Induced Breeding, Embryonic and Larval Development of Koi Carp in Recirculatory Aquaculture System
Induced Breeding, Embryonic and Larval Development of Koi Carp in Recirculatory Aquaculture System
Kalaiselvi Natarajan1, Karal Marx Karuppiah1, Sheena K Baby1,
Sakthivel Mohammed2, Shanmugam Seerappalli Aran1 and Suresh Eswaran1*
1Institute of Fisheries Postgraduate Studies, Tamil Nadu Dr. J. Jayalalithaa Fisheries University, OMR Campus, Chennai-603 103, India
2Mandapam Regional Centre of Central Marine Fisheries Research Institute, Mandapam, Tamil Nadu-623 520, India
ABSTRACT
Koi carp (Cyprinus carpio) is a colourful and economically valuable ornamental fish species, which has numerous strains. Even though culture of Koi carp is practiced at many tropical countries, there is a very little information on the seed production and embryonic development of the species especially in RAS. The present study was aimed to study the breeding, embryonic and larval development of Koi carp in RAS system. The matured brooders were administered with three synthetic hormone ovaprim, ovatide and WOVA -FH at 0.7 ml per kg of body weight. Comparatively WOVA-FH at 0.7 ml per kg showed better performance with higher number of ovulated eggs, fertilization and hatching rate than ovaprim and ovatide. For dose optimization, the matured brooders were administered with synthetic hormone WOVA -FH at three different doses viz., 0.3, 0.5 and 0.7 and 1.0 ml per kg of body weight at triplicate basis. The highest number of ovulated eggs (3380.5 ±202.75), fertilization rate (90± 0.8) and hatching rate (88±0.88) were observed in the brooders that were induced with 0.5ml per kg of WOVA -FH. The average diameter of the fertilized eggs ranged between 0.9 and 1.10 mm while the yolk sphere ranged between 0.6 and 0.8 mm. The larvae hatched out after 72 h of fertilization. The total length of the larvae ranged from 2.7 to 2.9 mm. This study aims to analyze the application of RAS technology for year-round seed production of Koi carp. Further this study aims in developing sustainable aquaculture practices to facilitate aquaculturists.
Article Information
Received 29 August 2022
Revised 15 September 2022
Accepted 08 October 2022
Available online 14 January 2023
(early access)
Published 25 March 2024
Authors’ Contribution
NK carried out the research work and drafted the manuscript. KKM narrated the work and executed the experiment. SKB reviewed the manuscript and checked the grammatical mistakes. MS analysed the data. SAS gave broad ideas in field sampling. ES reviewed the manuscript, condensed the matter and shared idea of analysis and interpretation part.
Key words
Koi carp, Induced breeding, Embryonic development, RAS
DOI: https://dx.doi.org/10.17582/journal.pjz/20220829060843
* Corresponding author: sura12@gmail.com
0030-9923/2024/0003-1059 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Ornamental fishes fetch a higher price than the food fish in international markets because of their aesthetic value and high commercial value in the export trade. Koi carp, Cyprinus carpio, is a well-known decorative fish. It is a variety of the common carp (Cyprinus carpio) species, a native of South East Asia. Koi carp produced from China and Japan fetches high market value for its excellent colour pattern. New varieties of colours and colour patterns in Koi carp have been developed over recent years. The common colours are white, cream, red, blue, yellow and black. Koi carps generally live up to 15-24 years. Being hardy, it is highly suitable for garden pools and aquarium keeping (Hussian et al., 2014).
Aquaculture faces many obstacles related to water quality and waste management. The waste can cause environmental pollution if it is not treated before disposal. Therefore, the development of aquaculture innovation is fundamentally essential to develop into sustainable aquaculture practices. One of the more intriguing strategies for intensifying production while simultaneously reducing wastes is the development of recirculating aquaculture systems (RASs). These systems are designed to collect and remove waste products, uneaten feed, and bacteria from the tank where the fish live so that water can be recycled back into the system. Even though many ornamental fish and invertebrates have been induced to spawn in captivity, the larvae of only a few species have been successfully reared in captivity (Holt, 2003; Olivotto et al., 2003). The bottleneck is the heavy mortality encountered in the larvae during the critical period of development, mostly noticed when the larvae change over from internal yolk storage to exogenous feed (Madhu et al., 2012). Breeding is a complex process which depends on several factors. In addition to the other environmental and biological factors, the condition of brooders is the major factor determining the success.
Selecting the appropriate inducing agents will enhance the overall reproductive success. Based on the literature, for many fish species synthetic hormone, Ovaprim (M/s Syndel Laboratories, Canada) has been successfully used since 1990. After 1997, Ovatide (M/s Hemmo Pharma, Mumbai), an indigenous synthetic hormone which is more affordable than Ovaprim was used. Nowadays, WOVA-FH (M/s.Biostadt India Limited, Mumbai) is used as an inducing agent for many ornamental fishes. Hence, the present study was conducted using three commercially available GnRH synthetic hormone viz., WOVA-FH, Ovaprim and Ovatide. Literature on the seed production and embryonic development of Koi carp is very scanty. Considering these facts, the present study was undertaken on broodstock development, induced breeding and larval development of Koi carp using RAS for sustainable aquaculture practices.
MATERIALS AND METHODS
Collection and conditioning of broodfish
Experiments were conducted at Aquatic Rainbow Technology Park, Madhavaram, Chennai, India. The brooders (Female: 250±50 g; Male: 200±50 g) were collected from commercial ornamental fish market in Kolathur, Chennai and were transported in oxygenated polythene bags.
Brood stock maintenance
The broodfishes were segregated into males of 2+ years and females of 4+ years. They were reared separately in 2 cemented grow out raceway tanks (20-ton capacity) with RAS facility. The brooders were fed with commercial GROWFIN supplementary feed (1.8 mm) containing 38% protein, at 5% of body weight, twice a day. Along with the supplementary diet, brooders were also fed with earthworm and Tubifex tubifex (sludge worm) to enhance maturity under captive condition for 4 months. Then the brooders were separated based on the secondary sexual characteristics (Table I). Matured males were selected by observing freely oozing milt (Fig. 1a). Matured females were observed with bulged belly region, slightly swollen genital aperture, and oozing ova upon gentle pressure on the abdomen (Fig. 1b).
Preparation of spawning tank
For induced breeding of fish, FRP (fibre-reinforced plastic) circular tanks of 1-ton capacity with RAS facility were filled (1ft of height) with water. The tanks surface was laid with artificial polyethylene strips (breeding mops) and nylon fibres. Nylon fibres act as a substrate to attach the eggs because Koi carp eggs are adhesive in nature.
Optimization of dosage for induced breeding
In the present study induced breeding trials were carried out using three different inducing agents to optimize the hormone on breeding performance. Ovatide, ovaprim and WOVA-FH were used as an inducing agent at 0.7 ml per kg of body weight (Table II). The synthetic hormone which showing better performance were administrated at 4 different doses viz., 0.3, 0.5, 0.7 ml, 1 ml per kg body weight intramuscularly for dose optimization. One control unit was also set up. Both males and females were induced by injecting single intramuscular injection, between the base of the dorsal fin and lateral line, in the evening (1730 h). After injecting the hormone, brooders were released at 2:1 (M: F) sex ratio into spawning tanks supplied with continuous aeration. The occurrence of spawning was monitored periodically by checking for the presence of eggs in the breeding hapa. Egg collection was done on the next day morning at 0530 h (Fig. 1e).
Table I. Sex identification characteristics in Koi carp.
|
Character |
Male |
Female |
|
Size |
Smaller |
Larger |
|
Body colour |
Bright |
Slightly dull |
|
Body shape |
Slimmer |
Broader |
|
Pectoral fin |
Large, rough |
Pointed, soft |
|
Secondary sexual characteristics |
Pink coloured vent Tubercles appears on the face Oozes milt |
Vent will be rounder and pinker than male Tubercles absent Oozes eggs |
Table II. Experimental design of dose optimization for induced breeding of Koi carp using different hormone in captive condition.
|
Treatment |
Hormonal dosage |
Latency period (h) |
No of ovulated eggs (Per unit body weight) |
Fertilization rate (%) |
Hatching rate (%) |
Breeding performance |
|
Ovaprim |
0.7 ml per kg |
9.15 |
1450 |
53 |
49 |
Average |
|
Ovatide |
0.7 ml per kg |
12.10 |
650 |
52 |
42 |
poor |
|
WOVA-FH |
0.7 ml per kg |
7.35 |
2200 |
72 |
60 |
Good |
Estimation of hormone efficiency
The efficiency of hormone was estimated based on parameters such as number of eggs spawned, fertilization rate and hatching rate. Number of spawned eggs was estimated by random sampling or by counting the eggs in each breeding mop or nylon fibres. The fertilization rate of eggs was determined by counting the egg having intact nucleus with yellowish colour. Fertilization rate and hatching rate was calibrated using the following formulae:
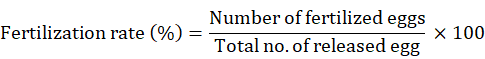
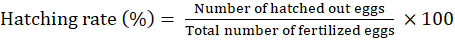
Data analysis
The data on number of ovulated eggs, fertilization, and hatching rates of different treatments were analysed by compared using one way ANOVA method using Statistical Package for the Social Sciences (SPSS) version 20.0. Duncan’s multiple range tests was used to compare the significant differences within and between the treatments. The significance level was set at p<0.05.
RESULTS AND DISCUSSION
In the present study, induced breeding trials were carried out using three different inducing agents ovaprim, ovatide and WOVA -FH at 0.7 ml per kg of body weight. Most carp species responded well at a dosage ranging between 0.4 and 0.70 ml per kg (Nandeesha et al., 1990; Das, 2003). Comparatively WOVA-FH at 0.7 ml per kg showed better performance with higher number of ovulated eggs, fertilization and hatching rate than ovaprim and ovatide (Table II). For dose optimization the matured brooders (250-300 g) were administered with the synthetic hormone WOVA-FH (M/s. Biostadt India Limited), Mumbai consisting of Synthetic Gonadotropin Releasing Hormone Analogue (SGnRH), Domperidone and Glycerol at four different doses 0.3, 0.5 and 0.7 ml,1 ml per kg of body weight. The ideal dose of synthetic hormone WOVA-FH for induced breeding of Koi carp was standardized and the results are summarised in terms of number of eggs per female, fertilization and hatching rate are given in Table III. During the entire culture period. the optimum water quality parameters such as pH and total dissolved solids were maintained as required for Koi fish (Table IV). The spawning was observed, in all breeding trials with significantly varied latency period (The time between administration of inducing agent and initiation of spawning) of 7-12 h. Similarly, variation in the latency period with dose and hormone in induced fish breeding was observed in Gonoproktopterus curmuca, Pethia manipurensis and Osteobrama belangeri (Padmakumar et al., 2014; Motilan et al., 2014; Das et al., 2016). This infers the effect of hormone and dose on the breeding performance in terms of pairing, courtship behaviour and spawning. In the present study, spawning activity was quicker at higher doses compared to lower doses; shows the difference in the mode of action and interaction of hormone. Similar observation was reported by Pandey et al. (2002) and Behera et al. (2007) in Labeo rohita and L. bata, respectively.
Table III. Induced breeding performance of Koi carp using WOVA-FH with different dose combination.
|
Treatment |
Hormonal dosage |
Latency period |
No of ovulated eggs (Per unit body weight) |
Fertilization rate (%) |
Hatching rate (%) |
Breeding performance |
|
No inducement |
0.00 |
0.00 |
0.00 |
0.00 |
No spawning |
|
|
Treatment 1 |
0.3 ml kg-1 |
9.25 |
1358±202.75a |
75±1.2c |
80±1.15b |
Partial spawning |
|
Treatment 2 |
0.5 ml kg-1 |
8.15 |
90±0.8d |
88±0.88c |
Complete spawning with high hatching rate |
|
|
Treatment 3 |
0.7 ml kg-1 |
7.35 |
2336±338.29b |
85±1.4b |
79±1.2b |
Complete spawning |
|
Treatment 4 |
1.0 ml kg-1 |
7.00 |
1579±202.75a |
70±0.3a |
69±0.88a |
Partial spawning |
*Values in the same column with different superscript are significantly different (p<0.05).
Table IV. Optimum water quality parameters.
|
Water quality parameters |
Observed range in spawning tank |
|
Temperature |
24± 2°C |
|
pH |
7.2± 1 |
|
Dissolved oxygen |
5.0± 0.1 mg/l |
|
Ammonia |
< 0.02 mg/l |
|
Total hardness |
150± 30mg/l |
|
Total alkalinity |
60 ± 5 mg/l |
The significant difference (P<0.05) was observed in egg output of the fishes bred with different hormone and doses. The better spawning was observed in WOVA-FH (0.5 ml per kg) and (0.7 ml per kg). Comparatively WOVA-FH at 0.5 ml per kg yielded (3380.5 ±202.75) higher number of eggs. Further, results showed that Ovatide at 0.7 ml per kg produced lower number of spawned eggs. It was observed that fertilization rate was also varied significantly (P<0.05) with different hormone and doses. The higher percentage of fertilization rate (90%) was observed in WOVA-FH (0.5 ml per kg). Lowest fertilization rate (52%) was observed in Ovatide (0.7 ml per kg). Rath et al. (2007) and Motilan et al. (2014) also reported better performance of breeding of IMC and P. manipurensis while administrating WOVA-FH at 0.4-0.5 ml per kg of body weight. The egg output by the female also depends on the release of the gonadotropin releasing hormone which can be stimulated by synthetic inducing agents, which is evidenced by the several studies such as cherry barb (Sundarabarathy et al., 2004), P. manipurensis (Motilan et al., 2014) and O. belangeri (Das et al., 2016). In the present study dose of different hormone affected the rate of fertilization apparently. Inducement of hormone at higher and lower doses causes poor fertilization that may be due to early milting and late inducement in males, respectively (Pandey et al., 2002; Das et al., 2016).
The higher percentage of hatching rate (88%) was observed in WOVA-FH (0.5 ml per kg) and lower percentage of hatching rate (42%) was observed in Ovatide (0.7 ml per kg). Further, results showed that hatching rate varied significantly with different hormone and dose administered. In a similar experiment, mature brooder was induced with three different dosages of ovaprim (0.4, 0.7 and 1.0 ml per kg) and it was found that Koi carp responds at all the three dosages but the best response was found at a higher dosage of 1.0 ml per kg (Ghosh et al., 2012). Koi brood fish injected with synthetic hormone ovaprim at 0.2 ml per kg for male and 0.5 ml per kg for female with aquatic macrophytes (Hydrilla verticillata) for attachment and development showed the number of ovulated eggs, fertilization and hatching rate as 100%, 75.2%, and 83.3%, respectively (Malik et al., 2014).
Several methods have been tried for induced spawning in fishes with various levels of success (Harvey and Hoar, 1979). Induced breeding of many Indian fishes was attempted by several workers however, the level of success varied based on the combination of the fish species and inducing agent (Ramaswamy and Sundaraj, 1969; JR et al., 1992; Alok et al., 1998). A study on different species of IMCs in comparison of spawning success with different inducing agents revealed that Catla catla responds more with Ovaprim, whereas, the results were better with Ovatide in L. rohita and Cirrhinus mrigala (Dhawan and Kamaldeep, 2004). Reddy and Mathur (2000) also reported similar results with ovatide in L. rohita and C. mrigala as compared to C. catla. Tiwana and Sudhanshu (2012) proved that Ovaprim gave 5.83 and 12.95% better performances over Ovatide and carp pituitary extract, respectively in terms of hatching rate of L. rohita eggs. The difference in effective dosage among different species was attributed to the varied levels of dopamine activity (Billard et al., 1983; Richard et al., 1986). Devi et al. (2009) attempted induced spawning and hatching of O. belangeri in its natural habitat using Ovatide (0.6 ml per kg for females and 0.3 ml per kg for males) and reported 95.0±1.05% fertilization and 88.8±1.04% hatching rates. A dosage of 1ml per kg body weight of Ovaprim was recommended for artificial spawning of Clarias gariepinus by Achionye and Israel (2012). Sharma et al. (2010) recommended 1 ml of Ovatide per kg body weight of female broodfish to be optimum among the three experimental doses, 0.6, 0.8 and 1ml per kg, for best breeding performance and egg quality in Clarias batrachus.
Comparing all the parameters, among the synthetic inducing agents used WOVA-FH at 0.5 ml per kg performed better in terms of number of spawned eggs, fertilization and hatching rate. Hence WOVA-FH at 0.7 ml per kg is the standardized hormone with standardized dose for induced breeding of Koi carp.
Embryonic and larval development
Changes in anatomical or morphological structure gives importance to study the difference between embryonic, larval and post-larval development (Kovac, 2000). The term hatchling, larvae and post larvae are used to indicate different stages of development from onset of hatchling to fingerling stage (Boglinoe et al., 1992) and is further divided into six stages namely embryo, hatchling, larva, post- larva, fry and fingerling and each stage was characterized by typical anatomical and physiological features (Jhingran and Pullin, 1985). In the present study, spawning was noticed 6-7 h after the injection and the eggs were hatched 68-72 h after fertilization at a water temperature of 25-26oC. The Koi carp eggs were spherical, demersal and adhesive in nature throughout their incubation period which made the observation of developmental stages more difficult. The diameter of the fertilized egg ranged between 0.9 and 1.10 mm while the yolk sphere ranged between 0.6 and 0.8 mm. The incubation period of eggs depends largely on water quality parameters such as salinity, temperature and pH. The embryonic developments were observed in three distinct phases viz., cleavage, embryonic and larval development stages (Tables V and VI). These were observed using trinocular microscope NLCD- 120E, Lawrence and Mayo (Fig. 2).
Cleavage phase
Embryonic development lasted up to 12 h after fertilization of eggs. It consisted of the formation of perivitelline space, bipolar differentiation and first multiplication of cells, formation of blastula, early gastrula, late gastrula, beginning of epiboly, germ ring, and embryonic shield. The blastodisc was noticed within 30 mins after fertilization (Fig. 3B) and first two-cell division occurred in the animal pole (posterior side) at 0.55 h post-fertilization (h.p.f). The four-cell division occurred at 1.20 h.p.f. The embryo attained an 8-celled stage, 16 -celled stage, 32-celled stage, 64-celled stage and 128-blastomeres stages within 2.45 h.p.f . The size of the blastomere reduced and appeared as cluster of solid cells called the morula. The blastoderm covered almost 1/3 of the yolk sac and formed dome like structure at 4.25 h.p.f. and entered to early gastrula stage. Germ ring was completed and Blastoderm started enveloping 3/4 of the yolk sphere at 7.45 h.p.f and entered into late gastrula stage. Blastoderm completely covered the yolk plug and epiboly came to an end at11.40 h.p.f.
Embryonic phase
The initiation of the embryonic body (organogenesis) formation was observed when the blastomeres covered the whole yolk. The embryo reached 5 somite stage; the tailbud formed at the posterior end of the yolk mass. A pair of kidney shaped optic capsules was visible in the optic vesicle and the lens started to differentiate. As the embryo increased in size, anterior and posterior region became distinguishable as broader cephalic region with the distinct forehead and tail. When the embryo reached 10-12 somite stages,
Table V. Embryonic developmental stages of Koi off springs.
the tailbud separated from the yolk sac and the heartbeat began @ 70-80 beat /minutes at 25.50 h.p.f. During the 13-19 somite stages, the optic vesicle became visible; gut started differentiated; major vein formation occurred; pigmentation started; crystalline lens formed; pectoral fin appeared at 42.10 h.p.f. During 20-25 somite stages, the lens was fully formed, heart was formed, embryo showed slight movement at 49.20 h.p.f. At 72 h.p.f., the embryo
Table VI. Larval developmental stages of Koi carp.
occupied the entire space of the capsule, while pigmentation became more apparent during the pre-hatching stage. The wriggling movement of embryos was more visible at this stage, and tail wrapped completely around the egg. The eyes were pigmented. All the embryos were ready to hatch at this stage. The embryo had undergone rapid development and was clearly visible through the transparent egg membrane just prior to hatching with large eyes, and an orange yolk sac. After the 3rd day of incubation (72 h), the embryo hatched out through the distal end of chorion by breaking the egg capsule with its active wriggling and the hatchlings emerged with tail first. The peak hatching took place between 06.00 to 08.00 h.
Larval metamorphosis
The newly hatched larvae measured 2.7-2.9 mm and were actively swimming near the corner of the water surface; devoid of mouth and anus. On the 3rd day post-hatch, the yolk sac was fully absorbed and the larvae started exogenous feeding. Jameson and Santhanam (1996) also reported that after 3 days, the larvae started exogenous feeding. During the 7th day post-hatch yellow pigmentation appeared in the body and the mouth size was 1.26 mm. After the 21st day post-hatch, various colour combinations appeared on the body. From the 35th day post- hatch, the larvae were fed with GROWFIN feed of size 0.6mm.
CONCLUSION
Koi carp is a colourful species which has numerous strains and it is an economically valuable ornamental species in aquaculture. Availability of quality seed is the basic and critical component of successful aqua culture practices. Induced breeding paves the way for continuous supply of seeds throughout the year. Result of the present study indicates that using WOVA-FH hormone at 0.5 ml per kg of body weight is the ideal dosage for inducing Koi carp using RAS system. This developed induced breeding technology would be helpful in producing year-round seed production with sustainability. The detailed description of embryonic development of Koi carp in this study will serve as baseline information for ornamental hatcheries due to its aquaculture importance.
ACKNOWLEDGEMENT
The authors are thankful to the Vice-Chancellor, Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam and PI of the ARTP project, Madhavaram Campus, Fisheries College and Research Institute, Ponneri for the facilities provided.
Funding
The study received no funding.
IRB approval
This research was carried out with the approval of University Students’ Research Guidance Workshop Committee (Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, Tamil Nadu, India).
Ethical statement
This study was approved by the ethical committee of Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, Tamil Nadu, India.
Data availability statement
The data that support the findings of this study are available within the article.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Achionye-Nzeh, C.G., and Obaroh, I.S.R.A.E.L., 2012. Ovaprim doses effects on eggs of African mudfish Clarias gariepinus. Int. J. Life Sci. Pharm. Res., 2: 6-9.
Alok, D., Krishnan, T., Talwar, G.P. and Garg, L.C., 1998. Multiple induced spawnings of the Indian catfish Heteropneustes fossilis (Bloch) within a prolonged spawning season. J. World Aquacult. Soc., 29: 252-258. https://doi.org/10.1111/j.1749-7345.1998.tb00985.x
Behera, B.K., Das, P., Singh, N.S. and Sahu, A.K., 2007. Observation on the induced breeding of Labeo bata (Hamilton) with Ovaprim and Ovatide as inducing agents with a note to its development. J. Aquacult., 15: 11-17.
Billard, R., Alagarswami, K., Peter, R.E. and Breton, B., 1983. Potentialisation par le pimozide des effects du LHRH-A sur la secretion gonadotrope hypophysaire, l’ovulation et la spermation chez la carpe commune (Cyprinus carpio). C. R. Acad. Sci. Sér. III, Sci., 296: 181-184.
Boglione, C., Bertolini, B., Russiello, M. and Cataudella, S., 1992. Embryonic and larval development of the thicklipped mullet (Chelon labrosus) under controlled reproduction conditions. Aquaculture, 101: 349-359. https://doi.org/10.1016/0044-8486(92)90037-L
Das, P., Behera, B.K., Meena, D.K., Singh, S.K., Mandal, S.C., Das, S.S., Yadav, A.K. and Bhattacharjya, B.K., 2016. Comparative efficacy of different inducing agents on breeding performance of a near threatened cyprinid Osteobrama belangeri in captivity. Aquacult. Rep., 4: 178-182. https://doi.org/10.1016/j.aqrep.2016.11.001
Devi, G.A., Devi, G.S., Singh, O.B., Munilkumar, S. and Reddy, A.K., 2009. Induced spawning and hatching of Osteobrama belangeri (Valenciennes) using Ovatide, an ovulating agent. Asian Fish. Sci., 22: 1107-1115. https://doi.org/10.33997/j.afs.2009.22.4.005
Dhawan, A. and Kaur, K., 2004. Comparative efficacy of ovaprim and ovatide in carp breeding. Indian J. Fish., 51: 227-228.
Ghosh, A.K., Biswas, S., Sarder, L., Sabbir, W. and Rahaman, S.M.B., 2012. Induced breeding, embryonic and larval development of Koi carp (Cyprinus carpio) in Khulna, Bangladesh. Mesopotamian J. Mar. Sci., 27: 1-14.
Harvey, B.J. and Hoar, W.S., 1979. Theory and practice of induced breeding in fish. IDRC, Ottawa, ON, CA.
Holt, G.J., 2003. Research on culturing the early life stages of marine ornamental fish. Mar. Ornament.Spp. Collect. Cult. Conserv., 251-254.
Hussain, T., Verma, A.K., Tiwari, V.K., Prakash, C., Rathore, G., Shete, A.P. and Nuwansi, K.K.T., 2014. Optimizing koi carp, Cyprinus carpio var. koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J. World Aquacult. Soc., 45: 652-661. https://doi.org/10.1111/jwas.12159
Jameson, J.D. and Santhanam, R., 1996. Manual of ornamental fishes and farming technologies. Tamil Nadu Veterinary and Animal Sciences University, Chennai, pp.50-60.
Jhingran, V.G. and Pullin, R.S., 1985. A hatchery manual for the common, Chinese, and Indian major carps (No. 252). Asian Development Bank. International Centre for Living Aquatic Resources Management. Manila, The Philippines. pp. 191.
Jr, M.Z., Furukawa, K. and Aida, K., 1992. Induction of ovulation by HCG injection in the tropical walking catfish Clarias batrachus reared under 23-25° C. Nippon Suisan Gakkaishi, 58: 1681-1685. https://doi.org/10.2331/suisan.58.1681
Kovac, V., 2000. Early development of Zingel streber. J. Fish Biol., 57: 1381-1403. https://doi.org/10.1111/j.1095-8649.2000.tb02219.x
Madhu, K., Madhu, R. and Retheesh, T., 2012. Broodstock development, breeding, embryonic development and larviculture of spine-cheek anemonefish, Premnas biaculeatus (Bloch, 1790). Indian J. Fish., 59: 65-75.
Malik, A., Abbasi, A.R., Kalhoro, I.B., Shah, S.A., Narejo, N.T. and Kalhoro, H., 2014. Effect of ovaprim hormone (Syndel Laboratory, Canada) on spawning of koi carp at fish hatchery Chilya Thatta, Sindh, Pakistan. Sindh Univ. Res. J. (Sci. Ser.), 46: 273-276.
Motilan, Y., Bedajit, Y. and Sunitibala, W., 2014. Captive breeding of endangered barbs Pethia manipurensis (Menon et al., 2000) by oral delivery of gonadotropic signaling molecular analogue WOVA-FH. Int. J. Sci. Res. Publ., 4: 296.
Nandeesha, M.C., Das, S.K., Nanthaniel, E. and Varghese, T.J., 1990. Project report on breeding of carps with Ovaprim in India. Special Publication No. 4. Asian Fisheries Society Indian Branch, Mangalore, pp. 41.
Olivotto, I., Yasumasu, S., Gioacchini, G., Maradonna, F., Cionna, C. and Carnevali, O., 2004. Cloning and expression of high choriolytic enzyme, a component of the hatching enzyme system, during embryonic development of the marine ornamental fish Chrysiptera parasema. Mar. Biol., 145: 1235-1241. https://doi.org/10.1007/s00227-004-1404-9
Padmakumar, K.G., Bindu, L., Sreerekha, P.S., Joseph, N., Manu, P.S. and Krishnan, A., 2014. First report on captive breeding of endemic red-tailed silver shark Gonoproktopterus curmuca (Cyprinidae: Hamilton-Buchanan 1807). Int. J. Res. Fish. Aquacult., 4: 156-160.
Pandey, A.K., Mohapatra, C.T., Kanungo, G., Sarkar, M., Sahoo, G.C. and Singh, B.N., 2002. Ovatide induced spawning in the Indian major carps, Labeo rohita (Hamilton-Buchanan). Aquaculture, 3: 1-4.
Ramaswamy, L.S. and Sundararaj, B.I., 1969. Induced spawning in the Indian catfish Heteropneustes fossilis with pituitary injection. Acta Anat., 31: 551-562. https://doi.org/10.1159/000141304
Rath, S.C., Sarkar, S.K., Gupta, S.D., and Sarangi, N., 2007. Comparative account of induced breeding of Indian major carps with ovaprim, ovatide, wova-FH and carp pituitary extract. Indian J. Anim. Sci., 77:1057-1060.
Reddy, A.K. and Mathur, K.B., 2000. Ovatide, a highly potent inducing agent for breeding of carps. First Indian Science Congress. September, 2000, Punjab University, Chandigarh. pp. 21-23.
Richard, E.P., John, P.C., Carol, S.N., Robert, J.O., Sokolowska, M., Stephen, H.S. and Roland, B., 1986. Interactions of catecholamines and GnRH in regulation of gonadotropin secretion in teleost fish. In: Proceedings of the 1985 laurentian hormone conference. Academic Press. pp. 513-548. https://doi.org/10.1016/B978-0-12-571142-5.50016-1
Sharma, K., Yadava, N.K. and Jindal, M., 2010. Effect of different doses of ovatide on the breeding performance of Clarias batrachus (Linn.). Livest. Res. Rural Dev., 22: 2010.
Sundarabarathy, T.V., Edirisinghe, U. and Dematawewa, C.M.B., 2004. Captive breeding and rearing of fry and juveniles of cherry barb (Puntius titteya Deraniyagala), a highly threatened endemic fish species in Sri Lanka.
Tiwana, G.S. and Raman, S., 2012. An economically viable approach for induced breeding of Labeo rohita by ovatide, ovaprim and carp pituitary extract. IOSR J. Agric. Vet. Sci., 1: 30-32. https://doi.org/10.9790/2380-0113032
To share on other social networks, click on any share button. What are these?










