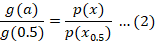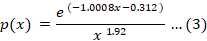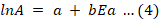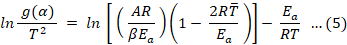Isoconversional Thermal Analysis of Cydonia oblonga Mucilage Composite Wound Dressing
Research Article
Aniqa Nasir1, Rashid Masih1, Shazma Massey1*, Laiba Arshad2, Sabi Ur Rehman2, Irva Waqar1 and Anwar Khalid3
1Department of Chemistry, Forman Christian College (A Chartered University), Lahore 54600, Pakistan; 2Department of Pharmacy, Forman Christian College (A Chartered University), Lahore 54600, Pakistan; 3Department of Chemistry, COMSATS University Islamabad, Abbottabad 22060, Pakistan.
Abstract | Stability and shelf life of commodities are crucial aspect in product development in the field of pharmaceutics, food, and other consumer goods. In today’s world, non-adherent bandages are essential for reducing the pain and discomfort of people after surgery. In the present study, Cydonia oblonga mucilage was used to prepare non-adherent wound dressing and its stability is determined by thermal analysis by using advanced isoconversional method. Isoconversional method is used to determine kinetic triplet including activation energy, pre-exponential factor and mechanism of thermal degradation. The prepared dressings were subjected to thermal analysis. Thermogravimetric analysis showed that the degradation of wound dressing occurs at 225˚C - 400˚C. Thus, the dressing was found to be stable up to wide ranges of temperature. Kinetics of thermal degradation was also studied by model-free isoconversional analysis. Activation energy, pre-exponential factor and decomposition mechanism model was determined by Starink method. The average activation energy of Cydonia oblonga wound dressing with and without drug was 111 kJ mol-1and 103 kJ mol-1 respectively and the pre-exponential factor values are 236751615 s-1 and 49975394 s-1 respectively. The wound dressing shows a distinct behavior of decomposition. Decomposition of wound dressing with drug followed two-dimensional diffusion mechanism model and wound dressing without drug followed three-dimensional diffusion mechanism. The value of kinetic parameters of wound dressing shows that both dressings with and without drug are stable but if these are compared with one another then with drug wound dressing is more stable as its activation energy is high.
Received | October 29, 2023; Accepted | November 23, 2023; Published | December 07, 2023
*Correspondence | Shazma Massey, Department of Chemistry, Forman Christian College (A Chartered University), Lahore 54600, Pakistan; Email: [email protected]
Citation | Nasir, A., R. Masih, S. Massey, L. Arshad, S.U. Rehman, I. Waqar and A. Khalid. 2023. Isoconversional thermal analysis of Cydonia oblonga mucilage composite wound dressing. Pakistan Journal of Agricultural Research, 36(4): 318-326.
DOI | https://dx.doi.org/10.17582/journal.pjar/2023/36.4.318.326
Keywords | Isoconversional analysis, Wound dressing, Thermal analysis, Activation energy, Starink method
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Cydonia oblonga (CO) is locally known as a common quince (Sharma et al., 2007). Its different parts are reported to be an ideal material to progress in many fields because of its wide range of pharmacological (antibacterial, anti-fungal, hepatoprotective, anti-diarrheal, cardiovascular, diuretic, anti-inflammatory, hypolipidemic, antidepressant, and hypoglycemic (Vaugham and Geissler, 1997; Kurian and Sankar, 2007; Rop et al., 2011; Ashraf et al., 2016), industrial (super-disintegrant, suspending agent, gelling agent, binder) and clinical (wound healing property) applications (Jaladat et al., 2015; Usher, 1974; Komarov, 1968). In order to make its mucilage more useful for the development in variety of applications, it is important to study its thermal stability and degradation behavior at different temperature ranges. Different approaches exist for investigating thermal effects, with thermo-gravimetric analysis (TGA) and differential scanning calorimetry (DSC) standing out as the prevailing thermo-analytical methods. Isoconversional thermal analysis is a popular technique these days to find out the activation energy and stability of food and pharmaceutical products.
Niu et al. (2017) isolated a novel heteropolysaccharide (PMH) from the seed husks of Plantago asiatica. Their findings indicated that PMH possesses thermal stability, a unique structural composition, and antioxidant properties, making it a promising candidate for use in functional foods. Gözke and Aciklin (2021) conducted a study on the pyrolysis properties of sour cherry stalk and flesh using non-isothermal thermo-gravimetric analysis at various heating rates. Their findings revealed that the particle size of the biomass and the obtained activation energy values play a significant role in pyrolysis kinetics. Moreover, this research suggested that sour cherry stalk and flesh could serve as a renewable resource for alternative energy production. Iqbal et al. (2013) had determined isoconversional thermal analysis of hydrogels from functional food for their application in pharmaceutical industry and results revealed that all the materials appear to be as stable as some of the important commercial materials used as pharmaceutical ingredients. Isoconversional methods will also be important for biomedical applications (Nosheen et al., 2021). A careful survey of literature reveals that no thermal degradation and stability study has been reported till to date on CO mucilage and its wound dressings.
In a previously published paper on wound healing, a bandage for wound healing was prepared, characterized, and reported in a publication (Massey et al., 2022) that was less painful while removing it. The patients used to bear a lot of pain on removal of the previously commercially used bandages and sometimes the patient is given anesthesia to remove the bandage so that they may not face the painful process. As compared to the previously used bandages, the newly prepared bandage could easily be removed. Surgical dressings and wound bandages are affected by varying temperature and relative humidity and moisture (Thomas, 2012). In this paper basically the effect of varying temperature on different parameters is considered. However, the gap was identified that there was a need to determine stability and the shelf life of that bandage. In this paper basically the effect of varying temperature on different parameters is considered. So that gap has been covered and reported in this paper. The thermal characterization analysis offers improved insights into the decomposition of polysaccharide films and provides a means to assess their stability by determining activation energy. The highest activation energy Ea is an indicator of the higher thermal stability (Jamaludin et al., 2017).
To determine the stability of CO wound healing bandages, isoconversional method is used, which demonstrate thermal behaviors and thermal decomposition kinetics of wound dressings. The study in this paper focuses on in-depth study of thermal degradation profile i.e., activation energy (Ea.), pre-exponential factor (A) and degradation mechanism model g(α) also known as kinetic triplet of the CO mucilage wound dressing obtained from plant seeds. These methods are accurate and considered to be the quickest way to find out kinetic parameters for complex reactions. By their use, it has become possible to predict the life expectancy of solid materials under isothermal conditions. TGA-DSC data obtained at multiple heating rates is helpful for the determination of activation energy, thermal stability, kinetic triplet, thermally activated reactions and shelf life using model based and model free isoconversional thermal methods. In Model-based methods, conversions (α) are reaction dependent and vary significantly in their consistency and reliability. Numerous model-free isoconversional methods (Simon, 2004) have also been reported for the determination of Ea from TGA-DSC data that includes Flynn-Wall-Ozawa (FWO) method (Niu et al., 2017; Mothe and Freitas, 2018), Kissinger-Akahira-Sunosei (KAS) method and Starink (Starink, 2003).
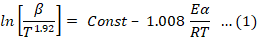
In this paper Starink method is used for the determination of Ea being the most accurate of all above methods. The Ea in Starink method is determined from the slope of straight-line plot between ln(β/ T1.92) and 1/T. Starink equation (Equation 1) is an optimized form of KAS equation.
This method is newly derived and optimized, and its accuracy is higher than other methods. The Flynn-Wall-Ozawa method is highly inaccurate and shows around 10% deviation in activation energy while Starink method shows less than 1% deviation in activation energy.
Materials and Methods
Material
CO seeds were procured from a local market in Lahore, Pakistan. Chemicals utilized were of analytical grade, chitin (Sigma-Aldrich), and glutaraldehyde (Sigma-Aldrich), and acetic acid (DAEJUNG). Ciprofloxacin was provided by the Department of Pharmacy at Forman Christian College (A Chartered University) Lahore, Pakistan. Distilled water was used throughout this research work.
Preparation of silver nanoparticles
Silver nanoparticles were prepared by Turkevich method (Massey et al., 2022; Rashid et al., 2013). 50 mL of AgNO3 (0.001iM) heated to boiling. Then, 5mL trisodium citrate (1%) was added dropwise into it. The solutions were stirred vigorously and heated until the color changed from colorless to pale yellow. After color change, the silver nanoparticle solution was cooled and recorded on UV/Visible spectrophotometer (UV-1800, Shimadzu, Japan).
Preparation of wound dressings
For the preparation of wound dressings, lyophilization technique was used. Different protocols were followed for preparing composite dressings and after optimization, the finest one was selected, which was prepared by the following method.
Chitin solution (1%) was prepared in acetic acid (1%) and stirred for 7 hours at 25℃. Subsequently, a measured quantity of CO was gently introduced into the solution while maintaining constant stirring to achieve thorough mixing and create a homogenized mixture. Following this, silver nanoparticles were incorporated into the reaction mixture and stirred continuously. After that, glutaraldehyde (1%) solution was added dropwise as crosslinking agent under constant and fast stirring by using homogenizer. Ultimately, the blend was transferred into petri dishes, frozen overnight, and subsequently subjected to lyophilization at -40oC for a duration of 48 hours. Water is usually removed from perishable products by a process called lyophilization, which prolongs the shelf life of materials. Lyophilized items are more stable than other products and are less prone to deteriorate or lose their qualities over time.
To prepare drug-loaded composite dressings, a 0.0005% ciprofloxacin drug solution was introduced into the aforementioned reaction mixture, followed by lyophilization using a freeze dryer. Then the dried wound dressing was subjected to thermal analysis.
Characterization
Thermogravimetric analyzer: Thermal analysis of composite wound dressings was performed on SDTQ600 (TAInstruments, USA) at different heating rates.
Thermogravimetric analysis
The composite wound dressing was analyzed at 5, 10, 15, 20oC min-1 heating rates from ambient to 650oC under nitrogen atmosphere (100cm3 min-1) by using alumina crucibles. TGA scans were obtained, and data was analyzed using Universal analysis 2000 software, version 4.2E (TA Instruments, USA), MS Excel® 2013 and isoconversional methods.
Isoconversional analysis
Isoconversional thermal analysis was performed using the data at multiple heating rates. To calculate Ea, A, g(α) and stability of composite wound dressings, isoconversional analysis was used along with Starink equation as it was fast and convenient.
Thermal degradation kinetics
In this study, we examined the thermal degradation kinetics of composite wound dressings made from CO using the optimized Starink method. Degradation mechanism, denoted as g(α) was determined through the utilization of the master plot method as described in Equation 2. In this method, straight-line plots were generated by plotting the ln (β/T1.92]) vs. 1/T with a slope parameter (B) equal to 1.92. From the slope of these plots, the Ea values were calculated.
Where, x represents Ea/RT, p(x) = ∫x∞ e-x/x2 dx, p (x0.5) and g (0.5) experimental and theoretical decomposition models at conversion α=0.5, respectively. The experimental and theoretical master plots (Table 1) were calculated by plotting p(x)/p(x0.5) and g(α)/g (0.5) verses α. P(x) integral was calculated by using Starink Equation 3.
Pre-exponential factor A was obtained by use of Equation 4.
In Equation 4 a and b are the compensation effect relationship parameters, which were calculated by using Coats-Redfern Equation 5 (Simon, 2004).
In Equation 5, T̅ is the average temperature. The g(α) models were used to solve Equation 5 from the plot of g(α)/T2 verses 1/T, a set of kinetic parameters, A and Eα is obtained. From the straight-line plots, the parameters a and b were calculated from the intercept and slope, between lnA and Eα values obtained from Equation 4.
Results and Discussion
The TGA thermograms of wound dressings with and without drug at 5, 10, 15 and 20˚C min-1 heating rates are shown in Figures 1 and 2. The major decomposition can be observed in the range of 220-405oC apparently in single step, while it may not be definitively true, this can be confirmed through isoconversional analysis, in which the Starink method is employed. Isoconversional methods are useful for determining kinetic triplets, comprising activation energy (Ea), pre-exponential factor (A), and the decomposition model g(α), which aids in assessing thermal stability. Ea can be derived from TGA data at various heating rates, calculated from the Arrhenius plot’s slope (Figures 5 and 6).
The average activation energy for the primary decomposition stages of CO mucilage wound dressing, determined using the Starink method, was found to be 103 kJ mol-1, while the average activation energy for wound dressing with a drug was 111 kJ mol-1 (Table 2). The Ea value for the wound dressing containing ciprofloxacin drug is 111 kJ mol-1, indicating greater stability compared to the drug-free wound dressing. The Starink method determines the activation energy (Eα) at various conversions (α). If activation energy remains consistent with changes in conversion (α), the decomposition is termed a single step. Conversely, if activation energy varies, it signifies a multi-step process. The gradual change in Ea was observed all over the conversion (α). This suggests that thermal degradation is a multi-step process. TGA data provided temperature vs. conversion (T-α) plots, as shown in Figures 3 and 4, illustrating that conversion is influenced by the heating rate. Decomposition mechanism models g(α) were
Table 2: Kinetic triplet data of major decomposition stage of wound dressings obtained from Cydonia oblonga mucilage.
|
Starink method |
Ea kJ (mol-1) |
g(α) |
A (s-1) |
|
Wound dressing |
103 kJ mol-1 |
[(1+α)⅓-1]² three-dimensional diffusion |
49975394 s-1 |
|
Wound dressing with drug |
111 kJ mol-1 |
α+(1-α)ln(1-α) two-dimensional diffusion |
236751615s-1 |
determined through master plots constructed from experimental data, offering the best correlation between g(α)/g (0.5) and α. The experimental master plots were independent of heating rates, confirming that g(α) varies with α without any effect of heating rates. A of the decomposition stages were determined by compensation effect relationship and Coats-Redfern equation. Although activation energy and reaction kinetics are provided by isoconversional methods, but they don’t give comprehensive insight into the intermediate species involved in the process. It takes experience to analyze the intricate data from isoconversional method.
Starink method is an optimized form of Kissinger-Akahira-Sunose method (KAS). In this method slope of plots ln(β/ T1.92) vs.1/T (Figures 5 and 6) was used to determine activation energy. The plots of Ea vs. α (Figures 7 and 8) shows multi-steps degradation of both wound dressings. For determination g(α) model plots of g(α) /g(0.5) vs. α (Figures 9 and 10) were constructed. The results showed that decomposition of Cydonia oblonga wound dressing followed three-dimensional diffusion [(1+α)1/3-1]2 mechanism and wound dressing with drug followed two-dimensional diffusion α+(1-α)ln(1-α) (Table 1). The value of A determined by Starink method for CO wound dressing was 49975394 s-1 and for wound dressing with drug was 236751615 s-1. The A and
g(α) models of major decomposition stages are given in Table 2. The high value of pre-exponential factor 236751615 s-1 which shows that the frequency of molecules collision for wound dressing with drug is higher as compared to the other wound dressing of CO mucilage. It means that the wound dressing with drug is more stable and long lasting. The kinetic parameters of wound dressings under investigation show that they can be used for applications where greater thermal stability is required.
The expression [(1+α)⅓ - 1]² is used to describe the rate of reaction in systems where three-dimensional diffusion plays a significant role. Three-dimensional diffusion is associated with reactions occurring in three dimensions of space, which can be important in systems with diffusion-limited reactions, for example, in certain catalytic reactions.
In the context of decomposition mechanism models, such as g(α), α, represents the extent of the chemical reaction. It’s value ranges from 0 (indicating no reaction has occurred) to 1 (indicating complete reaction or full conversion of reactants into products). It is a measure of how much the reaction has progressed (1 - α): This represents the fraction of the reactant that has not yet reacted.
In the context of a decomposition mechanism model, the expression α + (1 - α)ln(1 - α) represents a mathematical equation used to describe the kinetics of a decomposition reaction, specifically in the context of two-dimensional diffusion. Two-dimensional diffusion is relevant when chemical reactions occur in a system with surfaces, interfaces, or constrained dimensions, such as in thin films or on surfaces. In these cases, the movement of reactant molecules is confined to two dimensions, and the way they encounter each other can affect the reaction rate. Even the two-dimensional diffusion mechanism also proves that the wound healing bandage with drug is better. Generally, different diffusion mechanisms in wound dressings can significantly impact drug release rates, which in turn affects the healing process. For instance, a faster diffusion rate might be beneficial for rapidly treating acute wounds, whereas a slower, more controlled release could be advantageous for chronic wounds that require sustained medication delivery (Catanzano et al., 2021). Recent studies have shown advances in wound dressings, especially for diabetic wound healing (Niu et al., 2023). Research suggests that thermosensitive and antioxidant wound dressings that modulate TGFβ pathways may significantly improve diabetic wound healing outcomes. It is observed that these dressings reduce oxidative stress in diabetic wounds by decreasing ROS and improving antioxidant capacity, even without PTβR2I in the hydrogel. Even in diabetic wounds with high glucose levels, such dressings promote keratinocyte, fibroblast, and endothelial cell migration and proliferation. (Hawthorne et al., 2021).
The Eα, A and g(α) models of major decomposition stages prove that both wound dressings with and without drug are thermally stable and have good shelf life but in thermal stability wound dressing with drug is superior. In the context of stability and shelf life, knowing the activation energy allows you to predict how quickly degradation reactions will take place. Products with higher activation energies are more stable and have longer shelf lives because they deteriorate more slowly.
Both wound dressings with and without drug were found to be physically and chemically stable even after three years of storage at room temperature (25oC). No change in appearance was observed. Repeating the TGA after three years, same activation energy and pre-exponential factors were determined. The stability of wound dressings, indicated by activation energy, is a critical factor in their effectiveness. Dressings with higher activation energies are generally more stable and can maintain their integrity and therapeutic effectiveness over a longer period, which is especially important in settings where long-term storage is necessary. For dressings containing drugs, the stability also impacts the drug’s efficacy. A stable dressing ensures that the drug is released at a consistent rate, maintaining its therapeutic levels over the required duration. This can be particularly beneficial in treating chronic wounds where prolonged drug delivery is crucial.
Conversely, dressings without drugs, while not needing to account for drug release kinetics, still require stability to maintain their physical properties and protective functions over time. These dressings might be more suitable in situations where the primary requirement is physical wound protection or moisture maintenance, rather than active drug delivery.
Conclusions and Recommendations
Activation energy, pre-exponential factor and decomposition mechanism model of wound dressing isolated from CO mucilage was studied by isoconversional methods. The change in Ea values at different degree of conversion showing that the degradation of wound dressing is taking place in multi steps. The Ea values suggest that the dressing with drug is highly stable. Decomposition of wound dressing follows three-dimensional diffusion and wound dressing with drug follow two-dimensional diffusion model. This study shows that the wound dressing of mucilage with and without drug follows different decomposition mechanisms. When ciprofloxacin-loaded wound dressing is compared to CO mucilage-free wound dressing, the average Ea and pre-exponential factor are higher. This indicates that the drug-containing wound dressing is more stable, has a longer shelf life, and can be used to create commercial bandages that do not adhere to the wound.
Acknowledgement
The authors are thankful to Department of Chemistry, Forman Christian College (A Chartered University) Lahore for thermal analysis.
Novelty Statement
Activation energy and thermal stability of non-adherent wound dressing is determined by a Starink method.
Author’s Contribution
Aniqa Nasir: Performed experimental work, literature review, and wrote initial draft of manuscript.
Rashid Masih: Performed thermal analysis, helped in manuscript writing, calculations, drawing graphs and analyzing data.
Shazma Massey: Main concept of the study, supervised and coceptualized the work, analyzed the results and reviewed the manuscript.
Laiba Arshad: Helped in write up and validated the data.
Sabi Ur Rehman and Irva Waqar: Helped in write up and analyzed the data.
Anwar Khalid: Conducted literature review and helped in write up.
The authors have declared no conflict of interest.
References
Ashraf, M.U., G. Muhammad, M.A. Hussain and S.N. Bukhari. 2016. Cydonia oblonga M., a medicinal plant rich in phytonutrients for pharmaceuticals. Front. Pharmacol., 7: 163. https://doi.org/10.3389/fphar.2016.00163
Catanzano, O., F. Quaglia and J.S. Boateng. 2021. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert. Opin. Drug Deliv., 18(6): 737-759. https://doi.org/10.1080/17425247.2021.1867096
Gözke, G. and K. Açıkalın. 2021. Pyrolysis characteristics and kinetics of sour cherry stalk and flesh via thermogravimetric analysis using isoconversional methods. J. Therm. Anal. Calorim., 146(2): 893-910. https://doi.org/10.1007/s10973-020-10055-9
Hawthorne, B., J.K. Simmons, B. Stuart, R. Tung, D.S. Zamierowski and A.J. Mellott. 2021. Enhancing wound healing dressing development through interdisciplinary collaboration. J. Biomed. Mater. Res., 109(12): 1967–1985. https://doi.org/10.1002/jbm.b.34861
Iqbal, M.S., S. Massey, J. Akbar, C.M. Ashraf and R. Masih. 2013. Thermal analysis of some natural polysaccharide materials by isoconversional method. Food Chem., 140(1-2): 178-182. https://doi.org/10.1016/j.foodchem.2013.02.047
Jaladat, A.M., F. Atarzadeh, H. Rezaeizadeh, B. Mofid, A. Mosalaie, F. Farhan and G. Amin. 2015. Botanicals: An alternative remedy to radiotherapy-induced dysuria. Complement. Ther. Med., 23(1): 90-99. https://doi.org/10.1016/j.ctim.2014.11.004
Jamaludin, J., F. Adam, R.A. Rasid and Z. Hassan. 2017. Thermal studies on Arabic gum-carrageenan polysaccharides film. Chem. Eng. Res. Bull., 1: 19. https://doi.org/10.3329/cerb.v19i0.33800
Komarov, V.L., 1968. Asparagus L. Flora of the USSR. Pp. 325-339.
Kurian, A., and M.A. Sankar. 2007. Medicinal plants (Vol. 2). New India Publishing.
Massey, S., F. Iqbal, A.U. Rehman, M.S. Iqbal and F. Iram. 2022. Preparation, characterization and biological evaluation of silver nanoparticles and drug-loaded composites for wound dressings formed from Lallemantia royleana seeds’ mucilage. Biomater. Sci. Polymer. Ed., 33(4): 481-498. https://doi.org/10.1080/09205063.2021.1992590
Mothé, C.G. and J.S. de Freitas. 2018. Lifetime prediction and kinetic parameters of thermal decomposition of cashew gum by thermal analysis. J. Therm. Anal. Calorim., 131: 397-404. https://doi.org/10.1007/s10973-017-6844-9
Niu, H., Y. Guan, T. Zhong, L. Ma, M. Zayed and J. Guan. 2023. Thermosensitive and antioxidant wound dressings capable of adaptively regulating TGFβ pathways promote diabetic wound healing. NPJ Regen. Med., 8(1): 32. https://doi.org/10.1038/s41536-023-00313-3
Niu, Y., N. Li, S. Alaxi, G. Huang, L. Chen and Z. Feng. 2017. A new heteropolysaccharide from the seed husks of Plantago asiatica L. with its thermal and antioxidant properties. Food Funct., 8(12): 4611-4618. https://doi.org/10.1039/C7FO01171G
Nosheen, S., M. Irfan, S.H. Abid, Q. Syed, F. Habib, A. Asghar, B. Waseem, B. Soomro, H. Butt and M. Akram. 2021. A review: Development of magnetic nano vectors for biomedical applications. GSC. Adv. Res. Rev., 8(2): 85-110. https://doi.org/10.30574/gscarr.2021.8.2.0169
Rashid, M.U., M.K.H. Bhuiyan and Quayum ME. 2013. Synthesis of silver nanoparticles (Ag-NPs) and their uses for quantitative analysis of vitamin C tablets. Dhaka Univ. J. Pharm. Sci., 12: 29–33. https://doi.org/10.3329/dujps.v12i1.16297
Rop, O., J. Balik, V. Řezníček, T. Juríková, P. Škardová, P. Salaš and D. Kramářová. 2011. Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech J. Food Sci., 2: 65-73. https://doi.org/10.17221/212/2009-CJFS
Sharma, P.C., V. Bhatia, N. Bansal and A. Sharma. 2007. A review on Bael tree. Nat. Prod. Radiance. 6(2): 171-178.
Simon, P., 2004. Isoconversional methods fundamentals, meaning and application. J. Therm. Anal. Calorim., 76: 123–132. https://doi.org/10.1023/B:JTAN.0000027811.80036.6c
Starink, M.J., 2003. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta, 404(1-2): 163-176. https://doi.org/10.1016/S0040-6031(03)00144-8
Thomas, S., 2012. The effect of the weather and other environmental factors on the performance of surgical dressings. Wounds: Compend. Clin. Res. Pract., 24(12): 335-338.
Usher, G., 1974. A dictionary of plants used by man. Constable and Company Ltd.
Vaugham, J.G. and C.A. Geissler. 1997. The new oxford book of food plants. Oxford University Press.
To share on other social networks, click on any share button. What are these?