Is Use of Two Trichoderma Species Sole or Combined with a Plant Extract Effective for the Biocontrol Meloidogyne incognita on Potato and Soil Microorganisms Diversity?
Is Use of Two Trichoderma Species Sole or Combined with a Plant Extract Effective for the Biocontrol Meloidogyne incognita on Potato and Soil Microorganisms Diversity?
Mahmoud M.A. Youssef1, Wafaa M.A. El-Nagdi1*, Hassan Abd- El-Khair2, Usama S. Elkelany1, Mahfouz M.M. Abd-Elgawad1 and Mona G. Dawood3
1Department of Plant Pathology, Nematology Laboratory, National Research Centre, Dokki, 12622, Cairo, Egypt; 2Department of Plant Pathology, National Research Centre, Dokki, 12622, Cairo, Egypt; 3Department of Botany, National Research Centre, Dokki, 12622, Cairo, Egypt.
Abstract | Nature-friendly compounds are being explored to substitute chemically synthesized products for safe and effective control of crop pests. This study investigated the effects of Trichoderma viride (Tv) and T. virens (Tvr) as antifungal agents individually or combined with pomegranate peel aqueous extract (PP) on M. incognita infesting potato cv. Spunta. Based on the percentages juvenile reduction in soil at harvest time, the combined treatments of Tv or Tvr with PP recorded 66.8 or 60.5% nematode reductions, respectively, as each fungal agent was applied in soil before planting. However, such reductions were 83.7 and 78.7%, respectively, when Tv or Tvr were used as single treatments indicating more efficacy on M. incognita populations in the absence of PP. All treatments significantly (P ≤ 0.05) increased weights of plant branches and tubers; especially by individual than combined treatments. Biochemical compounds were influenced by different treatments of the tested materials. The diversity and community of aerobic bacteria, spore-forming bacteria and fungi and frequency% of fungi in potato rhizosphere varied among different treatments.
Received | May 02, 2023; Accepted | October 22, 2023; Published | November 15, 2023
*Correspondence | Wafaa M.A. El-Nagdi, Department of Plant Pathology, Nematology Laboratory, National Research Centre, Dokki, 12622, Cairo, Egypt; Email: [email protected]
Citation | Youssef, M.M.A., El-Nagdi, W.M.A., Abd-El- Khair, H., Elkelany, U.S., Abd-Elgawad, M.M.M. and Dawood, M.G., 2023. Is use of two Trichoderma species sole or combined with a plant extract effective for the biocontrol Meloidogyne incognita on potato and soil microorganisms diversity? Pakistan Journal of Nematology, 41(2): 153-164.
DOI | https://dx.doi.org/10.17582/journal.pjn/2023/41.2.153.164
Keywords | Antagonistic effect, Trichoderma viride, Trichoderma virens, Pomegranate residue extract, Meloidogyne incognita, Potato
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Globally, plant-parasitic nematodes (PPNs) cause significant plant diseases and consequently severe losses to economically important crops. Although the main parasitic nematodes of potato, Solanum tuberosum, plants may comprise species of Globodera, Ditylenchus, Pratylenchus and Nacobbus aberrans (Niere and Karuri, 2018), root-knot nematodes (RKNs), Meloidogyne spp. rank the highest among these serious groups causing substantial economic losses to many susceptible potato cultivars in Egypt (Abd-Elgawad, 2020), where M. incognita is the most dominant and dangerous species (Osman et al., 2022). The economic importance of increasing potato production is obvious as potatoes have various forms of local consumption (e.g., table potatoes, seed potatoes, chips, French fries, and processing potatoes), in addition to its exportation to provide direly needed foreign exchange revenue (Abd-Elgawad, 2020). Since M. incognita-infected plants show stunting in plant growth, leaf chlorosis, and reduction of water uptake and nutrients transport resulting in yield reduction (Niere and Karuri, 2018), it is necessary to develop safe and effective control measure against the RKNs (Šunjka and Mechora, 2023). Many growers use chemical nematicides, however, due to their side effects on the environmental and non-target beneficial species, it is mandatory to find benign and reliable alternative nematicides. Therefore, comprehensive and continuous investigations of the response (beneficial and harmful impacts) of such alternatives should be pre-requisites to products of biotechnology. Recently, the additive, synergistic, or antagonistic effects of agricultural inputs on RKNs on potato were reviewed (Abd-Elgawad, 2020) to offer novel insights for the nematode control.
Species of Trichoderma as biocontrol antifungal agents can share in integrated pest management (IPM) to control the pests and diseases in an environmentally friendly manner (Monte, 2001). A study by T. harzianum combined with Lantana camara, significantly reduced M. incognita population criteria on tomato which was reflected in increasing mean of yield criteria (Feyisa et al., 2015). Abd-El-Khair et al. (2018) reported that T. harzianum and T. virens as well as oil cakes of olive and castor bean residues alone or consortium reduced Fusarium solani root rot disease incidence and M. incognita on eggplants in pot experiment. El-Nagdi et al. (2019a; b) showed that three fungal species, T. harzianum, T. viride, and T. virens, and ground seeds and powdered leaves of certain plants as soil amendments in sole or combined treatments significantly (P ≤ 0.05) reduced M. incognita criteria and consequently enhanced plant growth parameters. The RKN reduction occurred by the combined treatments were higher than those obtained by single ones. Also, Youssef et al. (2017, 2020) stated that some fungal species acted as bioagents which reduced M. incognita on sugar beet in the first date and cowpea in the second date.
Punica granatum (pomegranate) was reported by Middleton et al. (2000) to be rich in antioxidant of polyphenolic class that includes tannins and flavonoids. Antioxidant activity has been suggested to have a vital role in various pharmacological activities such as anti-aging, anti-inflammatory aging, anti-atherosclerosis and other anti-activities. Regaieg et al. (2017) indicated that M. javanica infestation was reduced by aqueous extract of pomegranate that consequently improved plant growth, but its powder form exhibited a phytotoxicity symptoms compared to control. Youssef et al. (2014) and El-Nagdi and Youssef (2015) showed that reduction in the nematode parameters in roots of date palm for the first and sugar beet for the second authors infected by M. incognita occurred by pomegranate peel aqueous extract. However, no studies were carried out on the combining effect of Trichoderma and pomegarante extract on M. incognita population. Notwithstanding that the synergistic or additive effects of such benign factors on potato pests are needed, the combined effect of fungal bioagent with pomegranate peel aqueous extract might show antagonistic activities, given its aforementioned anti-activities. This does not negate the pressing need to look at novel solutions to enhance the crop productivity without harming the environment.
In this concern, two species of Trichoderma viride (Tv), T. virens (Tvr) as antifungal agents and pomegranate peel (PP) aqueous extract were singly used or each fungus combined with PP to biocontrol M. incognita and their influence on diversity and community of total microbes and fungal frequency% in potato soil under field conditions.
Materials and Methods
Test materials
Ministry of Agriculture and Land Reclamation, Vegetative Research Center, provided the source of potato (Solanum tuborsum L.) cv. Spunta tubers used in this experiment.
Plant extract
A weight of ١٠٠ g of crushed fruit peels of pomegranate (PP) was soaked in 1L distilled water for three days. Filtrates (10%) were then, prepared by filtration through Whatman filter paper no. 1 and were added to each replicate at the rate of 200 ml.
Source of fungal inocula
Trichoderma viride (Tv) and T. virens (Tvr) were singly reared on sterilized medium containing sorghum: sand: water (2:2:1 v/v/v). The sterilized medium was individually inoculated by each fungal species, using fungal disc (1-cm diameter) obtained from 7-day-old culture sourced from Plant Pathology Department, National Research Centre, Egypt. Then, the medium was incubated at 30±2 °C for 15 days. The resulting fungal inoculum was applied in the field.
Field trial
This study were performed in a field infested with M. incognita, with randomized complete block design, at El-Mansouryia village, Giza governorate, Egypt during the growing season of potato in winter season from January to May, 2020 season. The soil was divided into 3-m in length and 75-cm in width rows and the distance among plants (Hills) 20-cm. Each fungal bio-control agent (FCA) at the rate of 50g was added in each pi t( Hill) and pomegranate fruit peel aqueous extract at the rate of 200 ml was added by soil drenching in each replicate before planting as well as control (without treatment) followed by planting potato cv. Spunta tubers. Five replicates (hills) were used for each treatment and control. The treatments were: (1) Trichoderma viride (Tv)+ pomegarante peel (PP) residue extract, (2) T. virens (Tvr)+ PP residue extract, (3) PP residue extract + Sorghum (S) as carrier, (4) Tv, (5) Tvr, (6) PP residue extract, (7) Sorghum (S) (carrier or medium), and (8) Untreated control. Horticultural practices were followed as recommended (El-Anany et al., 2019).
M. incognita juvenile (J2s) numbers (initials) (at one week before planting), and others at mid-season (two months after planting and treatment) and at the end of growing season (four months after planting and treatment) were assessed from three soil subsamples which were mixed well to form an aliquot of one composite soil sample of 250 g soil. The number of J2s in soil was sieved and decanted according to Barker (1985). Numbers of galls and egg-masses in excised roots were recorded at harvest time in treated and untreated potato plants. The percentage of reduction for each nematode parameter was calculated according to the formula of Puntener (1981). The mean total percentages of nematode reductions (Observed effect) for each treatment was calculated to compare among different treatments. Lempel’s formula reported by Richer (1987) was used to calculate the percentage of the tested combined substances (expected effect) based on the percentages of reduction of juveniles in the soil in the combined treatments using the following formula:
E =X + Y - XY / 100
Where: E=effect that expected for the mixture. X and Y represent the effect due to each of single treatment A and Y alone, respectively.
The co-toxicity% was calculated to determine additive, synergistic, or antagonistic effect for each mixture according to the equation registered by Mansour et al. (1966) depending upon the percentages of nematode reduction for the number of final J2s in soil (Observed effect) as follows:
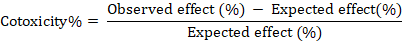
The percentages of co-toxicity values can be categorized based on the previously mentioned equation, where the value of 20 or more is considered potentiating, -20 or more show antagonistic and immediate values ranging from -20 to + 20 exhibit additive or synergistic action. Potato plant growth and yield attributes were recorded. For comparison among treatments, the percentages of increase in the weight of branches and number and weight of tubers per plant were calculated for each treatment.
The colorimetric method by Dubois et al. (1956) was followed to determine soluble carbohydrates and total carbohydrates in potato tubers. Total phenolic compounds from potato tubers were determined colorimetrically according to Snell and Snell (1953) and Folin Ciocalteu phenol reagent was used. Determination of chlorophyll a, chlorophyll b, and carotenoids as photosynthetic pigments in the fresh leaves followed Moran (1982).
As described by El-Nagdi et al. (2023), diversity and community of aerobic and, spore-forming bacteria and fungi in the rhizosphere of potato plant as affected by Tv and Tvr sole or combined with pomegranate peel were assessed as CFU per 10 g of soil at the aforementioned sampling dates by dilution method (Bridson, 1995; Ghini et al., 2007). On the basis of their morphological and cultural characters following Ellis (1971) and Barnett and Hunter (1972), the obtained fungi were identified to genus and species level and counted at the different sampling times. The frequency percentages of common fungi in the potato rhizosphere in the different treatments were determined (Ghini et al., 2007) as follows:
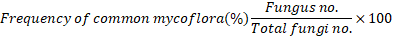
Statistical analysis of data
Analysis of the present experiment was achieved on the basis of one -way analysis of variance (ANOVA) to detect significance at probability 0.05 among the obtained data. Mean separation was evaluated by Duncan’s Multiple Range Test following Snedecor and Cochran (1989) by using computer statistical software (COSTAT).
Results and Discussion
Impact on Meloidogyne incognita parameters
The treatments of each of Tv and Tvr significantly (P ≤ 0.05) could decrease nematode parameters as indicated by the J2 numbers in soil at mid-season and numbers of J2s in soil and roots and egg masses and galls on roots at harvest time (Table 1). Based on the percentages of nematode reductions in soil, the treatments differed in the J2s numbers at mid-season. At harvest time, the combined treatments of Tv or Tvr with PP recorded 66.8 and 60.5% nematode reductions, respectively which were less than 83.7 and 78.7% nematode reductions caused by the respective Trichoderma spp. when used as single treatments. Both PP and S registered nematode reductions in soil, 50.9 and 46.7%, respectively compared to PP extract + S as recorded 49.9%. Other nematode parameters differed in their reductions according the tested materials.
Antagonistic effects for the percentages of juvenile reductions on potato were shown by PP. Their assessed co-toxicity values (-27.6 and -32.6) on the respective fungi, when combined with Tv or Tvr were implied in soil at harvest stage. The combination between PP + S showed antagonistic interaction too (Table 2).
Table 1: Trichoderma viride, T. virens and pomegranate peels in sole or in combination and their effects on Meloidogyne incognita parameters in potato under field conditions.
|
Treatment |
Initial and mid- season J2s /250 g soil |
No. of nematode parameters and galls/5 g roots and 250 g soil at harvest time |
% mean of total percentages of nematode reduction |
|||
|
Initial |
Mid -season |
Final J2s in soil |
Egg masses |
Galls |
||
|
Trichoderma viride+ pomegranate peel residue extract(Tv + PP) |
430a |
294d (84.7%) |
640f (66.8%) |
396b (29.0%) |
578b (18.4%) |
47.5 |
|
Trichoderma virens+ pomegranate peel residue extract(Tvr + PP) |
428a |
336c (82.5%) |
758e (60.5%) |
100e (82.1%) |
175f (75.3%) |
74.4 |
|
Pomegranate residue extract + Sorghum (carrier)(PP + S) |
452a |
350b (82.7%) |
780d (61.5%) |
290c (48.0%) |
435d (38.6%) |
49.9 |
|
T. viride (Tv) |
450a |
251e (87.6%) |
328h (83.7%) |
300c (46.2%) |
510c (28.0%) |
59.8 |
|
T. virens (Tvr) |
434a |
180f (90.8%) |
415g (78.7%) |
200d (64.2%) |
370e (47.7%) |
58.2 |
|
Pomegranate residue extract(PP) |
438a |
352b (82.1%) |
938b (52.2%) |
72e (87.1%) |
171f (75.8%) |
50.9 |
|
Sorghum (carrier or medium)(S) |
444a |
253e (87.3%) |
840c (57.8%) |
285c (48.9%) |
378e (46.6%) |
46.7 |
|
Untreated control |
435a |
1950a (0) |
1950a (0) |
558a (0) |
708a (0) |
- (0) |
1Each value is average of 5 replicates. 2Means in each column, denoted by the same small letter are not significantly different according to Duncan`s multiple range test (P = 0.05). Values of initial population were transformed to √x before statistical analysis. 3Values between brackets indicate the percentages nematode reductions.
Table 2: Interaction type among the combined treatments on the percentages nematode reduction of root-knot nematode, Meloidogyne incognita on potato at harvest stage.
|
Treatment |
Effect of observed and expected percentages of nematode reduction at harvest stage |
Co toxicity |
Type of interaction |
|
|
Expected% |
Observed% |
|||
|
Trichoderma viride+ Pomegranate residue extract(Tv+ PP) Trichoderma virens + pomegranate residue extract(Tvr+PP) Pomegranate residue extract + sorghum (carrier)(PP+S) |
92.21 89.8 79.8 |
66.80 60.5 61.5 |
-27.6 -32.6 -22.9 |
antagonistic antagonistic antagonistic |
Table 3: Growth parameters of potato infested by Meloidogyne incognita as affected by Trichoderma viride, T. virens and pomegranate extract in sole or in combination under field conditions.
|
Treatments |
Mid-season |
Harvest season |
||||
|
Length of branches (cm)/ plant |
No. of leaves/ plant |
Length of branches (cm)/pant |
No. of leaves/ plant |
Weight of branches (g)/plant |
% Increase |
|
|
Trichoderma viride+ Pomegranate residue extract (Tv + PP) |
14ab |
35a |
36a |
39a |
46bc |
130.0 |
|
Trichoderma virens + Pomegranate residue extract (Tvr + PP) |
13ab |
20b |
36a |
22bc |
43bc |
115.0 |
|
Pomegranate residue extract+ Sorghum(carrier)(PP+S) |
17a |
12c |
35a |
14cd |
45bc |
125.0 |
|
Trichoderma viride (Tv) |
12ab |
8d |
33a |
10d |
52b |
160.0 |
|
Trichoderma virens (Tvr) |
15ab |
20b |
36a |
23b |
80a |
300.0 |
|
Pomegranate residue extract (PP) |
16ab |
13c |
34a |
16bc |
35cd |
75.0 |
|
Sorghum (carrier)(S) |
14ab |
10c |
35a |
16bc |
33d |
65.0 |
|
Untreated Control |
11b |
7d |
29b |
9d |
20e |
0 |
1Each value is average of 5 replicates. 2Means in each column, denoted by the same small letter(s) are not significantly different according to Duncan`s multiple range test (P = 0.05).
Table 4: Trichoderma viride, T. virnes and pomegranate peel extract in single or in combination and their effects on yield parameters of potato infested by M. incognita under field conditions.
|
Treatments |
No of tubers/plant |
% Increase |
Weight of tubers/plant (g) |
% Increase |
|
Trichoderma viride+ pomegranate residue extract(Tv + PP) |
3a |
50 |
219.3d |
78.3 |
|
T. virens + pomegranate residue extract(Tvr + PP) |
5a |
100 |
300.0bc |
143.9 |
|
Pomegranate residue extract + Sorghum (carrier) (PP + S) |
3a |
50 |
285.0c |
131.7 |
|
Trichoderma viride(Tv) |
3a |
50 |
340.0b |
176.4 |
|
Trichoderma virens(Tvr) |
3a |
50 |
480.0a |
290.2 |
|
Pomegranate residue extract(PP) |
3a |
50 |
290.0c |
135.8 |
|
Sorghum(carrier)(S) |
4a |
100 |
200.0d |
62.6 |
|
Untreated control |
2a |
0 |
123.0e |
0 |
1Each value is average of 5 replicates. 2Means in each column, denoted by the same small letter are not significantly different according to Duncan`s multiple range test (P = 0.05).
Impact on potato plant growth
As for plant growth of potato plants in Table 3, all treatments significantly (P ≤ 0.05) increased plant growth criteria as indicated by length of branches, number of leaves and weight of branches per plant compared to control. On the basis of the percentages of increases of weight of branches, they recorded the highest percentages of increases; 160 and 300% by using either Tv or Tvr as single treatments, respectively. The combined treatments of each of the two fungal species + PP aqueous extract caused less percentages of branch weight increases, 130 and 115%, respectively compared to those of single treatments and control.
Impact on potato tuber yield
The sole treatments (Tv or Tvr) achieved 176.4 and 290.2%, increases in weight of tubers, respectively (Table 4). Whereas the combined treatments of either Tv or Tvr with PP recorded less percentages of mean weights of tubers.
Impact on photosynthetic pigments
Chorophyll a, chlorophyl b, and carotenoid contents were differently affected by treatments (Table 5). Their total contents recorded the maximum via sorghum (S) followed by PP residue extract, and Tvr combined with PP compared to the other applied treatments.
Impact on phytochemical changes
Total carbohydrates, polysaccharides, and phenolic compounds were influenced by different treatments of the tested materials (Table 5). Their contents were enhanced by applying different treatments as compared to those of the untreated check. The combined treatment; pomegranate peel residue extract (PP) + S (Sorghum) recorded maximum contents of the previous contents followed by the treatment pomegranate peel residue extract (PP). T. viride treated plants had the lowest contents of total carbohydrates and polysaccharides. As for soluble carbohydrates, the combined treatment; Tvr + PP recorded the highest content followed by Tv + PP residue extract. The lowest content of soluble carbohydrates was recorded in plants treated by Tv only.
Impact on diversity and community of microbiota
Averages of microbial counts at the different sampling dates were illustrated in Table 6. All treatments at mid- and end- growing seasons, increased the total numbers of aerobic bacterial counts in the ranges of 6.95 to 7.28 and 7.35 log10 CFU/10g soil, respectively comparable to untreated control (7.00) (Table 6). The maximum aerobic bacterial counts during end- season were recorded by Tvr + PP (7.35), followed by PP only (7.33), PP+S (7.30), Tv + PP (7.22), Tvr (7.21), Tv (7.12) at the same period compared to untreated control (7.00). The total spore-forming bacterial counts also increased in the ranges of 4.95 to 5.09 and 5.25 log10 CFU/10g soil with the tested treatments at mid- and end- growing seasons, relative to untreated control which recorded 5.00 and 5.11 CFU/10g soil, respectively. At end of the growing season, the greatest spore-forming bacterial counts were recorded with Tvr + PP (5.25) and PP only (5.25) followed by PP+S (5.18), Tvr (5.18), Tv (5.14), and Tv + PP (5.08) compared to the untreated control. The total fungal counts also increased in the ranges of 5.00 to 5.11 and
Table 6: Trichoderma viride and Trichoderma virens alone or combined with pomegranate extract and their effects on microbial diversity and composition in potato soil at various sampling dates under field conditions.
|
Treatments |
Log 10 CFU Total microbial counts1 |
||||||||
|
Aerobic bacteria 106 |
Spore-forming bacteria 104 |
Fungi 104 |
|||||||
|
B |
M |
E |
B |
M |
E |
B |
M |
E |
|
|
Trichoderma viride + Pomegranate peel extract (Tv + PP) |
6.73 de |
7.11 bcd |
7.22 b |
4.68 ab |
4.95 a |
5.08b |
4.78bc |
5.05ab |
5.17 abc |
|
Trichoderma virens + Pomegranate peel extract (Tvr + PP) |
7.11 a |
7.28a |
7.35a |
4.99a |
5.05a |
5.25a |
4.98 ab |
5.00abc |
5.19 ab |
|
Pomegranate peel + Sorghum (PP + S) |
6.94 abcd |
7.20 ab |
7.30 ab |
4.78 b |
5.02a |
5.18ab |
4.86ab |
5.04abc |
5.20a |
|
Trichoderma viride (Tv) |
6.79 cde |
6.95 d |
7.12 c |
4.79 ab |
4.98a |
5.14ab |
5.01a |
5.11a |
5.22ab |
|
Trichoderma virens (Tvr) |
6.70 abc |
7.10 bcd |
7.21 b |
4.84 ab |
5.01a |
5.18ab |
4.95ab |
5.05ab |
5.19ab |
|
Pomegranate peel (PP) |
7.05 ab |
7.13 abc |
7.33 a |
4.99 a |
5.09 a |
5.25a |
4.97ab |
5.01bc |
5.12bc |
|
Sorghum (S) |
6.88c |
7.07c |
7.42c |
4.96bc |
5.25bc |
5.48a |
4.95bc |
5.00bc |
5.16cd |
|
Untreated control |
6.62 d |
6.78 d |
7.00 f |
4.78 cd |
5.00e |
5.11c |
4.59e |
4.90c |
5.04e |
1Each value is average of 5 replicates. 2Means in each column, denoted by the same small letter are not significantly different according to Duncan`s multiple range test (P = 0.05). B = before sowing, M= at mid- growing season and E= at harvest time.
5.22 log10 CFU/10g soil with the tested treatments at mid-season and season-end, higher than 4.90-5.04 CFU/10g soil in the untreated control, respectively. The maximum fungal count was shown with Tv (5.22), followed by PP+S (5.20), Tvr (5.19), Tvr+PP (5.19), Tv+PP (5.17), S(5.16) and PP(5.12), all were higher than untreated check (Table 6).
Impact on percentage of fungal frequency
Results indicated that Aspergillus spp., A. niger, Penicillium spp., P. chrosogenum, P. citrinium, Rhizopus nigricans, Fusarium spp., Trichoderma spp., and Rhizoctonia spp. were found to be the most frequently fungi in the rhizosphere of potato. Data of the frequency percentages of fungi were listed in Table 7. The treatments of PP, S, and PP + S could markedly recorded high % frequency of Aspergillus spp, at mid-season (19.1, 18.1 and 15.8, higher than their frequency before planting (13.6, 9.5, and 14.3), respectively and untreated check (12.05). The treatment of Tv decreased Aspergillus spp. frequency % at the end- growing season (12%), being less than that before planting (16.7%) and untreated check (12.05%), but Tvr was not effective against the mentioned fungal species. The treatments of Tv or Tvr + PP reduced the frequency % of these fungal species during growing season, especially at the season-end relative to the corresponding frequency before planting. Results revealed that the treatments of Tvr, PP, and S reduced the frequency of Penicillium spp., especially at mid-season, being less than the control, however, these fungal species were reduced by Tv or Tv + PP at season-end, being less than at mid- and before planting season. The treatments of Tvr + PP (19.2%) and PP + S (21.1%) highly increased the frequency of Penicillum spp., especially at mid-season. The treatments of Tv + PP (45.8%), Tvr (40.0%) and Tv (37.9%), boosted the frequency % of Trichoderma spp., especially at mid-season, compared to the two other dates, being higher than check. The treatments of PP highly enhanced the frequency % of these fungi at the end- season relative to both those at mid-season and before planting, as they were higher than the check. On the other hand, the treatments of PP + S and S reduced that fungal frequency % during the growing season compared to the check. Most of the tested treatments remarkably decreased the frequency% of Fusarium spp. during the growing season, especially at mid-season relative to other dates. However, PP+S recorded high frequency% (15.8%) at mid-season, but was less than the check. Likewise, most of the tested treatments minimized the frequency percent of Rhizoctonia spp. at mid-season compared to the check (Table 7).
The present investigation indicated that that each of Tv, Tvr, and pomegranate peel extract as individual or combined treatments suppressed M. incognita population parameters as well as boosted the growth and yield of potato. The reduction in nematode numbers may be due to that the tested fungi can directly parasitize on M. incognita population and secrete protease and chitinase enzymes that can inhibit egg hatching as reviewed by Abd-Elgawad and Askary (2018). Also, the present results were similar to those recorded by Olabiyi and Gbadamosi (2013), as T. harzianum was combined with composted soil amendments, T. virens mixed with plant debris (Moradi et al., 2015), and T. harzianum was combined with decomposed neem (Khan et al., 2012). On the basis of the mean percentages of reductions in soil concerning M. incognita population the combined treatment of each tested fungal species + pomegranate extract showed less nematode reduction at harvest stage than that achieved by each sole fungal treatment. This may refer to a possible antagonistic effect that occurred, when each fungal species was combined with pomegranate peel extract. Such a difference in reductions of M. incognita population densities was reflected on percentages of branches and tuber weight; both had more increase in single treatments than in their combined ones. The effect of pomegranate extract alone in reducing RKN population was documented by several workers (Korayem et al., 1993; Youssef et al., 2014; El-Nagdi and Youssef, 2015) which corroborated that reported herein. Nevertheless, similar antagonism induced by PP was also shown by Dahham et al. (2010). They reported Bacillus coagulans, B. cereus, B. subtilis, and Staphylococcus aureus as the most biocontrol agents were adversely affected by using pomegranate extract. Prashanth et al. (2001) proved that certain bacteria were negatively affected by using pomegranate fruit rind extract too. In accordance to the previous study, co-toxicity% for the applied bioagents +PP extract on M. incognita at harvest stage in the present study recorded antagonistic effect on potato. Similar interpretation was also reported by Regaieg et al. (2017) on tomato and by El-Nagdi et al. (2023) on potatoes. Thus, higher increases of branches and tuber weights by single fungal treatments than those by their combination with PP may refer to that nutrient absorption was faster via potato roots in the former than the latter case as reported by El-Nagdi et al. (2023).
As for biochemical changes, the compounds measured herein increased in the tubers at the different treatments relative to the check, enriching the nutritional tuber value. Chlorophyll (a + b) and carotenoid contents in the fresh leaves were elevated by most treatments; similar to other results on some antagonistic bacteria (Akhtar et al., 2012; Abd-El-Khair et al., 2019; El-Nagdi et al., 2019a). Some phenolic substances were involved to cause resistance against PPNs (Bajaj and Mahajan, 1977; Giebel, 1982) and have been correlated with levels of phenol in certain plant roots (Narayana and Reddy, 1980).
Likewise, all treatments increased the diversity and community composition of some monitored microbes. The antagonistic fungi, Aspergillus spp., Penicillium spp., and Trichoderma spp. were differently increased in rhizosphere of the growing potato. These results agreed with those obtained by El-Nagdi et al. (2023) on potatoes infested by M. incognita and treated by two bacterial species. Sikandar et al. (2020) mentioned that P. chrysogenum (Snef1216) caused inhibition of the egg hatching and increased mortality of M. incognita, relying on increasing the concentration and exposure time to the fungus filtrate; as a novel nematicidal agent against the root-knot nematode. Korayem et al. (2019) stated that Aspergillus spp., A. niger, Fusarium spp., Penicillium spp., and Trichoderma spp. were common fungi in the rhizosphere of wheat crop grown in different governorates of Egypt. In another study, T. hamatum and T. album significantly reduced the occurrence of the plant pathogens, Rhizoctonia solani and F. solani and these treatments increased the frequency of Aspergillus spp. than A. niger, Penicillium spp., and Trichoderma spp. compared to the controls. (Abd-El-Khair and El-Nagdi, 2014). Eventually, two of the most serious potato diseases in Egypt, root-knot and potato brown rot could be controlled by several bacterial bio-control agents (BCAs) (Kabeil et al., 2016). Various factors such as soil depth, organic matter, porosity, oxygen and carbon dioxide concentrations and soil pH can influence the diversity and population levels of such BCAs in soil horizons (Bhattarai et al., 2015).
Conclusions and Recommendations
It could be concluded from the present study that Trichoderma viride or T. virens as single treatments inhibited M. incognita development and reproduction on potato and increased potato growth and yield parameters. Each fungal species achieved higher increases of these parameters than those obtained by combining a fungal species with PP. This study revealed that the total counts of aerobic bacteria, spore-forming bacteria, and fungi existing in potato rhizosphere increased with different treatments used herein. The antagonistic faunas as Aspergillus spp., Penicillium spp. and Trichoderma spp. may serve as bioagents against parasitic nematodes and other plant pathogens leading to their inhibition on potato plants.
Acknowledgements
This research work was supported in part by the In-house Project No. ١٢٠٥٠١٠٥ entitled “Pesticide alternatives against soilborne pathogens and pests attacking economically important Solanaceous crops” carried out by National Research Centre, Egypt.
Novelty Statement
The current study indicated that two isolates of the antagonistic fungi, Trichoderma viride and T. virens, when used sole or in combination with aquatic extract of pomegranate peel could control root-knot nematode, M. incognita on potato as well as increase plant growth and yield and biochemical substances, especially as sole treatment. Also, soil microorganisms’ diversity improved. These fungal isolates proved to be acted as a biocontrol agent within sustainable pest management.
Author’s Contribution
MMAY and WMAEN were equal in the design and execution of this experiment. MMAY work out and wrote the manuscript. HAEK isolated, reared and identified the tested fungal species. USEK carried out the experiment in the field. MMMAE provided the facilities during this work and reviewed the manuscript. MGD carried out biochemical analysis. All authors read and approved the final manuscript.
List of abbreviations
Tv: Trichoderma viride; Tvr: Trichoderma virens; FCA: Fungal bio-control agent; PP: Pomegarante peel; S: Sorghum (medium); CFU/ml: colony-forming unit per millilitre.
Conflict of interest
The authors have declared no conflict of interest.
References
Abd-Elgawad, M.M.M., 2020. Biological control agents in the integrated nematode management of potato in Egypt. Egypt. J. Biol. Pest Cont., 30: 121. https://doi.org/10.1186/s41938-020-00325-x
Abd-Elgawad, M.M.M., and Askary, T.H., 2018. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest. Cont., 28: 74. https://doi.org/10.1186/s41938-018-0080-x
Abd-El-Khair, H., and El-Nagdi, W.M.A., 2014. Field application of bio-control agents for controlling fungal root rot and root-knot nematode in potato. Arch. Phytopathol. Plant Prot., 47(10): 1218–1230. https://doi.org/10.1080/03235408.2013.837632
Abd-El-Khair, H., El-Nagdi, W.M.A., Youssef, M.M.A., Abd-Elgawad, M.M.M., and Dawood, M.G., 2019. Protective effect of Bacillus subtilis, B. pumilus, and Pseudomonas fluorescens isolates against root- knot nematode, Meloidogyne incognita on cowpea. Bull. Nat. Res. Centre, 43(64): 1-7. https://doi.org/10.1186/s42269-019-0108-8
Abd-El-Khair, H., El-Nagdi, Wafaa, M.A., and Hammam, M.M.A., 2018. Effect of olive and castor bean oil cakes singly or combined with Trichoderma spp. on Fusarium solani and Meloidogyne incognita infecting eggplant. Middle East J. Appl. Sci., 8(2): 465–473.
Akhtar, A., Abbasi, H., and Rushda, S., 2012. Antagonistic effects of Pseudomonas fluorescens and Bacillus subtilis on Meloidogyne incognita infecting Vigna mungo L. Intern. J. Plant Anim. Environ. Sci., 2(1): 55-63.
Bajaj, K.L., and Mahajan, R., 1977. Phenolic compounds in tomato susceptible and resistant to M. incognita (Kofoid et White) Chitwood. Nematol. Medit., 5: 329-333.
Barker, K.R., 1985. Nematode extraction and bioassays. In: An advanced treatise on Meloidogyne Vol. II. Methodology (Eds.) Barker, K.R., Carter, C.C., and Sasser, J.N. North Carolina State University Graphics, USA, pp. 19-35.
Barnett, H.L. and Hunter, B.B., 1972. Illustrated genera of imperfect fungi. Burgess Publ. Co., Minnesota, pp. 241.
Bhattarai, A., Bhattarai, B. and Pandey, S., 2015. Variation of soil microbial population in different soil horizons. J. Microbiol. Exp., 22(2): 75-78. https://doi.org/10.15406/jmen.2015.02.00044
Bridson, E.Y., 1995. The oxide manual 7th Ed., Published by Unipath Limited, Wade Koad, Basingstoke Hampshire, RG 248 PW, England.
Dahham, S.S., Ali, M.N., Tabassum, H., and Khan, M., 2010. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.). Am. Euras. J. Agric. Environ. Sci., 9: 273-281.
Dubois, M., Cilles, K.A., Hamilton, J., Rebers, R., and Smith, F.M., 1956. Colorimetric method of determination of sugars and related substances. Anal. Chem., 28: 350-356. https://doi.org/10.1021/ac60111a017
El-Anany, A.M., Abdel-Aziz, F., and Khafagy, E.Y., 2019. Potato cultivation and production. Technical issue No. 1376 (In Arabic), Central Administration of Agricultural Extension, Ministry of Agriculture, Egypt.
Ellis, M.B., 1971. Dematiaceous hyphomycetes. Commw. Mycol. Inst. Kew. Surrey, England.
El-Nagdi, Wafaa M.A., and Youssef, M.M.A., 2015. Nematicidal effect of some aqueous extracts of botanicals and a commercial bacterial byproduct for biocontrolling root knot nematode, Meloidogyne incognita infecting sugar beet. Sci. Agric., 10: 55-58. https://doi.org/10.15192/PSCP.SA.2015.10.2.5558
El-Nagdi, W.M.A., Youssef, M.M.A., Abd-El-Khair, H., Abd-Elgawad, M.M.M., and Dawood, M.G., 2019a. Effectiveness of Bacillus subtilis, B. pumilus, Pseudomonas fluorescens on Meloidogyne incognita infecting cowpea. Pak. J. Nematol., 37(1): 35-43. https://doi.org/10.18681/pjn.v37.i01.p35-43
El-Nagdi, W.M.A., Youssef, M.M.A., Abd El-Khair, H., and Abd-Elgawad, M.M., 2019b. Effect of certain organic amendments and Trichoderma species on the root-knot nematode, Meloidogyne incognita infecting pea (Pisum sativum L.) plants. Egypt. J. Biol. Pest Contr., 29(1): 1–9. https://doi.org/10.1186/s41938-019-0182-0
El-Nagdi, W.M.A., Youssef, M.M.A., Abd-El-Khair, H., Elkelany, U.S., Abd-Elgawad, M.M.M., and Dawood, M.G., 2023. Effect of integration of two bacterial bioagents and a plant residue extract for biocontrolling a root-knot nematode, Meloidogyne incognita infesting potatoes. Egypt. Pharma. J., 22: 67-77. https://doi.org/10.4103/epj.epj_119_22
Feyisa, B., Lencho, A., Selvaraj, T., and Getaneh, G. 2015. Evaluation of some botanicals and Trichoderma harzianum for the management of tomato root-knot nematode [Meloidogyne Incognita (Kofoid and White) Chitwood]. Adv. Crop Sci. Tech., 4: 201. https://doi.org/10.4172/2329-8863.1000201
Ghini, R.F., Patrico, R.A., Bettiol, W., de Almeida, M.G., and Maia, A.H.N., 2007. Effect of sewage sludge on suppressiveness to soil-borne plant pathogens. Soil Biol. Biochem., 39: 2797-2805. https://doi.org/10.1016/j.soilbio.2007.06.002
Giebel, J., 1982. Mechanism of resistance to plant nematodes. Ann. Rev. Phytopathol., 20: 257-279. https://doi.org/10.1146/annurev.py.20.090182.001353
Kabeil, S.A., Hammam, M.M.A., Mohamed, M.M.M., and Abd-Elgawad, M.M.M., 2016. Biocontrol of potato brown rot and root-knot diseases in Egypt. Egypt. J. Biol. Pest Cont., 26: 209-215.
Khan, M.R., Mohiddin, F.A., Ejaz, M.N., and Khan, M.M., 2012. Management of root-knot disease in eggplant through the application of biocontrol fungi and dry neem leaves. Turk. J. Biol., 36: 161–169. https://doi.org/10.3906/biy-1008-72
Korayem, A.M., Hasabo, S.A., and Ameen, H.H., 1993. Effects and mode of action of some plant extracts on certain plant parasitic nematodes. Anz. Schad. Pflanz., 66: 32-36. https://doi.org/10.1007/BF01909140
Korayem, A.M., Mohamed, M.M.M., Noweer, E.M.A., Abd-El-Khair, H., and Hammam, M.M.A., 2019. Occurrence of nematode-antagonistic fungi and bacteria associated with phytonematodes in the rhizosphere of wheat grown in different governorates of Egypt. Plant Arch., 19 (Supplement 2): 780-787.
Mansour, N.A., El-Dafrawi, M.E., Tappozada, A., and Zeid, M.I., 1966. Toxicological studies on the Egyptian cotton leaf worm, Proedenia litura. Potentiation and antagonism of organophosphorus and carbaamate insecticides. J. Econ. Entomol., 59: 307-311. https://doi.org/10.1093/jee/59.2.307
Middleton E., Kandaswami, C., and Theoharides, T.C., 2000. The effects of plant flavonoids on mammalian cells: Implication for inflammation, heart disease and cancer. Pharmacol. Rev., 52: 673-681.
Monte, E., 2001. Understanding Trichoderma between biotechnology and microbial ecology. Int. Microbiol., 4: 1-4.
Moradi, R., Moradi, F., Mirehki, K., and Abdollahi, M., 2015. Plant debris of oak forest as soil amendment, to improve the biocontrol activity of Pseudomonas fluorescens and Trichoderma vierns against Meloidogyne javanica, in tomato. J. Crop Prot., 4(3): 373–384.
Moran, R., 1982. Formulae for determination of chlorophyllous pigments extracted with N, N dimethylformamide. Plant Physiol., 69: 1376–1381. https://doi.org/10.1104/pp.69.6.1376
Narayana, Y.D., and Reddy, D.D.R., 1980. The role of nitrogen amino acids and phenols in resistance of tomato to root knot-nematodes. Nematol. Medit., 8: 51-52.
Niere, B., and Karuri, H.W., 2018. Nematode parasites of potato and sweet potato. In: Sikora, R.A., Coyne, D., Hallmann, J., and Timper, P. (eds) Plant parasitic nematodes in subtropical and tropical agriculture. CAB International, Boston, USA, pp. 222-251. https://doi.org/10.1079/9781786391247.0222
Olabiyi, T.I., and Gbadamosi, A.R., 2013. The effect of four compost soil amendments based on Trichoderma harzianum on nematode pests of sesame. Int. J. Agro. Plant Prod., 4(5): 3859–386.
Osman, H.A.I., Ameen, H.H., Hammam, M.M.A., El-Sayed, G.M., Elkelany, U.S., and Abd-Elgawad, M.M.M., 2022. Antagonistic potential of an Egyptian entomopathogenic nematode, compost and two native endophytic bacteria isolates against the root-knot nematode (Meloidogyne incognita) infecting potato under field conditions. Egypt. J. Biol. Pest Cont., 32: 137. https://doi.org/10.1186/s41938-022-00635-2
Prashanth, D.J., Asha, M.K., and Amit, A., 2001. Antibacterial activity of Punica granatum. Fitoterapia, 72: 171-173. https://doi.org/10.1016/S0367-326X(00)00270-7
Puntener, W., 1981. Manual for field trials in plant protection paste. Agric. Div. Ciba-Geigy limited, Swizerland, pp. 205.
Regaieg, H., Boujila, M., Hajji, L., Larayadh, A., Chihini, N., Guessmi-Mzoughi, I., and Horrigue-Raouani, N., 2017. Evaluation of pomegranate (Punica granatum L. var. Gabsi) peel extract for control of root-knot nematode Meloidogyne javanica on tomato. Arch. Phytopathol. Plant Prot., 50: 839-849. https://doi.org/10.1080/03235408.2017.1396721
Richer, D.L., 1987. Synergism, a patent view. Pest Sci., 19: 309-315. https://doi.org/10.1002/ps.2780190408
Sikandar, A., Zhang, M., Wang, Y., Zhu, X., Liu, X., Fan, H., Xuan, Y., Chen, L., and Duan, Y., 2020. In vitro evaluation of Penicillium chrysogenum Snef1216 against Meloidogyne incognita (root-knot nematode). Sci. Rep., 10: 8342. https://doi.org/10.1038/s41598-020-65262-z
Snedecor, G.W., and Cochran, W.G., 1989. Statistical methods. 8th ed. Iowa State University Press, Ames, Iowa.
Snell, F.D., and Snell, C.T., 1953. Colorimetric method. Vol. III, Van Nostrand Company, London, pp. 606.
Šunjka, D., and Mechora, Š., 2023. Advances in alternative measures in plant protection. Plants, 12: 805. https://doi.org/10.3390/plants12040805
Youssef, M.M.A., Abd-El-Khair, H., and El-Nagdi, W.M.A., 2017. Management of root knot nematode, Meloidogyne incognita infecting sugar beet as affected by certain bacterial and fungal suspensions. Agric. Eng. Int. CIGR J., Special issue: 293–301.
Youssef, M.M.A., El-Nagdi, Wafaa M.A., and Eissa, M.F.M., 2014. Population density of root knot nematode, Meloidogyne incognita infecting date palm under stress of aqueous extracts of some botanicals and a commercial bacterial byproduct. Middle East J. Appl. Sci., 4(4): 802-805.
Youssef, M.M.A., El-Nagdi, W.M.A., and Lotfy, D.E.M., 2020. Evaluation of the fungal activity of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus as biocontrol agents against root-knot nematode, Meloidogyne incognita on cowpea. Bull. Nat. Res. Centre, 44: 112. https://doi.org/10.1186/s42269-020-00367-z
To share on other social networks, click on any share button. What are these?




