Isolation, Purification and Application of Siderophore Producing Bacteria to Improve Wheat Growth
Research Article
Isolation, Purification and Application of Siderophore Producing Bacteria to Improve Wheat Growth
Shabana Ehsan1*, Aneela Riaz2, Muhammad Amjad Qureshi1, Abid Ali1, Ifra Saleem3, Muhammad Aftab3, Khalid Mehmood4, Fakhar Mujeeb1, Muhammad Asif Ali1, Hina Javed1, Fraza Ijaz1, Anwar-ul-Haq5, Khaliq-ur-Rehman3 and M. Usman Saleem4
1Soil Bacteriology Section, Ayub Agricultural Research Institute, Faisalabad, Pakistan; 2Soil and Water Testing Lab, Nankana Sb, Pakistan; 3Institute of Soil Chemistry and Environmental Sciences, Ayub Agricultural Research Institute, Faisalabad, Pakistan; 4Soil and Water Testing Lab, Toba Tek Singh, Pakistan; 5Soil and Water Testing Lab, Pakpatan, Pakistan.
Abstract | Iron (Fe), being an essential micronutrient, is necessary for human health and to maintain the integrity and development of the plant. In Fe-limiting conditions, plants and plant growth-promoting rhizobacterial (PGPR) have a siderophore production mechanism. Inoculation with seed soaking of such siderophore-producing bacteria can be a cost-effective biofortification technique. The current study includes the collection of rhizobacterial isolates from wheat, maize, sorghum, millet, and maize rhizosphere soil of Rawalpindi and Sargodha divisions. The screening of bacterial isolates for siderophore production through CAS-shuttle assay (quantitative) and CAS-agar (qualitative) was done. Isolates were further characterized for Fe and phosphorus solubilization, indole acidic acid (IAA) equivalents, and organic acid production. The growth chamber and field study was planned to evaluate the effectiveness of these isolates on the growth and yield parameters of wheat. Total bacterial isolates were 50, out of which 15 isolates were found significantly positive for the production of siderophore and solubilizing of nutrients. The (SPS10) produced a comparatively high percentage of 46.2 % siderophore units, as shown by results between positive isolates. Out of 15 positive, 7 isolates significantly improved root/shoot growth over control in the growth chamber study. Inoculation with siderophore-producing bacteria showed a significant increase in plant height, grain yield, spike length, grain weight, no. of tillers plant-1, and wheat quality in a field trial. The results from the current study proposed that in the plant, rhizobacteria can also play a beneficial role in nutrient translocation to plants efficiently and nutrients uptake from the soil insoluble form.
Received | November 03, 2021; Accepted | June 06, 2022; Published | June 28, 2022
*Correspondence | Shabana Ehsan, Soil Bacteriology Section, Ayub Agricultural Research Institute, Faisalabad, Pakistan; Email: [email protected]
Citation | Ehsan, S., A. Riaz, M.A. Qureshi, A. Ali, I. Saleem, M. Aftab, K. Mehmood, F. Mujeeb, M.A. Ali, H. Javed, F. Ijaz, A. Haq, K. Rehman and M.U. Saleem. 2022. Isolation, purification and application of siderophore producing bacteria to improve wheat growth. Pakistan Journal of Agricultural Research, 35(2): 449-459.
DOI | https://dx.doi.org/10.17582/journal.pjar/2022/35.2.449.459
Keywords | Biofortification, Siderophore producing bacteria, Wheat, Field study
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Micronutrients, especially iron and zinc, are an important part of the food chain. Although these are needed in very small quantities, their very little use in dietary foods is the cause of serious health issues (Ramzan et al., 2020; Hussan et al., 2022). It has been reported by Riaz et al. (2021) that Fe, an integral part of chlorophyll, plays a substantial role in the plant through physiological and biochemical pathways and improves human and livestock health. The gap between demand and availability of the soluble form of Fe by plants are the primary causes of Fe deficiency which is linked with malnutrition. Injudicious use of mineral fertilizers, improper agronomic practices, and soil erosion are the leading causes of deficiency of micronutrients in Pakistan. Such practices also limit the nutrient mobilization of iron and phosphorus in calcareous and alkaline soils. The low biological activity of iron in well-aerated soils is due to the abundance of insoluble ferric iron compounds even at neutral pH levels (Gyana, 2015). In Pakistan, Zulfiqar et al. (2020) revealed that Pakistani soils are iron (Fe) deficient and approximately 30% are calcareous because of bicarbonates and high pH. To improve malnutrition in a micronutrient, Kaur et al. (2020) stated that biofortification of nutrients using microbes is the most efficient, emerging, and cost-effective approach in wheat (Sun et al., 2021). Anwar et al. (2022) introduced fortification, dietary supplementation, and food-diversification approaches to overcome Fe malnutrition. Previously, for the development of biofortified crops, molecular breeding and foliar spray has been employed. Still, in cereal crops, the use of siderophore producing microbes for effective uptake of iron (Fe) and efficient translocation of iron is assured need to integrate.
Siderophores (SP) produced by PGPR are helpful in the fortification of iron (Fe) in cereal crops. Furthermore, Khalid et al. (2015) stated the functions of siderophore (SP), which include improvement in fertility of the soil, crop growth, and good health of plants. In addition, inoculation of crop plants with various strains of (PGPR) enhances not only the quality of different medicinal plants such as spice crops (Sahay and Patara, 2014) but also improves the yield of the crop (Kumar et al., 2013). Zhang et al. (2020) has observed that the translocation and mobilization of iron (Fe) in plants frequently happens through; (i) in the form of free ions (Fe+2) of iron, and/or (ii) Fe chelation process along with Phyto-siderophores (PS). The rhizobacteria are valuable soil microorganisms naturally occurring in the soil rhizosphere. Mushtaq et al. (2021) stated that rhizobacteria play crucial roles in plants, such as enhancing the nutrient use efficiency (NUE), improving development, and increasing plant growth (Mehmood et al., 2018), which increase the crop yield and eventually improves the physiological process in plants. Scientists demonstrated that there are two categories of plants i.e., non-gramineous and gramineous plants. The non-graminaceous used a reduction-based strategy to solubilize insoluble form of iron (Fe) by phenolic compounds from roots and proton extrusion by roots that reduce the ferric ions (Fe3+) to a soluble form of ferrous ions (Fe2+) (Nathiya et al., 2020). While gramineous plants employed a siderophore chelation-based strategy which after complex with insoluble iron form siderophore ferric ions (PS–Fe3+) soluble complex taken up by plants with high-affinity uptake system.
There are also two major transport proteins genes observed by (Boukhalfa and Crumbliss, 2002), (1) the Ferric reduction oxidase gene (FRO2), and (2) the Iron-regulated transporter gene (IRT1). Both are engaged in ferric chelates reduction and absorption of ferric chelates across the membrane of root plasma. On the other hand, the main form of phytosiderophores (PSs) excretes via plants that have a strong affinity for nicotianamine (NA) form and Fe (III) aremugineic acids (MA) (Boukhalfa and Crumbliss, 2002). However, excellent siderophore (SP) producers i.e., Azotobactor, Pseudomonas, and Bacillus spp. which perform vital roles in boosting the growth of plants. They solubilized Fe and phosphate using increased nitrogen (N) and potassium (K) uptake. Chaudhary et al. (2017) observed variability in the amount of siderophores production in soil with environmental conditions.
Wheat is as major staple food contributing to more than 50% of the diet. The concentration of iron is low in the wheat grain like all other staple crops. Globally, about 2 billion people consuming wheat as staple food have iron deficiency (Qureshi et al., 2017), where wheat is the only protein-energy and essential micronutrient food, highlighting the importance of staple crop (Velu et al., 2017). To meet the dietary needs of the adults, the concentration of iron should be in the grain of wheat about 59 mg kg-1. On the other hand, wheat varieties are low in Fe concentrations which are about 22-26 mg kg-1(WHO, 2017). The potential of wheat to reduce iron malnutrition can be covered by microbial biofortification. Even though fortified wheat genetically and agronomically gives promising results, a more long-term solution can be the microbial biofortification of wheat, which is based on the siderophore producing bacteria. PGPR are non-pathogenic bacteria that perform a prominent role in biofortification. Therefore, microbial-assisted biofortification through seed inoculation with siderophore-producing bacteria was used for the growth and development of iron-enriched wheat. This is thought to be a cost-effective and eco-friendly technique that can play a role in overcoming the gap of Fe deficiency.
Materials and Methods
Around 50 rhizobacterial isolates from wheat, maize, millet, and sorghum rhizospheres of Rawalpindi and Sargodha divisions were collected. The isolates were preserved in an icebox to minimize microbial activity and brought to the laboratory for further analysis and characterization.
The dilution plate technique method was used for isolation. Around 10 g soil was weighed, dissolved in (99 mL) of D.I (deionized water) and agitated for 5-10 min. This shaking brought rhizosphere bacteria into soil solution. Then, take 1mL of that soil solution in 250 mL conical flasks, which already contained 99 mL of autoclaved D.I water to acquire 10-2 dilutions. After this, the process was repeated to get 10-6 dilutions. LB agar medium in autoclaved petri plates was used for pouring 100µL of each dilution through the sterilized nozzle and moved in the shape of 8 for proper spreading and placed in an incubator at 28±2oC for 24-48 hours. Then picking and streaking colonies of rhizobacteria on LB agar medium was used for purification. The process is repeated 2-3 times until getting pure strains. In the end, pure strains are preserved in eppendrophs tubes containing broth and stored at -20oC for further characterization.
Mineral iron solubilizing activity of bacterial isolates was evaluated using a specific media demonstrated by Nishio and Ishida (1989). Specific media have the insoluble source of Fe. Then LB agar medium was prepared in Petri plates. Plates has [(1 g) FePO4.4H2O + (20 g) of glucose]. Every single bacterial isolate was streaked in the zigzag form on petri plates. After that, Petri plates were kept in an incubator at 28±2 oC. The bacteria showed a change in color after 3-4 weeks due to reducing insoluble iron form to a soluble form. In the end, it was marked as positive for iron solubilization.
For qualitative estimation of siderophores, Chrome Azurol S (CAS) plates were used. For spot inoculation of different bacterial isolates, five equidistance places on the plates (CAS-agar medium) were made. For proper growth of bacteria, kept the plates in the incubator for 48 hrs at (28±2 oC). The appearance of halo zones around the colonies was an indication of siderophore (SP) production. This qualitative estimation was made using Milagres et al. (1999) and Chaiharn et al. (2009).
Kotasthane et al. (2017) described a CAS-shuttle assay for quantification of siderophore (SP) production. For this purpose, about 0.5 mL of aliquots of culture filtrate was added. After that, 0.5 mL of CAS reagent was added in reference/blank. Then [(0.5 mL) of CAS reagent + (0.5 mL) of uninoculated medium] was also added. Finally, a color change was noticed and measured colourimetry. The percentage (%) siderophore unit was calculated by using a formula given below:
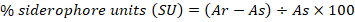
As= Sample absorbance at the wavelength of (600 nm); Ar= Reference/blank absorbance at the wavelength of (600 nm).
An agar medium was prepared with 1% tryptophan + 1% peptone water for the determination of IAA (indole acetic acid). The concentration of auxin (IAA) equivalents was predictable by using the standard solution of IAA (0.1 mg/ml). The loopful cultured isolates were embedded in the medium and incubated for 24 hours at (28±2 oC). To clear the supernatant solution centrifuged the samples, in which 4 mLof Salkowski’s reagent [(1 mL of 0.5 N FeCl3 solution + 50 mL of perchloric acid solution (35%))] and 2 drops of orthophosphoric acid was added for color development (Brick et al., 1991). Then optical density was calculated at the wavelength of 540 nm.
A method described by Nautiyal (1999) was used to determine P-solubilization. For this purpose, a tri-calcium phosphate solution was added to NBRIP medium. Then, the preserved bacterial isolates culture was inoculated in NBRIP medium; (a loopful of each isolated culture was embedded on the agar Petri plates). After this, to provide favorable environmental conditions, placed the plates in the incubator for 7 days at (28±1oC). After (6-7) days, a clearing zone was noticed around the colonies. Then from colony and halo zone diameter, the solubilization index (SI) was measured (Sadiq et al., 2014). Petri plates were incubated for 7 days at (28±2oC) using Sambrook and Russell (2001) method for organic acid production measurement. The organisms were inoculated on (MM9) agar medium with a pH indicator (methyl red dye). A red color indication is marked as positive for organic acid production while yellow for negative.
For screening, a germination experiment was carried out in a growth chamber. Based on growth promotion activity under axenic conditions in the growth chamber, bacterial isolates were screened out. For the lab screening experiment, 7 isolates (top-performing isolates) were chosen on the basis of microbial characteristics. For germination test, an inoculum of particular bacterial strains was prepared in volumetric conical flasks of (250 mL). Then, the flask was kept in an incubator for 3 days with continued shaking. Surface sterilization of seeds was done using 3% hypochlorite solution (for 2-3 minutes). Three times washing of seeds was done in D.I water. After that, dipped these seeds in selective bacterial inoculums, and growth parameters were recorded after (10) days of seed germination. Agronomic traits include (root length, shoot length, root fresh weight, shoot fresh weight, shoot dry weight, and root dry weight) were measured.
The field study was conducted to analyze the effectiveness of siderophore-producing bacteria to chelate insoluble iron and their ultimate impact on wheat’s growth and yield parameters for two years (2019-2021). Wheat variety Faisalabad 2008 was used in the field experiment. In control (un-inoculated) treatment, seed coating was carried out using mixture of 10% sugar solution + sterilized broth + sterilized peat. Seed inoculation was done with peat enriched siderophore (SP) producing bacteria plus sterilized 10% sugar) as sucrose solution as (10:1) ratio. The recommended dose of NPK fertilizers was applied at sowing time, and doses were used @ (N: 120, P: 90, K: 60 kg ha-1). The soil used for the experiment was free from salinity and sodicity hazards, deficient in organic matter contents while adequate in phosphorus, potassium, and iron contents (Table 1). The statistical design was a randomized complete block design (RCBD) having three replicates. Treatments were (T1=control, T2=SPP1, T3=SPP2, T4=SPP3, T5=SPP4, T6=SPP5, T7=SPS9 and T6=SPS10) respectively. At harvesting yield, parameters were recorded related to grain weight, plant height, spike length, no. of tillers per plant, and grain yield. Randomized Complete Block Design (RCBD) was employed in the current study The LSD test (least significant difference) was used for sums of means. Statistically analysis was performed using Statistics v. 8.1 (Steel et al., 1997).
Results and Discussion
Plants only take up iron in the ferrous form (Fe2+). So, the reduction of a ferric form of iron (Fe3+) into soluble iron form (Fe2+) was occurred due to alteration in rhizosphere soil pH. Microbes release specific organic acid, which helps alter the rhizosphere soil pH. Out of 50 bacterial isolates, 15 isolates were significantly positive for solubilizing iron in test tubes. Likewise, 7 isolates out of 15 positive isolates indicated the maximum change in color for Fe solubilization illustrated in (Table 2).
Through the appearance of transparent halo-zone in petri plate’s agar medium, siderophores (SP) production was noticeable positive. Only 15 isolates were confirmed for production of SP demonstrated in (Table 2).
Table 1: Physico-chemical attributes of soil.
|
Characteristics soil depth |
Unit (cm) |
Value (2019-20) |
Value (2020-21) |
References |
||
|
0-15 |
15-30 |
0-15 |
15-30 |
|||
|
pH |
8.01 |
7.99 |
8.00 |
7.99 |
(McLean and Page, 1983) |
|
|
EC |
(dSm-1) |
1.71 |
1.55 |
1.72 |
1.56 |
|
|
Organic matter |
(gkg-1) |
0.61 |
0.55 |
0.63 |
0.58 |
(Walkley, 1947) |
|
Available P |
(mgkg-1) |
8.13 |
7.12 |
8.25 |
7.62 |
(Olsen P, 1954) |
|
Extractable K |
(mgkg-1) |
180 |
174 |
182 |
172 |
(Rowell, 1994) |
|
DTPA extractable Fe |
(mgkg-1) |
4.59 |
4.12 |
5.12 |
4.34 |
(Soltanpourand Workmen, 1977) |
Table 2: Some basic stats of isolated strains of siderophore producing bacteria.
|
Location unit |
Isolate |
CAS-assay |
Solubilization index |
IAA (µg mL-1) |
Siderophore (%) |
FeCl3 Test |
Organic acid |
|
Rawalpindi |
SPP1 |
+ |
2.81 |
2.18 |
41.51 |
+ |
+++ |
|
SPP2 |
+ |
2.96 |
2.01 |
24.41 |
++ |
+++ |
|
|
SPP3 |
++ |
3.18 |
2.11 |
31.57 |
++ |
++ |
|
|
SPP4 |
+ |
2.88 |
2.15 |
38.88 |
++ |
+ |
|
|
SPP5 |
+ |
2.85 |
2.09 |
26.66 |
++ |
+ |
|
|
Sargodha |
SPS1 |
+ |
2.41 |
1.18 |
22.54 |
+ |
+ |
|
SPS2 |
++ |
2.57 |
1.08 |
22.00 |
+ |
++ |
|
|
SPS3 |
++ |
2.30 |
1.11 |
19.15 |
+ |
+ |
|
|
SPS4 |
+ |
2.31 |
1.15 |
12.24 |
++ |
+ |
|
|
SPS5 |
++ |
2.32 |
1.29 |
18.00 |
++ |
+ |
|
|
SPS6 |
++ |
2.34 |
1.19 |
10.12 |
+ |
- |
|
|
SPS7 |
+ |
2.48 |
1.11 |
10.22 |
+ |
+ |
|
|
SPS8 |
+ |
2.16 |
1.23 |
22.20 |
++ |
- |
|
|
SPS9 |
++ |
2.76 |
1.98 |
36.64 |
++ |
++ |
|
|
SPS10 |
+++ |
2.72 |
1.89 |
46.24 |
++ |
+++ |
Quantitative estimation showed that siderophores are produced in the range of 10.12-46.2%, out of which SPP1, SPP2, SPP3, SPP4, SPP5, SPS1, SPS2 produced a very high percentage of siderophores (Table 2).
Auxin (IAA) is a natural phytohormone that controls plant physiological activities. It has a vital role in elongation, gene regulation, cell differentiation, and cell division. Results of current research works showed that all isolates of bacteria were accomplished of producing equivalents of indole acetic acid (IAA) observed in (Table 2). The maximum auxin was produced by application of SPP1 at 2.18 µg mL-1 as compared to SPP4 at 2.15 µg mL-1. Salkowski reagent indicates pink color, revealing the potential of bacterial isolates to produce IAA.
Cereal crops are facing phosphorus deficiency due to fixation on alkaline conditions in Pakistani soils. Phosphorus (P) is the second primary mineral nutrient for the plant’s root development and appropriate growth. The maximum solubilization index was found in SPP3 followed by SPP4 and SPP5 (Table 2). When examined particular isolates of bacteria, all isolates were found accomplished for P solubilizing trait (ranged from 2.16 to 3.18).
Inoculation of SP producing bacteria and Fe solubilization indicated a significant increase in agronomic attributes of wheat crop (Table 3). Wheat root and shoot traits positively correlated to inoculation with Fe solubilizing, and SP isolates over control. Shoot weight was also found statistically significant and high in SPS10 (371.3 cm). In strain (SPP4) and (SPS9), the shoot length of wheat was significantly increased by about 86.7 cm and 91.7 cm as compared to other isolates. Similarly, inoculation of selective bacterial isolates improved root length and root weight, with SPP5 performing best than all other isolates. The root attributes are manifested through inoculation with selective iron and phosphorus solubilizing isolates. The root length was increased two-fold through inoculation with SPS9 and root weight up to 27% increase with SPP5 strain over the control (Table 3).
Table 3: Effect of Siderophore producing bacteria on root-shoot parameters in lab conditions.
|
Treatments |
Shoot length (cm) |
Root length (cm) |
Shoot mass (g) |
Root mass (g) |
|
Control |
35.0 c |
27.3 d |
266.0 b |
332.7 bc |
|
SPP1 |
42.3 c |
40.0 bc |
295.3 ab |
339.3 bc |
|
SPP2 |
53.3 b |
40.3 bc |
295.3 ab |
369.3 b |
|
SPP3 |
39.0 c |
37.0 c |
296.0 ab |
264.7 d |
|
SPP4 |
86.7 a |
45.0 b |
282.0 ab |
330.0 bc |
|
SPP5 |
60.7 b |
44.7 b |
362.0 a |
460.3 a |
|
SPS9 |
91.7 a |
57.0 a |
335.3 ab |
292.3 cd |
|
SPS10 |
85.7 a |
57.0 a |
371.3 a |
302.3 cd |
|
LSD |
8.57 |
7.39 |
91.5 |
53.07 |
Different letters within the same column indicate statistically significant differences at p≤0.05
Moreover, positive response was observed regarding yield and yield parameters over control through inoculation with SP and Fe solubilization in field trials. Data showed that plant height increased in the second year with a 3% maximum increase in plant height compared to the first-year maximum height (Figure 1). In the first year, T6 (102 cm) performed best overall than other strains, while in the second year, T5 and T7 gave the best performance with 105.3 cm plant height. In T6, where SPP5 bacterial isolates were used, significantly increased no. of tillers plant-1 over the control with maximum No. of tillers (330) showed in (Figure 2) A significant increase in spike length of the wheat plant was found with siderophore producing bacterial strain (Figure 3). In the first year of the field experiment, maximum spike length was found in T2 (14.8 cm) and T8 (14.7 cm), while in the second year, T6 and T4 performed best with 17.2 and 17.1 cm spike length is 20% and 19% more as compared to control. In the first-year maximum yield of 3.47 t/ha was found in T7 (SPS9), while in the second year, T6, T4, and T8 showed a significant increase in wheat yield with 4.60, 4.58, and 4.08 t/ha, respectively (Figure 4). Grain weight indirectly shows the improvement in the quality of wheat. Application of microbial strains and a recommended dose of fertilizers improved the grain weight of wheat. In both years, an increase in grain weight remained in the range of 26.7-33.9 g, which is statistically significant over control where the sterilized broth was used (Figure 5).
Iron deficiency is an emerging threat to humanity, affecting health, mental/physical behavior, and activities. It is the most spreading mal-nutrition overwhelming the world. Plant growth-promoting rhizobacteria (PGPR) secrets specialized Fe chelating compounds having low molecular weight known as siderophores as mentioned by Khalid et al. (2015). These compounds have the ability to enhance the solubility and availability of Fe to plants. Siderophore producing microbial-mediated biofortification is an emerging approach to overcome malnutrition.
Further, Kaur et al. (2020) reported that the inadequacy of essential micronutrients including vit. A, Zn, and Fe. The PGPR can fortify Fe content in soil rhizosphere by SP production and Fe solubilization. He et al. (2020) observed that some wheat-linked microbes primarily the microbes of rhizospheric soil produce SP and other metabolites which increase the solubility of Fe in the soil. The present study was conducted to isolate and purify siderophore-producing bacteria from different locations and then see their impact on the physiological and yield impact of wheat. The microbial characteristics determination showed that out of 50 collected strains, 15 produced a higher amount of siderophore, having phosphorus solubilization, and auxin production activity. Similar results were found in (Kumari et al., 2021) experiment that (SPS10) strain showed a significant relationship and produced a comparatively high level of SP about 46.2 (SU %).
Germination test assay showed increased shoot/root length and weight in inoculated seeds over un-inoculated (control) seeds. Satish et al. (2020) reported that the growth of plants could affect the roots of the plant upon contact with microbiota. Previous researchers reported that SP improved the Fe uptake in plants which caused an increase in chlorophyll contents, leaf area, and the photosynthetic rate (Mushtaq, 2020; Saleem et al., 2018). The current scenario may imply that an increase in physiological traits might be due to increased phosphorus solubilization, solubilization/ uptake/ transloaction of iron, auxin and phytohormone production (Etesami, 2020; Delaporte-Quintana et al., 2020; Mushtaq et al., 2021; Yavarian et al., 2021). The addition of plant growth regulators (PGRs) to plant growth-promoting rhizosphere bacteria (PGPRs) showed improvements in chlorophyll content, leaf area, sugar content, oxidative stress, and reduction in peroxidation of lipid (Khan et al., 2019). These findings of the current study are in line with the observation of Ekin (2019) and Dawwam et al. (2013).
Field studies demonstrated around 34 % increase in wheat grain yield, 40% in the number of productive tillers per plant, 20% increase in plant height and spike length after inoculation with siderophore producing bacteria especially in the second year. Such an increase in germination and yield attributes provides a baseline to test these siderophore and iron solubilizing PGPR for other cereal crops. Furthermore, around 40% increase in grain Fe contents and 20% increase in thousand grain weight (TGW) was observed in inoculated plants compared with uninoculated control. These outcomes are in harmony with the results of Yadav et al. (2020) and Singh and Parsanna (2020). Similar types of improvements in potato (Solanum tuberosum L.) rhizosphere was observed by Mushtaq et al. (2021). Interaction of plant-microbe is the primary factor determining productivity, plant health, and soil fertility. Kabiraj et al. (2020) cited that bacteria inoculants can increase the agronomic parameters significantly, which helps alleviate the cost of production and environmental pollution Souza et al. (2015). Microbes proved as cost-effective, efficient, more promising, and sustainable approaches and contribute to plants development. Mushtaq et al. (2020) reported that microbes promoted nutrients concentration, plant physiological processes, plant development, yield and growth using the various (direct or indirect) methods such as hormonal production, including (cytokinin, gibberellins, and auxin IAA). Similar findings were reported by Prasanna et al. (2015).
Biofortification of wheat (Triticum aestivum L.) through seed inoculation with siderophore-producing bacteria is an alternative approach to fulfill desired micronutrients deficiency in the human diet in rural areas (Riaz et al., 2021). Khalid et al. (2015) and his colleagues explained that, gene expression of ferritin persuades as uptake mechanisms of Fe. Radzki et al. (2013) found a reason for iron uptake enhancement. They reported that SP with low molecular weight binds the Fe and transports it into root cells via protein membrane. Like the current study, Khalid et al. (2015) found a 13-18% increase in wheat grain yield, 12-16% plant shoot, 6-11% root length, and 34-60% chlorophyll contents by the inoculation of siderophore producing Pseudomonas in wheat. The E. coli (SP producing bacteria) noted that significantly increases yield and growth of sugarcane and ryegrass (Gangwar and Kaur, 2009), and on the other side, in tea plants, a substantial decline in pathogenicity was observed by Bacillus megaterium (also SP producing bacteria) (Chakraborty and Iwatsuki, 2006).
Conclusions and Recommendations
It has been proven from the research experiment that siderophore producing bacteria not only proformed efficient role in solubilizing mineral nutirents, uptake and translocation to plant but also improved yield attributing traits of wheat. Thus, it can be concluded that inoculation of seed with growth-promoting (GP) character of siderophore (SP) producing microbes can increase solubilization of insoluble (Fe) and bring about improvement in development and growth of the wheat crop in alkaline calcareous soil.
Novelty Statement
High siderophore producing bacteria collected from silty loam areas of Sargodha division perform better in improving yield traits of wheat under very fine sandy loam soils of Faisalabad division. The siderophore producing bacteria can successfully be used to enhance availability of iron and improve the crop yields.
Author’s Contribution
Shabana Ehsan: Principal author and researcher.
Aneela Riaz: Conductance of 1st year experiment.
Muhammad Amjad Qureshi: Planned the research experiment and methodology.
Abid Ali: Supervised the research experiment.
Ifra Saleem: Assisted in writing a manuscript.
Muhammad Aftab: Assisted in conducting experiment.
Khalid Mehmood: Collected some references.
Fakhar Mujeeb: Supervision and facilitate in conducting experiment.
Muhammad Asif Ali: Supply of all chemicals/ apparatus needed for analysis.
Hina Javed and Fraza Ijaz: Analysis of biochemical tests.
Anwar-ul-Haq: Helped in data analysis.
Khaliq-ur-Rehman: Helped in results and discussion.
M. Usman Saleem: Helped in revising the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Anwar, Z., Z. Basharat, M.B. Hafeez, S. Khan, N. Zahra, Z. Rafique and M. Maqsood. 2022. Biofortification of maize with zinc and iron not only enhances crop growth but also improves grain quality. Asian J. Agric. Biol., 2022(2): 1-9.
Boukhalfa, H. and A.L. Crumbliss. 2002. Chemical aspects of siderophore mediated iron transport. Biometals, 15(4): 325–339. https://doi.org/10.1023/A:1020218608266
Brick, J.M., R.M. Bostock and S.E. Silversone. 1991. Rapid in situ assay for Indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl. Environ. Microbiol., 57: 535-538. https://doi.org/10.1128/aem.57.2.535-538.1991
Chaiharn, M., S.C. Chanon and S. Lumyong. 2009. Scrrening siderophore producing bacteria as potential biocontrol agents for fungal rice pathogens in Thailand. World J. Microbiol. Biotechnol., 25: 1919-1928. https://doi.org/10.1007/s11274-009-0090-7
Chakraborty, A. and Y. Iwatsuki. 2006. Genetic variation at the mitochondrial 16S rRNA gene among Trichiurus lepturus (Teleostei: Trichiuridae) from various localities: preliminary evidence of a new species from West coast of Africa. Hydrobiology, 563(1): 501–513. https://doi.org/10.1007/s10750-006-0105-4
Chaudhary, D.Y., P. Gosavi and A. Durve-Gupta. 2017. Isolation and application of siderophore producing bacteria. Int. J. Agric. Res., 3(4): 246-50.
Dawwam, G.E., A. Elbeltagy, H.M. Emara, I.H. Abbas and M.M. Hassan. 2013. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci., 58(2): 195–201. https://doi.org/10.1016/j.aoas.2013.07.007
Delaporte-Quintana, P., N.C. Lovaisa, V.A. Rapisarda and R.O. Pedraza. 2020. The plant growth-promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Reg., 91: 185. https://doi.org/10.1007/s10725-020-00598-0
Ekin, Z., 2019. Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability, 11(12): 3417. https://doi.org/10.3390/su11123417
Etesami, H., 2020. Halotolerant plant growth promoting bacteria: prospects for alleviating salinity stress in plants. Environ. Exp. Bot., 178: 104–124. https://doi.org/10.1016/j.envexpbot.2020.104124
Gangwar, M. and G. Kaur. 2009. Isolation and characterization of endophytic bacteria from endorhizosphere of sugarcane and ryegrass. Int. J. Microbiol. 7(1): 139–144. https://doi.org/10.5580/181d
Gyana, R., 2015. Role of iron in plant growth and metabolism. Rev. Agric. Sci., 3: 1-2. https://doi.org/10.7831/ras.3.1
He, L., Z. Yue, C. Chen, C. Li, J. Li and Z. Sun. 2020. Enhancing iron uptake and alleviating iron toxicity in wheat by plant growth-promoting bacteria: Theories and practices. Int. J. Agric. Biol., 23: 190–196.
Hussan, M., M. Hafeez, M. Saleem, S. Khan, S. Hussain and N. Ahmad. 2022. Impact of soil applied humic acid, zinc and boron supplementation on the growth, yield and zinc translocation in winter wheat. Asian J. Agric. Biol., pp. 1-8.
Kabiraj, A., K. Majhi, U. Halder, M. Let and R. Bandopadhyay. 2020. Role of plant growth-promoting rhizobacteria (PGPR) for crop stress management. In: Sustainable agriculture in the era of climate change. Springer, Cham. pp. 367-389. https://doi.org/10.1007/978-3-030-45669-6_17
Kaur, T., K.L. Rana, D. Kour, I. Sheikh, N. Yadav, V. Kumar, A.N. Yadav, H.S. Dhaliwal and A.K. Saxena. 2020. Microbe-mediated biofortification for micronutrients: Present status and future challenges. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier, pp. 1-17. https://doi.org/10.1016/B978-0-12-820528-0.00002-8
Khalid, S., H.N. Asghar, M.J. Akhtar, A. Aslam and Z.A. Zahir. 2015. Biofortification of iron in chickpea by plant growth-promoting rhizobacteria. Pak. J. Bot., 47(3): 1191–1194.
Khan, N., A. Bano, M.A. Rahman, J. Guo, Z. Kang, and M.A. Babar. 2019. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep., 9(1): 1–19. https://doi.org/10.1038/s41598-019-38702-8
Kotasthane, A.S., T. Agrawal, N.W. Zaidi and U. Singh. 2017. Identification of siderophore producing and cynogenic fluorescent Pseudomonas and a simple confrontation assay to identify potential bio-control agent for collar rot of chickpea. Biotech, 7(3): 1-8. https://doi.org/10.1007/s13205-017-0761-2
Kumar, M., R. Prasanna, N. Bidyarani, S. Babu, B.K. Mishra, A. Kumar, A. Adak, S. Jauhari, K. Yadav, R. Singh and A.K. Saxena. 2013. Evaluating the plant growth promoting ability of thermotolerant bacteria and cyanobacteria and their interactions with seed spice crops. Sci. Hortic., 164: 94-101. https://doi.org/10.1016/j.scienta.2013.09.014
Kumari, S., S. Kiran, S. Kumari, P. Kumar and A. Singh. 2021. Optimization of Siderophore production by bacillus subtilis DR2 and its Effect on growth of Coriandrum Sativum. Res. Sq., https://doi.org/10.21203/rs.3.rs-567897/v1
McLean, E., 1983. Soil pH and lime requirement. Methods of soil analysis: Part 2 Chemical and microbiological properties. 9: 199–224. https://doi.org/10.2134/agronmonogr9.2.2ed.c12
Mehmood, U., M. Inam-ul-Haq, M. Saeed, A. Altaf, F. Azam and S. Hayat. 2018. A brief review on plant growth promoting Rhizobacteria (PGPR): A key role in plant growth promotion. Plant Protect., 2(2): 77-82.
Milagres, A.M.F., D. Napoleão and A. Machuca. 1999. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Method, 37: 1-6. https://doi.org/10.1016/S0167-7012(99)00028-7
Mushtaq, Z., 2020. PGPR: present role, mechanism of action and future prospects along bottlenecks in commercialization. EQA Int. J. Environ. Qual., 41: 9-15.
Mushtaq, Z., H.N. Asghar and Z.A. Zahir. 2021. Comparative growth analysis of okra (Abelmoschus esculentus) in the presence of PGPR and press mud in chromium contaminated soil. Chemosph, 262: 127865. https://doi.org/10.1016/j.chemosphere.2020.127865
Nathiya, S., R. Janani and V.R. Kannan 2020. Potential of plant growth promoting Rhizobacteria to overcome the exposure of pesticide in Trigonella foenum-graecum (fenugreek leaves). Biocat. Agric. Biotechnol., 23: 101493. https://doi.org/10.1016/j.bcab.2020.101493
Nautiyal, C.S., 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbial. Lett., 170: 265-270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Nishio, T., and Y. Ishida. 1989. Distribution of iron-solubilizing bacteria in the sediment of a small lagoon. Nippon Suisan Gakkaishi. 55(11): 1955-1960. https://doi.org/10.2331/suisan.55.1955
Olsen, S. and Sommers, L., 1982. Phosphorus. In: Al-Page et al. (ed.) methods of soil analysis. Part 2. 2nd ed. Agron. Monogr. 9. ASA and SSSA, Madison, WI. pp. 403–430. https://doi.org/10.2134/agronmonogr9.2.2ed.c24
Prasanna, R., N. Bidyarani, S. Babu, F. Hossain, Y.S. Shivay and L. Nain. 2015. Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food Agric., 1(1): 998507. https://doi.org/10.1080/23311932.2014.998507
Qureshi, M.A., F. Mujeeb, M.A. Anjum, M.A. Ali and A. Khan. 2017. Influence of growth stimulants with rhizobium inoculation on the yield of mung bean (Vigna radiata L.). Pak. J. Agric. Sci., 54(3): 523-529. https://doi.org/10.21162/PAKJAS/17.1610
Radzki, W., F.G. Manero, E. Algar, J.L. Garcia, A. Garcia-Villaraco and B.R. Solano. 2013. Bacterial siderophores efficiently provide iron to iron starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek, 104(3): 321–330. https://doi.org/10.1007/s10482-013-9954-9
Ramzan, Y., M.B. Hafeez, S. Khan, M. Nadeem, S. Batool and J. Ahmad. 2020. Biofortification with zinc and iron improves the grain quality and yield of wheat crop. Int. J. Plant Prod., 14(3): 501-510. https://doi.org/10.1007/s42106-020-00100-w
Riaz, U., M. Ghulam, A. Wajiha, S. Tayyaba, S. Muhammad, M. Nazir and M. Zulqernain. 2021. Plant growth-promoting rhizobacteria (PGPR) as biofertilizers and biopesticides. In: Microbiota and Biofertilizers. Spinger, pp. 181-196. https://doi.org/10.1007/978-3-030-48771-3_11
Rowell, D.L., 1994. Soil science. Methods and application. Longman Scientific and Technical, UK.
Sadiq, H.M., G.Z. Jahangir, I.A. Nasir, M. Iqtidar and M. Iqbal. 2014. Isolation and characterization of phosphate solubilizing bacteria from rhizosphere soil. Biotech. Biotech. Equipment, 27: 4248-4255. https://doi.org/10.5504/BBEQ.2013.0091
Sahay, R. and D.D. Patra. 2014. Identification and performance of sodicity tolerant phosphate solubilizing bacterial isolates on Ocimum basilicum in sodic soil. Ecol. Eng., 71: 639-643. https://doi.org/10.1016/j.ecoleng.2014.08.007
Saleem, I., S. Javid, F. Bibi, S. Ehsan, A. Niaz and Z.A. Ahmad. 2016. Biofortification of maize grain with zinc and iron by using fertilizing approach. J. Agric. Ecol. Res. Int., 8: 1-6. https://doi.org/10.9734/JAERI/2016/24532
Saleem, M., H.N. Asghar, Z.A. Zahir and M. Shahid. 2018. Impact of lead tolerant plant growth-promoting rhizobacteria on growth, physiology, antioxidant activities, yield, and lead content in sunflower in lead-contaminated soil. Chemosphere, 195: 606–614. https://doi.org/10.1016/j.chemosphere.2017.12.117
Sambrook, J., and D.W. Russell. 2001. Molecular cloning: A laboratory manual. Cold Spring Harbor; New York.
Satish, L., S. Shamili, S. Yolcu, G. Lavanya, H. Alavilli and M.K. Swamy. 2020. Biosynthesis of secondary metabolites in plants as influenced by different factors. In: (ed. M. Swamy Plant-derived bioactives. Springer, Singapore. pp. 61–100. https://doi.org/10.1007/978-981-15-1761-7_3
Singh, D., and R. Prasanna. 2020. Potential of microbes in the biofortification of Zn and Fe in dietary food grains. A review. Agron. Sustain. Dev., 40: 1–21. https://doi.org/10.1007/s13593-020-00619-2
Soltanpour, P.N., A. Khan and W.L. Lindsay. 1976. Factors affecting DTPA extractable Zn, Fe, Mn, and Cu from soils. Commun. Soil Sci. Plant Anal., 7(9): 797–821. https://doi.org/10.1080/00103627609366689
Souza, R.D., A. Ambrosini and L.M. Passaglia. 2015. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol., 38: 401-419. https://doi.org/10.1590/S1415-475738420150053
Steel, R.G.D., J.H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics: A biometrical approach, 3rd Ed. McGraw Hill Co. New York, USA.
Sun, Z., Z. Yue, H. Liu, K. Ma and C. Li. 2021. Microbial-assisted wheat iron biofortification using endophytic bacillus altitudinis WR10. Front. Nutr., 8: 704030. https://doi.org/10.3389/fnut.2021.704030
Velu, G., R.P. Singh, J. Huerta and C. Guzman. 2017. Genetic impact of Rht dwarfing genes on grain micronutrients concentration in wheat. Field Crops Res., 214: 373-377. https://doi.org/10.1016/j.fcr.2017.09.030
Walky, A., 1947. A critical examination of a rapid method for determining organic carbon in soil effect of variation in digestion condition and inorganic soil constant. Soil Sci., 63: 241–264. https://doi.org/10.1097/00010694-194704000-00001
WHO, 2017. World Health Organization. The double burden of malnutrition. Policy Breif.,
Yadav, R., P. Ror, P. Rathore, S. Kumar and W. Ramakrishna. 2020. Bacillus subtilis CP4, isolated from native soil in combination with arbuscular mycorrhizal fungi promotes biofortification, yield and metabolite production in wheat under field conditions. J. Appl. Microbiol., 131: 339–359. https://doi.org/10.1111/jam.14951
Yavarian, S., P. Jafari, N. Akbari and M.M. Feizabadi. 2021. Selective screening and characterization of plant growth promoting bacteria for growth enhancement of tomato, Lycopersicon esculentum. Iran. J. Microbiol., 13(1): 121. https://doi.org/10.18502/ijm.v13i1.5502
Zhang, Y.Y., R. Stockmann, K. Ng and S. Ajlouni. 2020. Revisiting phytate-element interactions: implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Crit. Rev. Food Sci. Nutr., 16: 1–17.
Zulfiqar, U., M. Maqsood, S. Hussain and M. Anwar-ul-Haq. 2020. Iron nutrition improves productivity, profitability, and biofortification of bread wheat under conventional and conservation tillage systems. J. Soil Sci. Plant Nutr., 20: 1298. https://doi.org/10.1007/s42729-020-00213-1
To share on other social networks, click on any share button. What are these?






