Lemon Juice and Microwave Assisted Modification of Date Seed Husk for Arsenic Biosorption
Lemon Juice and Microwave Assisted Modification of Date Seed Husk for Arsenic Biosorption
Tahira Moeen Khan*, Irum Riaz, Shahida Hameed and Bushra Khan
Department of Chemistry, Lahore College for Women University, Jail Road Lahore, 54000, Pakistan.
Abstract | Arsenic poisoning and its removal from drinking water has become a serious issue now a days. For arsenic removal batch studies were conducted using low cost adsorbent (raw date seeds husk and lemon juice microwave activated date seeds husk) by taking 25ppm/50ml initial concentration of NaAsO2. Best removal (90%) was achieved for Lemon juice microwave activated date seed husk LMDS (time= 30mins, agitation speed= 150 rpm, pH= 5, adsorbent dose= 0.1 g). While 85% removal efficiency was observed for Raw date seed husk RDS (time= 45mins, agitation speed=250 rpm, pH= 4 and adsorbent dose= 0.5 g). The adsorption isotherms (Freundlich and Langmuir) and adsorption dynamic kinetic studies were also conducted. The comparison between the FTIR, EDX and SEM of RDS and LMDS revealed that with lemon juice microwave activation of adsorbent there were activation of more active sites which in turn increased the removal efficiency.
Received | May 29, 2019; Accepted | December 23, 2019; Published | December 29, 2019
*Correspondence | Tahira Moeen Khan, Department of Chemistry, Lahore College for Women University, Jail Road Lahore, 54000, Pakistan; Email: [email protected]
Citation | Khan, T.M., Riaz, I., Hameed, S. and Khan, B., 2019. Lemon juice and microwave assisted modification of date seed husk for arsenic biosorption. Journal of Innovative Sciences, 5(2): 106-114.
DOI | http://dx.doi.org/10.17582/journal.jis/2019/5.2.106.114
Keywords | Biosorption, RDS, LMDS, Natural modification, SEM, EDX, Batch studies
1. Introduction
Arsenic toxicity is now recognized as a global health problem associated with almost more than 21 countries (Smith et al., 2000). In nature arsenic exists in the form of ores (powdery, amorphous and crystalline). Different anthropogenic and natural sources (mining and smelting processes, pesticide use and coal combustion) are responsible for water contamination by arsenic (Raj et al., 2013). Arsenic in environment (groundwater and in turn in drinking water) is not only creating the other health problems (respiratory, renal, neurological, cardiovascular, mutagenic etc.) but also has a prominent role in creating various types of cancer (Hall, 2002; Roy et al., 2013; Saha et al.,1999; Asif and Chen, 2017) in several developing regions (Brouwer et al., 2007). Ground water arsenic is present in the form of As V (arsenate) and As III (arsenite) (Kumari et al., 2005; Wasiuddin et al., 2002; Amin et al., 2006; Roy et al., 2013).
For arsenic remediation various techniques involving adsorption were considered (Sharma and Bhattacharya, 2017). Various adsorbents used for arsenic removal are DE-4 resin (Qureshi and Sahabuddin, 2012), red mud (Bauxsol), a waste from aluminum manufacturing (Genc et al., 2003), iron coated brown seaweed (Sargassum muticum) (Vieira et al., 2017), Ceria (CeO2) coated powdered activated carbon (Sawana et al., 2017), modified fungal biomass of Aspergillus niger coated with iron oxide (Pokhrel and Viraraghavan, 2006), sand was coated with ferric chloride and used as filtering media (Devi et al., 2014), limestone-based material is effective for reducing arsenic (Davis et al. , 2018) and silica based chitosan beads (Malwal and Packirismy, 2017).
These conventional methods are reported to be noneconomical, less efficient and not safe environmentally (Eccles, 1999; Barakat, 2011; Azimi et al., 2016). A best and alternative method for arsenic remediation (Kratochvil and Volesky, 1998; Asif and Chen, 2017) is biosorption which utilizes natural, dead biomass (plants, agricultural wastes) or microorganisms (Roy et al., 2017; Ozer et al., 1998; Dimme et al., 2017; Amin et al., 2017; Sidhu et al., 2014; Javanbakht et al., 2014). These natural materials are excessively available having low or no cost and also have high decontamination ability. Biosorbents for removal of arsenic can be used in activated or in natural form (activated cashew shells, millet stalks, seed powder (Raj et al., 2013), Azadirachta indica bark powder (Roy et al., 2017), rice husk (Asif and Chen, 2017) and biochars derived from rice husk (Agrafioti et al., 2014).
The metal decontamination ability is attributed due to the presence (Basso et al., 2002; Chen et al., 2010), activation and modification of various groups (-COOH, OH, NH2, SO4-2, PO4-3) (Deng et al., 2003). These groups when introduced or activated can enhance the adsorption power of adsorbent (Oliveira et al., 2009). Metal entrapment by these natural residues is due to metal substrate interactions by various processes (Srivastava and Anil, 2016). So far biosorbents can be activated by using different chemicals like FeCl3, ZnCl2 (Liu et al., 2016), H2O2, H2SO4, NaOH (Shwantes et al., 2016), urea (Rehman et al., 2013) and thiourea (Salman et al., 2014).
Various biosorbents were successfully being used for removing heavy metals due to their different up take capacities (qmax values) Table 1. Present study was designed to check the effectiveness of Phoenix dactylifera seeds (date seeds) belonging to family Arecaceae, an agricultural waste in removing arsenic ions from aqueous solution. There are many areas in Sindh and Baluchistan which are producing high quality dates of different varieties. Their seeds can be reused in different ways. In present study two types of biosorbents i.e., raw date seeds husk (RDS) and microwave assisted lemon juice modified date seed husk (LMDS) were used for the first time as a new biosorbent and its modification with lemon juice is totally ecofriendly instead of using various chemicals as modifiers or activators.
Table 1: Comparison of sorption capacities of different biosorbents for the removal of Heavy metals.
| Biosorbent | Heavy metal |
qmax mg/g |
Reference |
| Oil palm shell | Pb (II) | 3.39 |
(Chong et al., 2013) |
| Oil palm shell | Cu (II) | 1.75 |
(Chong et al., 2013) |
| Palm shell | Hg (II) | 83.33 |
(Ismaiel et al., 2013) |
| Corn straw | Cd (II) | 38.91 |
(Chi et al., 2017) |
| Corn straw | Pb (II) | 28.99 |
(Chi et al., 2017) |
| Raw date seeds | As (III) | 1.3332 | Present study |
| Lemon juice microwave activated date seeds | As (III) | 1.4828 | Present Study |
By adding lemon juice there is the introduction of additional -COOH groups on adsorbent surface (date seed husk) and with microwave radiation these groups are much more activated as COO- thus providing more adsorbent sites for arsenic ions removal.
The capability of RDS and LMDS for arsenic ions removal was checked by studying isothermal, kinetic and batch studies.
2. Materials and Methods
Shaker (yellow line), Atomic Absorption spectrometer, PH meter, Microwave oven (2,450 MHz), Midac FTIR 2000 spectrometer (406-7800cm-1), EDX, SEM, 0.1M NaOH, 0.1M HCL (MERCK), Sodium Arsenite salt (NaAsO2) and lemon juice.
2.1 Preparation of solution
Stock solution of 1M Sodium Arsenite salt (NaAsO2) was prepared. From this stock solution 25 ppm/50 ml volume of solution was prepared and mixed into the activated as well as non-activated date seed husk.
2.2 Preparation of biosorbent
Preparation of raw date seed husk: Date seeds (Phoenix Dactylifera) an easily available raw material were purchased from seed bank of Lahore. These were then washed, dried, grinded and sieved (60 ASTM). This was raw date seed husk (RDS).
Preparation of modified Date Seed husk: 60g of the preserved raw date seed husk (RDS) and lemon juice (1:1) was mixed fairly. This was then subjected to microwave oven for 25 min. to get lemon juice microwave activated date seed husk (LMDS) Scheme 1.
3. Results and Discussion
FTIR Analysis: FTIR spectra of raw date seed husk (RDS) and lemon juice microwave activated date seed husk (LMDS) Figure 1 showing various functional groups on the surface of RDS and LMDS. LMDS showed the presence of additional peaks at 1746.57 cm-1 (C=O Stretching), 1458.21cm-1 (C-H Stretching), 1373.34 cm-1(O-H Bending), 1151.52 cm-1(C-O Stretching), 1010.72 cm-1 (C-N Stretching) and 721.39 cm-1 (C=C Bending). RDS showed different peaks in the region of 1616.38 cm-1 (C=C Stretching), 1445.67 cm-1(C-H Bending), 1249.89 cm-1(C-O Stretching), 1154.42 cm-1 (C-O Stretching), 1036.76cm-1(S=O Stretching), 869.91 cm-1(C=C Bending), 816.87cm-1(C=C Bending). There was also shift in peaks of RDS from 2923.17 cm-1 to 2922.21 cm-1, 1743.68cm-1 to 1746.57 cm-1, 1445.67 cm-1 to 1458.21 cm-1, 1154.42 cm-1 to 1151.52 cm-1 and 1036.76 cm-1to 1010.72 cm-1 after modification with lemon juice and microwave activation. Thus the presence of these groups were involved in biosorption of As (III) by Phoenix Dactylifera. LMDS had more active sites and had more functional groups indicating the involvement of these groups in biosorption of As (III).
SEM Analysis: It was noticed that the surface of RDS was rough and consisted of particles of various shapes and sizes. Particles were large in size providing small surface area Figure 2a. After lemon juice microwave activation surface of LMDS had become irregular and wavy and had holes which were unevenly spread on the small size particles of LMDS Figure 2b. After activation of sample with lemon juice surface morphology was changed and increases in surface area consequently giving higher sorption capacity of the sorbent.
EDX Analysis: EDX image of RDS Figure 3a shows its elemental composition as having Ca, K, Na and no arsenic but for LMDS an additional prominent peak is observed due to retention of arsenic on the surface of biosorbent Figure 3b.
3.1 Batch studies
Effect of shaking speed: It was observed that adsorption of Sodium Arsenite (NaAsO2) (25ppm /50ml) had increased with the increased shaking speed (50 – 400 rpm) for both RDS and LMDS. At 250 rpm for RDS adsorption reaches a maximum of 60.50 % and at 150 rpm for LMDS maximum adsorption was 90.25 % Figure 4a. Moderate speed provided more active sites as compared to low speed and further increase in speed decreased the contact time resulting in desorption.
Effect of contact time: Removing capacity (%) of RDS and LMDS was increased with the increase in their time of contact Figure 4b. Maximum removal efficiency (%) of RDS was 82.25 % at 45 minutes contact time whereas LMDS had 90.59 % removal efficiency at 30 minutes contact time. Therefore, the 30 minutes contact time was chosen for further batch adsorption experiment.
Effect of adsorbent dose: Adsorption behaviour of RDS and LMDS was studied by keeping the adsorbent amount from 0.1g–0.9g Figure 4c. Maximum adsorption occurred when RDS is 0.5 g and LMDS was only 0.1 g increase in adsorption was due to the increased sites and then decrease was due to their aggregation and decreased surface area.
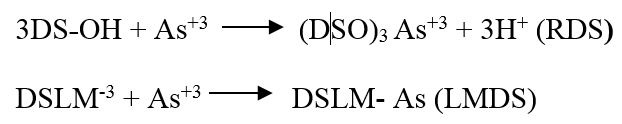
Effect of pH: The pH (3-9) also effected the adsorption capacity of RDS and LMDS due to the presence of different forms of carboxylic group on biosorbent surface at different pH. As+3 adsorption was low at pH 3 because at pH 3 the dominating specie was –COOH and it then increases and reaches the highest at pH 5 where –COO- was mainly present on adsorbent surface. For RDS As+3 maximum removal efficiency (%) was at pH 4 (84.625 %) and for LMDS at pH 5 (89.75 %) Figure 4d. At still higher pH (6.0) adsorption decreases due to the precipitation of As+3as insoluble metal hydroxides. The mechanism of As+3biosorption is:
Temperature dependence: Temperature is an important parameter to study extent of adsorption. Experiment were conducted in the temperature range 283.0 K - 333.0 K in which 293.0 K was the suitable temperature for both the sorption of As+3 using RDS (90.16 %) and LMDS 93.53 % Figure 4e. Above 293.0 K adsorption decreases due to very high movement of As+3and decrease in interaction between adsorbent surface and adsorbate. The value of ΔG for RDS and LMDS showed that the reaction is spontaneous and its greater value for LMDS than RDS means that adsorption was more favorable with LMDS Figure 5.
3.2 Isothermal study
Langmuir Isotherm: qmax values for RDS and LMDS were 1.3332 mg g-1 and 1.4828 mg g-1 Table 2. Greater qmax for LMDS had more active sites as compared with RDS. RL values of LMDS and RDS were 0.0237 and 0.0335 respectively which indicated that data is not according Langmuir Figure 6a.
Table 2: Isothermal parameters for Arsenic sorption onto LMDS and RDS.
| Adsorbent | Langmuir | Freundlich | ||
| LMDS |
q max(mg/g) |
1.4828 | n | 0.094 |
| b(L/g) | 1.6503 |
KF |
2.7064 | |
|
R2 |
0.7581 |
R2 |
0.9245 | |
|
RL |
0.0237 | 1/n | 10.638 | |
| RDS |
q max(mg/g) |
1.3332 | n | 0.2135 |
| b(L/g) | 1.1527 |
KF |
2.605 | |
|
R2 |
0.7855 |
R2 |
0.9226 | |
|
RL |
0.0335 | 1/n | 4.683 | |
Freundlich isotherm: Magnitude of KF and n (Freundlich constant) indicated high adsorptive capability of Phoenix Dactylifera. The value of KF for LMDS and RDS were 2.7064 and 2.605 respectively Table 2. The value of R2 for LMDS and RDS were 0.9245 and 0.9226 respectively which is a good fit of Frendulich isotherm Figure 6b.
Table 3: Kinetic Study for Arsenic sorption onto LMDS and RDS.
| Pseudo first order kinetic Model | ||||
| Adsorbent |
qe (exp) |
K1 |
qe (cal) |
R2 |
| RDS | 11.79 | 0.00007 | 2.582 | 0.0425 |
| LMDS | 12.4896 | 0.0002 | 2.6072 | 0.3419 |
| Pseudo second-order kinetic model | ||||
| Adsorbent | qe (exp) |
K2 |
qe (cal) |
R2 |
| RDS | 1.92864 | 0.2151 | 0.216 | 0.9936 |
| LMDS | 3.6049 | 0.2151 | 0.4381 | 0.9966 |
Kinetics studies: Phoenix Dactylifera kinetics studies were not according to pseudo first order reaction but is according to second order Table 3 as the experimental values of K1 and qe were not according to calculated values and the R2 (correlation coefficients) were low Figure 7a. Whereas k2 (pseudo second order rate constant) and qe values agreed with experimental data and R2 values is a good fit for pseudo second order reaction Figure 7b.
Conclusions and Recommendations
On the basis of the result it can be concluded that Date seed (Phoenix Dactylifera) could be successfully used for As (III) removal from aqueous solutions. The results obtained suggest that LMDS is a good adsorbent as compared to RDS because it had more active sites. Experimental data fitted very well to the Freundlich adsorption isotherm models. FTIR spectra identified different functional group present in the Phoenix Dactylifera. Process adopted is simple and economically valueable. It could be used for sorption of different other heavy metals like Cd, Pb etc. and also for different dyes such as methylene blue etc. from water. Activation by lemon juice was very effective, low cost and at the end of reaction sludge or by products were not formed. This method is easily applicable in agricultural country where a lot of agricultural waste is present and lemon juice is also abundantly available. This method can be applied on industrial scale to remove contaminants from waste water.
Acknowledgement
All this research work was conducted and supported by the Central Lab. of Lahore College for Women University, Jail Road Lahore Pakistan.
Authors Contribution
All this work was planned and supervised throughout by Dr. Tahira Moeen Khan. Iram Riaz and Shahida Hameed helped in sample preparation and statistical data. Prof. Dr. Bushra Khan helped in the analyzing of data.
Conflict of interest
The authors have declared no conflict of interest.
References
Agrafioti, E., Kalderis, D., and Diamadopoulos, E., 2014. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. Journal of environmental management. 133: 309-314. https://doi.org/10.1016/j.jenvman.2013.12.007
Amin, M.N., Kaneco, S., Kitagawa, T., Begum, A., Katsumata, H., Suzuki, T. and Ohta, K., 2006. Removal of arsenic in aqueous solutions by adsorption onto waste rice husk. Industrial and Engineering Chemistry Research. 45(24): 8105–8110. https://doi.org/10.1021/ie060344j
Amin, T.M., Alazba, A.A., Amin, N.M. and Polish. 2017. Absorption Behaviours of copper, lead, and arsenic in aqueous solution using date palm fibres and orange peel: Kinetics and thermodynamics. Polish Journal of Environmental Studies. 26(2): 543-557. https://doi.org/10.15244/pjoes/66963
Asif, Z. and Chen, Z., 2017. Removal of arsenic from drinking water using rice husk. Applied Water Science. 7(3): 1449-1458. https://doi.org/10.1007/s13201-015-0323-x
Azimi, A., Azari, A., Rezakazemi, M. and Ansarpour, M., 2016. Removal of Heavy Metals from Industrial Wastewaters: A Review. Chem. Bio. Eng. Reviews. 4 (1): 1-24. https://doi.org/10.1002/cben.201600010
Barakat, M.A., 2011. New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry, 4(4): 361-377. https://doi.org/10.1016/j.arabjc.2010.07.019
Basso, M.C., Cerrella, E.G. and Cukierman, A.L. 2002. Lignocellulosic Materials as Potential Biosorbents of Trace Toxic Metals from Wastewater. Industrial and Engineering Chemistry Research. 41(15): 3580-3585. https://doi.org/10.1021/ie020023h
Brouwer, R., Akter, S., Brander, L. and Haque, E., 2007. Socioeconomic vulnerability and adaptation to environmental risk: a case study of climate change and flooding in Bangladesh. Risk Analysis. 27(2): 313-326. https://doi.org/10.1111/j.1539-6924.2007.00884.x
Chen, S., Yue, Q., Gao, B. and Xu, X., 2010. Equilibrium and kinetic adsorption study of the adsorptive removal of Cr (VI) using modified wheat residue. Journal of Colloid and Interface Science. 349(1): 256-264. https://doi.org/10.1016/j.jcis.2010.05.057
Chi, T., Zuo, J. and Liu, F., 2017. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Frontiers of Environmental Science and Engineering. 11(2): 15. https://doi.org/10.1007/s11783-017-0921-y
Chong, H.L.H., Chia, P.S., and Ahmad, M.N., 2013. The adsorption of heavy metal by Bornean oil palm shell and its potential application as constructed wetland media. Bioresource technology. 130: 181-186. https://doi.org/10.1016/j.biortech.2012.11.136
Davis, D.A., Webb C., Sorensen, L.J. and Dixon, J.D., 2018. Thermodynamic constraints on limestone-based arsenic removal from water. Environmental Earth Sciences. 77(2): 1-9. https://doi.org/10.1007/s12665-017-7204-6
Deng, S., Bai, R. and Chen, J.P., 2003. Behaviors and mechanisms of copper adsorption on hydrolyzed polyacrylonitrile fibers. Journal of Colloid and Interface Science. 260(2): 265-272. https://doi.org/10.1016/S0021-9797(02)00243-6
Devi, R.R., Umlong, M.I., Das, B., Borah, K., Thakur, J.A., Raul, K.P. and Singh, L., 2014. Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Applied Water Science. 4: 175-182. https://doi.org/10.1007/s13201-013-0139-5
Dieme, M.M., Villot, A., Gerente, C., Andres, Y., Diop, S.N. and Diawara, C.K., 2017. Sustainable conversion of agriculture wastes into activated carbons: energy balance and arsenic removal from water. Environmental Technology. 38(3): 353-360. https://doi.org/10.1080/09593330.2016.1193225
Eccles, H., 1999. Treatment of metal-contaminated wastes: why select a biological process? Trends in Biotechnology. 17(12): 462. https://doi.org/10.1016/S0167-7799(99)01381-5
Genç, H., Tjell, C.J., McConchie, D. and Schuiling, O., 2003. Adsorption of arsenate from water using neutralized red mud. Journal of Colloid and Interface Science. 264(2): 327-334. https://doi.org/10.1016/S0021-9797(03)00447-8
Hall, A.H., 2002. Chronic arsenic poisoning. Toxicology Letters. 128(1-3): 69-72. https://doi.org/10.1016/S0378-4274(01)00534-3
Ismaiel, A.A., Aroua, M.K. and Yusoff, R., 2013. Palm shell activated carbon impregnated with task-specific ionic-liquids as a novel adsorbent for the removal of mercury from contaminated water. Chemical Engineering Journal. 225: 306-314. https://doi.org/10.1016/j.cej.2013.03.082
Javanbakht, V., Alavi, S.A. and Zilouei, H., 2014. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Science Technology. 69(9): 1775-1787. https://doi.org/10.2166/wst.2013.718
Kratochvil, D. and Volesky, B., 1998. Advances in biosorption of heavy metals. Trends in Biotechnology. 16(7): 291-300. https://doi.org/10.1016/S0167-7799(98)01218-9
Kumari, P., Sharma, P., Srivastava, S. and Srivastava, M.M., 2005. Arsenic removal from the aqueous system using plant biomass: a bioremedial approach. Journal of Industrial Microbiology and Biotechnology. 32(11-12): 521–526. https://doi.org/10.1007/s10295-005-0042-7
Liu, Z., Huang, Y., and Zhao, G., 2016. Preparation and characterization of activated carbon fibers from liquefied wood by ZnCl2 activation. BioResources. 11(2): 3178-3190. https://doi.org/10.15376/biores.11.2.3178-3190
Malwal, D., and Packirismy, G., 2017. Silica stabilized magnetic-chitosan beads for removal of arsenic from water. Colloid and Interface Science Communications. 19: 14-19. https://doi.org/10.1016/j.colcom.2017.06.003
Oliveira, L.C., Pereira, E., Guimaraes, I.R., Vallone, A., Pereira, M., Mesquita, J.P. and Sapag, K., 2009. Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. Journal of Hazardous Materials. 165(1-3): 87-94. https://doi.org/10.1016/j.jhazmat.2008.09.064
Ozer, A., Tanyildizi, M.S., and Tumen, F., 1998. Study of cadmium adsorption from aqueous solution on activated carbon from sugar beet pulp. Enviornmental Technology. 19(11): 1119-1125. https://doi.org/10.1080/09593331908616770
Pokhrel, D., and Viraraghavan, T., 2006. Arsenic removal from an aqueous solution by a modified fungal biomass. Water Research. 40(3): 549-552. https://doi.org/10.1016/j.watres.2005.11.040
Qureshi, I. and Shahabuddin, M., 2012. Synthesis and application of calixarene-based functional material for arsenic removal from water. Applied Water Science 2(3): 177-186. https://doi.org/10.1007/s13201-012-0035-4
Raj, K.R., Kardam, A. and Srivastava, S., 2013. PEI modified Leucaena leucocephala seed powder, a potential biosorbent for the decontamination of arsenic species from water bodies: bioremediation. Applied Water Science. 3(1): 327-333. https://doi.org/10.1007/s13201-012-0057-y
Rehman, R., Mahmud, T., Kanwal, F., Aslam, M.N., Latif, R. and Nasir, H., 2013. Chemical modification of Oryza sativa linnaeus husk with urea for removal of brilliant vital red and murexide dyes from water by adsorption in environmentally benign way. Journal of Chemical Society of Pakistan. 35 (2): 510-515.
Roy, P., Dey, U., Chattoraj, S., Mukhopadhyay, D., and Mondal, N.K., 2017. Modeling of the adsorptive removal of arsenic (III) using plant biomass: a bioremedial approach. Applied Water Science. 1307-1321. https://doi.org/10.1007/s13201-015-0339-2
Roy, P., Mondal, N.K., Bhattacharya, S., Das, B. and Das, K., 2013. Removal of arsenic (III) and arsenic (V) on chemically modified low-cost adsorbent: batch and column operations. Applied Water Science. 3(1): 293-309. https://doi.org/10.1007/s13201-013-0082-5
Saha, J.C., Dikshit, A.K., Bandyopadhyay, M. and Saha, K.C., 1999. A Review of Arsenic Poisoning and its Effects on Human Health. Critical Reviews in Environmental Science and Technology, 29 (3): 281-313. https://doi.org/10.1080/10643389991259227
Salman, M., Athar, M. Farooq, U., Rauf, S. and Habiba, U., 2014. A new approach to modification of an agro-based raw material for Pb (II) adsorption. Korean Journal of Chemical Engineering. 31 (3): 467-474. https://doi.org/10.1007/s11814-013-0264-8
Sawana, R., Somasundar, Y., Iyer, S.V. and Baruwati, B., 2017. Ceria modified activated carbon: an efficient arsenic removal adsorbent for drinking water purification. Applied Water Science. 7(3): 1223-1230. https://doi.org/10.1007/s13201-016-0398-z
Schwantes, D., Goncalves, C.A.J., Coelho, F.G., Campagnolo, A.M., Dragunski, C.D., Tarley, T.R.C., Miola, J.A. and Leismann, V.A.E., 2016. Chemical Modifications of Cassava Peel as Adsorbent Material for Metals Ions from Wastewater. Journal of Chemistry, 2016: 15 pages. https://doi.org/10.1155/2016/3694174
Sharma, S. and Bhattacharya, A., 2017. Drinking water contamination and treatment techniques. Applied Water Science. 7(3): 1043-1067. https://doi.org/10.1007/s13201-016-0455-7
Sidhu, M., Sama, P., Parmar, J. and Bhatt, M.S., 2014. Biosorption of Arsenic (III) from drinking water by using low cost biosorbents derived from peels of Oranges, Turnip and Peanut shells. International Journal of Pharmaceutical Research and Drug Development. 1(1): 66-69.
Smith, H.A., Lingas, O.E. and Rahman, M., 2000.Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bulletin of the World Health Organization. The International Journal of Public Health. 78 (9): 1093-1103.
Srivastava, S. and Anil, D.K., 2016. Biological wastes the tool for biosorption of arsenic. Journal of Bioremediation and Biodegradation. 7(1): 323. https://doi.org/10.4172/2155-6199.1000323
Vieira, B., Pintor, A., Boaventura, R.A., Botelho, C.M. and Santos, S., 2017. Arsenic removal from water using iron-coated seaweeds. Journal of environmental management. 192: 224-233. https://doi.org/10.1016/j.jenvman.2017.01.054
Wasiuddin, N.M., Tango, M. and Islam, M.R., 2002. A novel method for arsenic removal at low concentrations. Energy Sources. 24(11): 1031-1041. https://doi.org/10.1080/00908310290086914
To share on other social networks, click on any share button. What are these?





