucial journey continue, Harold Raistrick in 1949 started work on the metabolites of fungi and made many useful contributions. A billion dollar anticancer drug Taxol was for the first time discovered from an endophytic fungus; Taxomyces andreanae, (Moustafa, 2011). Likewise, many other important antibiotics and drugs like Vanomycin and griseofulvin are obtained from Nocardia orientalis and Penicillium griseofulvum, respectively. Similarly, many other human significant compounds (antioxidant, anticancer, antimicrobial and antiviral, etc.) have been extracted from fungi in the past. The spectroscopic analysis of these compounds showed that they belong to different groups of secondary metabolites including flavonoids, steroids, quinines, terpenoids and phenol etc. (Yu et al., 2010). This shows that fungi have outstanding potential in drug discovery of clinical, industrial and agriculture importance (Qadri et al., 2013).
Keeping in consideration hazards caused by different phytopathogens in crops and to find a possible solution in order to prevent attack of these diseases causing agents in order to improve quality and quantity of our food, this study is designed to screen various fungal species including Alternaria alternata, Acremonium sp. and Pythium sp., Alternaria brasiscicola and Aspergillus flavus using dual culture assays and to select culture media for more active secondary metabolites production of potent fungus against wilt causing including Fusarium oxysporum, Clavebactor michiganensis and Xanthomonas campestris.
Materials and Method
This experimental work was carried out in the laboratory of Agriculture Chemistry, The University of Agriculture Peshawar during February to August, 2015.
Microbial specimen collection
Specimens of test fungi including A. alternata, Acremonium sp. and Pythium sp. were collected from soil samples belonged to different Districts (including Charsadda, Peshawar and Bannu) of KPK while A. brassicicola and A. flavus were obtained from The Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture Peshawar, Pakistan. The vascular Wilt causing bacteria i.e. Clavibactor michiganensis and Xanthomonas campestris and fungus i.e. Fusarium oxysporum were obtained from The Department of Plant Pathology, The University of Agriculture Peshawar, Pakistan. Selected microbes were cultured on Potato Dextrose Agar (PDA) media.
Dual Culture Assays
In vitro antagonistic activity of the test fungi against F. oxysporum: For identification of potent fungi against the selected Wilt disease causing phytopathogens, sterilized PDA media plates were inoculated with 5mm plug taken from 15 days old culture of F. oxysporum. After 3 days of incubation at 28 °C, the same plate was inoculated with 5 mm plug of the selected test fungi. The fungal plug was placed 3 mm away from the previous pathogenic fungal plug and was incubated at 28°C for 12 days again. Control plate for this activity contained only F. oxysporum (Sonawane et al., 2015; Siameto et al., 2010). The antagonistic efficacy of selected test fungus was observed each day and percent growth of the phytopathogenic fungus i.e. Fusarium oxysporum was calculated as follows:
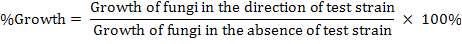
In vitro antagonistic activity of the test fungi against phytopathogenic bacteria: Dual culture assay of the selected test fungal species was also carried out against Xanthomonas campestris and Clavibactor michiganensis. Selected bacteria were streaked on PDA (20 mL) plate and incubated for 48 hrs at 35°C. After complete growth of each type of bacteria on PDA, a 5 mm plug of test fungi was inserted in the center. These cultural plates were again incubated at 28°C. After 5 days of incubation, the antagonistic activity of test fungi was observed.
Preparation of Fungal Broth Culture: In order to find out culture medium whose secondary metabolites possessing comparatively more inhibition against the selected Wilt causing phytopathogens, A. flavus was cultured on potato dextrose broth (PDB) and Glucose nutrient broth (GNB) media in conical flasks (1 L). After incubation for 10 days at 28°C, fungal mycelia were separated from broth media under vacume. The fungal mycelia were extracted with Ethyl acetate (v/v) and the solvent was dried using rotary evaporator at 40°C (Joel et al., 2013; Sanchez et al., 2013).
Antifungal Assay:For antifungal assay well diffusion method was used (Sonawane et al., 2015). Crude extract (1 ug.mL-1) of each selected medium was poured in the 5 mm well pre-made in the center of PDA plate. Positive (mencozeb) and negative references (EtAOc) were also run in parallel. The cultures were inoculated with 5 mm plug of the Fusarium oxysporum and incubated at 28°C for 7 days. Growth inhibition (%) was measured as:
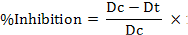
Dc stands for total inhibition in Blank while Dt for total inhibition in test solution.
Antibacterial Assay: Disc diffusion method was used for the determination of antibacterial activity of the extracts according to the protocol of the National Committee for Clinical Laboratory Standard (NCCLS, 2002). The bacterial strains, i.e. Xanthomonas campestris and Clavibactor michiganensis were streaked on sterilized PDA plates and sterile discs (5mm) saturated with 10 µL of fungal extract (1 ug.mL-1) were placed at the center of the plates under aseptic conditions. Streptomycin solution (1 ug.mL-1) was used as positive while EtOAc as negative reference. After incubation at 35°C for 48 hrs, mean zone of bacterial inhibition was measured in mm.
Statistical Analysis
Experiments were repeated three times along with blank and standard. In the results data values were represented as mean with standard deviation (mean ± SD).
Results and Discussion
In the present work antimicrobial potential of A. flavus, A. alternata, A. brassicicola, Acremonium sp. and Pythium sp. were screened against the selected Wilt causing phytopathogenic bacteria and fungi. Furthermore, we were also interested to determine a culture medium on which test fungal showed better inhibition against the selected pathogenic microbes.
Dual Culture Assays
Among all tested fungi, Aspergillus flavus was found most potent to inhibit the growth of Wilt causing phytopathogenic bacteria, i.e. Clavibactor michiganensis and Xanthomonas campestris and the fungus i.e. Fusarium oxysporum.
During dual culture incubation period, A. flavus showed no growth inhibition of the pre-cultured (3 days ago than A. flavus) pathogen in initial two days. Inhibition in the growth of Fusarium sp. (pathogen) was noticed at day 3 that followed upto day 12. On day 12 of incubation period, Fusarium oxysporum showed 12.50 ± 0.34% growth in the direction of the test fungi (i.e. Aspergillus flavus), where in the control plate, Fusarium oxysporum showed full growth (100%).
A. flavus was also found potent against selected phytopathogenic bacteria when tested through dual culture assay and showed more than 50% antagonistic activity. Initially upto two days, A. flavus showed no growth inhibition toward pre-cultured selected bacteria (48 hrs), just like its response to F. oxysporum. However, on day 5 it showed 67 ± 0.46% and 72 ± 1.02% inhibition against Xanthomonas campestris and Clavibactor michiganensis, respectively (Table 1).
Table 1: In vitro antagonistic activity of A. flavus against selected vascular Wilt causing phytopathogens (mean ± S.D)
| S.No | Pathogens | Inhibition (%) |
| 1. | Fusarium oxysporum | 87.50 ± 0.11 |
| 2. | Xanthamonas campestris | 67.00 ± 0.46 |
| 3. | Clavebactor michiganensis | 72.00 ± 1.02 |
The genus Aspergillus is widely known for its production of secondary metabolites having useful activities for application in medicine and agriculture (Frisvad et al., 2008). Durairaj et al. (2015) reported the biosynthesis of Titanium dioxide nanopaarticles from Aspergillus niger which were proven more potent antimicrobial compared to synthetic ones showing 65 mm and 21 mm zone of inhibition against T. reesei and Pseudomonas sp. respectively. Some species of this genus including A. flavus were also reported to produce some toxic metabolites (such as aflatoxin, aspertoxin, aspergillic acid, sterigamatocystin and β-nitroproponic acid, etc.) in human food and animal feed. However, today some non-toxic strains of A. flavus have also been known that produced some beneficial compounds (Hedayati et al., 2007).
From dual culture assay it was indicated that A. flavus grew faster than Fusarium oxysporum, rolled around this pathogenic fungus in a parasitic manner and inhibited its growth up to 87.5 ± 0.11%. Moreover an inhibitory boundary was observed between A. flavus and Fusarium oxysporum. Regarding Fusarium oxysporum the present results were found similar to the results of Siameto et al. (2010) using Trichoderma harzianum as test fungus. Siameto et al. (2010) performed dual culture assay of the various isolates of Trichoderma harzianum against different plant pathogenic fungi including Fusarium oxysporum. All isolates of T. harzianum inhibited Fusarium oxysporum growth in a parasitic manner and sporulated its pathogen colony. Pereira et al. (2013) also analyzed the antagonistic effect of Hypholoma fasciculare (Huds.) P. Kumm against yeast to determine the antimicrobial potential of filamentous fungi. They found that at the start of incubation period Hypholoma fasciculare (Huds.) P. Kumm showed slow growth towards S. cerevisiae, however, with the passage of time increase in the growth of filamentous fungus along with anti-yeast zone (up to1.3 fold more) was recorded.
Although the dual culture assay requires more time to determine antagonism because of slow fungal growth. However, for testing inhibition potential against other pathogenic microorganisms, it is a common practice as it provides a quick and more consistent approach for testing antimicrobial potential compared to crude extracts (Pereira et al., 2013). Intially A. flavus showed no growth inhibition against pre-cultured pathogens. With the passage of time as it grown, gradually inhibition toward selected phytopathogenic fungus and bacteria occurred which was maximum at day 12 for F. oxysporum and at day 5 for selected pathogenic bateria. This increase in growth inhibition of A. flavus against the plant pathogenic bacteria might be due to the biosynthesis of secondary metabolites. These secondary metabolites might be diffused into medium and inhibited the growth of selected pathogenic microbes. On the basis of growth inhibitory activity, the A. flavus was selected for further bioassays.
Antifungal Assay: Mean zone of growth inhibition (%) showed by selected fungal (A. flavus) crude extract in PDB medium and GNB medium against Fusarium oxysporum were 62.20 ± 2.51% and 53.79 ± 3.36%, respectively as given in Table 2. The same concentration of standard antifungal solution (Mencozeb) inhibited the growth up to 70 ± 1.44% and pure ethyl acetate caused 0.00 ± 0.00% inhibition in growth of F. oxysporum.
Table 2: Growth inhibition (%) potential of A. flavus crude extracted from two different medium against Fusarium oxysporum using well diffusion method (mean ± S.D)
| % Inhibition of Fusarium Oxysporum (mean ± S.D) | ||||
|
Treatment 1 ug.mL-1 |
||||
| S.No | Media | Crude | Standard | Control |
| 1. | PDB | 62.20 ± 2.51 | 70.00 ± 1.44 | 0.00 ± 0.00 |
| 2. | GNB | 53.79 ± 3.36 | 70.00 ± 1.44 | 0.00 ± 0.00 |
Antibacterial Assay:Against Xanthomonas campestris, PDB medium crude extract (1 µg.mL-1) showed 2.50 ± 0.64 mm zone of inhibition in 48 hrs of incubation at 35°C, even higher than the standard antibacterial agent (Streptomycin) that caused 2.42 ± 1.00 mm mean zone of inhibition in the growth while using pure EtOAc, the recorded mean inhibition zone of
Table 3: Mean zone of growth inhibition (mm) of A. flavus EtOAc crude extracts obtained from two different media against selected phytopathogenic bacteria compared to control (EtOAc) and standard (Streptomycin) solutions using disc diffusion method (mean ± S.D)
| Zone of growth inhibition in mm (mean ± S.D) | ||||||||
| X.campestris | C.michiganensis | |||||||
|
Treatment 1 µg.mL-1 |
||||||||
| Media | Crude | Standard | Control | Crude | Standard | Control | ||
| PDB | 2.50 ± 0.64 | 2.44 ± 1.27 | 0.00 ± 0.00 | 1.97 ± 0.21 | 2.37 ± 1.32 | 0.00 ± 0.00 | ||
| GNB | 2.12 ± 1.01 | 2.44 ± 1.36 | 0.00 ± 0.00 | 1.75 ± 0.83 | 2.23 ± 1.71 | 0.00 ± 0.00 | ||
this bacterium was 0.00 ± 0.00 mm. However, GNB medium crude showed 2.12 ± 1.01 mm mean zone of inhibition against X. campestris as compared to control and standard, which showed 0.00 ± 0.00 mm and 2.44 ± 1.00 mm mean inhibition zone respectively against X. campestris.
In case of C. michiganensis, at 35°C the crude extract of Aspergillus flavus obtained from PDB and GNB media showed 1.97 ± 0.21 mm and 1.75 ± 0.83 mm mean zone of inhibition respectively in 48 hrs of incubation period. While the standard antibacterial solution (Streptomycin) caused 2.37 ± 1.32 mm and 2.23 ± 1.71 mm mean zone of inhibition against this bacterium and pure EtOAc 0.00 ± 0.00 mm (Table 3).
It was obvious from Tables 2 and 3 that the crude extract obtained from PDB fermented mycelia exhibited greater inhibition potential against both types of selected vascular Wilt causing phytopathogens (bacteria and fungi) than GNB. Mean inhibition zones of 2.50 ± 0.64 mm and 1.97 ± 0.21 mm were recorded for X. campestris and C. michiganensis respectively, and 62.2 ± 2.51% inhibition for F. oxysporum. While GNB medium showed mean inhibition zone of 2.12 ± 1.01 mm and 1.75 ± 0.83 mm for X. campestris and C. michiganensis respectively, and 53.79 ± 3.36% growth inhibition for F. oxysporum at given incubation condition. Literature, presented many such reports, where potato dextrose broth medium proved better for bioactive secondary metabolites comparative to other media. Pereira et al. (2013) studied two different media including Potato Dexrose Agar (PDA) and modified Melin-Norkrans (MMN) along with temperature and inoculum size to determine suitable conditions for antagonistic effects of filamentous fungus i.e. H. fasciculare against yeast. They reported that H. fasciculare has been possessed antagonistic activity at all tested temperature (20°C, 25°C, 30°C, 35°C) except 40°C with small inoculum size when fermented on both types of selected media. Depending on the size of the growth diameter of fungi and inhibition halo (1.4 fold higher for PDA) PDA media was selected as better medium for assay. Pradeep et al. (2013) also studied the effects of 14 different culture media (eight solid and six liquid media) including OMA (Oat Meal Agar), RBA (Rose Bengel Agar), NA (Nutrient Agar), MB (Malt extract Agar), YMA (Yeast Malt Agar), PDA (Potato Dextrose Agar), CDA (Czapex’s Dox Agar), PGB (Peptone Glycerol Broth), SDB (Sabouraud Dextrose Broth), PDB (Potato Dextrose Broth), NB (Nutrient Broth), MB (Malt extract Broth), YMB (Yeast extract Malt extract Broth) on secondary metabolites production of Fusarium moniliforme KUMBF1201 isolated from paddy field soil at 28°C and pH of 5.5. Out of all tested solid media, PDA (mean max. growth of 74.96±0.35 mm) followed by MA and OMA (mean max. growth of 74.03±0.25 and 74.03±0.15 mm, respectively) were recorded best for pigment production possessing industrial importance from Fusarium moniliforme KUMBF1201. Similarly among liquid, media Potato Dextrose Broth (PDB) proved better with 52.3 g/L mycellial growth for production of pigments when analyzed through double beam spectrophotometer at 500 nm.
Secondary metabolites production is the result of interference between genotype of microbes and environmental factors (temperature light, pH, etc). Different culture media provide different nutrients which interact differently with environmental factors and so control the growth and metabolism of microbes in different ways. As a result metabolites of different types have been synthesized which showed different responses for the same organism and also for other organisms present in the environment (Salvador et al., 2003). This indicated that the response of microbial secondary metabolites depended on media.
In the present work considering the difference between inhibition potential of both media crude extracts against selected phytopathogens, PDB fermented A. flavus found was more active (8.4% more for F. oxysporum and 0.38 mm and 0.22 mm more for X. campestris and C. michiganensis respectively) than other. Based on its comparatively better antimicrobial activity, PDB (Potato Dextrose Broth) medium was suggested for further fermentation of A. flavus in this work. Moreover, in this medium the selected test fungus produced more secondary metabolites than GNB (Glucose Nutrient Broth) medium.
Conclusion
From the present work it was concluded that A. flavus produced more active metabolites against F. oxysporum, X. campestris and C.mechiganensis when fermented on PDB media compared to GNB. Further work on the identification, isolation and structural elucidation of responsible antimicrobial compounds using advance spectroscopic techniques including HR-MS and NMR is suggested, that would help in the development of safer and potent antimicrobial compounds of agriculture and pharmaceutical importance.
Acknowledgements
The authors wish to extend their appreciation to the Higher Education Commission of Pakistan (Project No. (20.2059/R and D/11/282) and Pakistan Science Foundation (Project No. PSF/NSLP/KP-AU-421) for providing financial grant to complete this research.
Authors Contribution
Sana Irshad Khan performed overall research activities. She also wrote the manuscript. Dr. Zafar Iqbal thoroughly organized whole research work as project supervisor. He also developed research methodology for column chromatography and HPLC and proof read the manuscript. Prof. Dr. Hamid Ullah Shah was a member of supervisory committee and helped in the proof reading of this article. Prof. Dr. Shaukat Hussain was also a member of supervisory committee and helped in the identification of screened fungal species. He further helped in the cultivation of fungal species and provision of pathogenic fungus i.e. Fusarium oxysporum.
References
Cachinero, J.M., A. Hervas, R.M. Jimenez-Díaz and M. Tena. 2002. Plant defence reactions against fusarium wilt in chickpea induced by incompatible race 0 of Fusarium oxysporum f. sp. Ciceris and nonhost isolates of F. oxysporum. Plant Pathol. 51:765–776. https://doi.org/10.1046/j.1365-3059.2002.00760.x
Chakraborty, S., A.V. Tiedemann and P.S. Teng. 2000. Climate change: potential impact on plant diseases. Environ. Pol. 108: 317-326.
Durairaj, B., S. Muthu and T. Xavier. 2015. Antimicrobial activity of Aspergillus niger synthesized titanium dioxide nanoparticles. Adv. App. Sci. Res. 6(1): 45-48.
Frisvad, J.C., B. Andersen and U. Thrane. 2008. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol. Res. 112: 231–240. https://doi.org/10.1016/j.mycres.2007.08.018
Hedayati, M.T., A.C. Pasqualorro, P.A. Warn, P. Bowyer and D.W. Denning. 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. J. Microbiol. 153(6): 1677-1692. https://doi.org/10.1099/mic.0.2007/007641-0
Joel, E.L. and V. Bhimba. 2013. A secondary metabolite with antibacterial activity produced by mangrove foliar fungus Schizophyllum commune. Int. J. Chem. Environ. Biol. Sci. 1(1): 2320 -4087.
Kalyebara, M.R., P.E. Ragama, G.H. Kagezi, J. Kubiriba, F. Bagamba, K.C. Nankinga and W. Tushemerirwe. 2006. Economic importance of the banana bacterial Wilt in Uganda. Afr. Crop Sci. J. 14(2): 93-103.
Konakovsky, V. 2012. Optimization of fungal fermentation for the preparation of bioactive metabolites. Master thesis. Boku - University of natural resources and life sciences, Vienna. 1-18.
Minz, S., C.O. Samuel, and S.C. Tripathi. 2012. The effect of plant extracts on the growth of Wilt causing fungi Fusarium oxysporum. IOSR-JPBS. 4(1): 13-16. https://doi.org/10.9790/3008-0411316
Moustafa, M.F.E. 2011. Novel and bioactive natural products from the marine-derived endophyticfungi Coniothyrium cereale, Phaeosphaeria spartinae and Auxarthron reticulatum.
National Committee for Clinical Laboratory Standard (NCCLS). 2002. Performance Standards for antimicrobial disk susceptibility tests: Approval Standard M2-A7 7th edition, Pennsylvania; Clinical and laboratory Standard Institute.
Pereira, E., A. Santos, F. Reis, R.M. Tavares, P. Baptista, T. Lino-Neto and C.A. Aguiar. 2013. A new effective assay to detect antimicrobial activity of filamentous fungi. Microbiol. Res. 168: 1–5. https://doi.org/10.1016/j.micres.2012.06.008
Pradeep, F.S., M.S. Begam, M. Palaniswamy and B.V. Pradeep. 2013. Influence of culture media on growth and pigment production by Fusarium moniliforme KUMBF1201 isolated from paddy field soil. W. Appl. Sci. J. 22 (1): 70-77.
Prieto, J.A., J.O. Patino, E.A. Plazas, L.C. Pabon, M.C. Avila, J.D. Guzman, W.A. Delgado and L.E. Cuca. 2013. Natural Products from Plants as Potential Source Agents for Controlling Fusarium. Chapter 10. 233-278. (http:// creativecommons. org/ licenses/by/3.0)
Qadri, M., S. Johri, B.A. Shah, A. Khajuria, T. Sidiq, S.K. Lattoo, M.Z. Abdin and S.R.U. Hassan. 2013. Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. Springer Plus. 2: 1-8. https://doi.org/10.1186/2193-1801-2-8
Sabat, J. and N. Gupta. 2009. Development of modified medium for the enhancement in antifungal activity of P. steckii (MF1 Mangrove Fungi) against Verticillium Wilt pathogenic fungi of rose. Braz. Arch. Biol. Technol. 52 (4): 809-818. https://doi.org/10.1590/S1516-89132009000400003
Salvador, M.J., P.S. Pereira, S.C. França, R.C. Candido, I.Y. Ito and D.A. Dias. 2003. Comparative study of antibacterial and antifugal activity of callus culture and adult plants extracts from Alternanthera maritima (Amaranthaceae). Braz. j. Microbiol. 34: 131-136. ISSN 1517-8382-131.
Sanchez, J.F. and C.C.C. Wang. 2013. The chemical identification and analysis of Aspergillus nidulans secondary metabolites. Meth. Mol. Biol. (Clifton, N.J.), 944:97-109. http://dx.doi.org/10.1007/978-1-62703-122-6_6
Sarker, S.D., Z. Latif and A.I. Gray. 2005. Natural Product Isolation. Method. Biotechnol. 20: 1-25.
Shanmugam, V. and N. Kanoujiaa. 2011. Biological management of vascular Wilt of tomato caused by Fusarium oxysporum f. sp. lycospersici by plant growth-promoting Rhizobacterial mixture. Biol. Control. 57(2): 85–93. https://doi.org/10.1016/j.biocontrol.2011.02.001
Sharma, B., P. Kumar. 2009. In vitro antifungal potency of some plant extracts against Fusarium oxysporum. Int. J. Green Pharm. 3(1): 63-65. https://doi.org/10.4103/0973-8258.49377
Siameto, E.N., S. Okoth, N.O. Amugune and N.C. Chege. 2010. Antagonism of Trichoderma farzianum isolates on soil borne plant pathogenic fungi from Embu District, Kenya. J. Yeast Fungal Res. 1(3): 47-54.
Sinha, K., R. Hegde and A. Kush. 2014. Exploration on native actinomycetes strains and their potential against fungal plant pathogens. Int. J. Curr. Microbiol. App. Sci. 3(11): 37-45.
Sonawane, A., M. Mahajan and S. Renake.2015. Antifungal activity of a fungal isolates against pomegranate Wilt pathogen Fusarium. Int. J. Curr. Microbiol. App. Sci. (2): 48-57.
Tripathi, P. and N.K. Dubey. 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Post Harv. Bio. Technol. 32: 235–245. https://doi.org/10.1016/j.postharvbio.2003.11.005
Yu, H., L. Zhang, L. Li, C. Zheng, L. Guo, W. Li, P. Sun and L. Qin. 2010. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. J. Microbiol. Res. 165: 437-449. https://doi.org/10.1016/j.micres.2009.11.009
To share on other social networks, click on any share button. What are these?








