Livestock Forage Resources in Manokwari Lowland Valley; A Case Study of West Papua’ Abundancy, Richness, and Potential
Research Article
Livestock Forage Resources in Manokwari Lowland Valley; A Case Study of West Papua’ Abundancy, Richness, and Potential
Deny Anjelus Iyai1*, Ambo Ako2, Sitti Nurani Siradjuddin2, Budiman Nohong2
1Ph.D. of Animal Science Study Program. Animal Science Faculty, Universitas Hasanuddin, Jl. Tamalanrea. Km. 10. Makassar-Indonesia; 2Animal Science Faculty, Hasanuddin University, Jl. Tamalanrea. Km. 10. Makassar-Indonesia
Abstract | Typical areas of oil palm plantations and land-use change are open gates to biodiversity loss. The aim of the research was to find out the types of plants that can be a source of natural food for livestock both inside and outside the oil palm land. The study area was purposively selected from 9 districts (subdistricts) as many as 4 districts (44.44%). Withdrawal of grass clippings is done using a quadrant measuring 1 x 1 m2. Quadrant laying is done diagonally in a land area of 100 m2. Dominance Index, Species abundance using Shannon-Weiner Diversity Index, Similarity index, Species richness). The number of plant families identified was 751 families spread over 4 districts, with 890 species of grass, legume and non-grass/non-legume plants. There were 11 families found in total in the observation plots in each district, namely Compositae, Poaceae, Fabaceae, Rubiaceae, Cyperaceae, Moraceae, Lamiaceae, Melastomataceae, Acantaceae, Peperomiaceae and Verbenaceae. Dominant plant species are in the range of 0.01-0.03, abundance is in the range of 1.65-3.87, evenness is in the range of 0.76-1.67 and species richness is in range of numbers 2.74-4.66. The range of scarcity numbers is in the numbers 1-29 (2.08-60.42%), followed by the adequacy status of animal feed is in the range 144-248 (19.17% -33.02%), while the abundance of animal feed livestock is in the range of 0.00% -66.67%. Cattle and goat feed is more available with a range of 39-84 (16.96% -36.52%). Whereas for pigs it is quite low, which is in the range of 0-3 (0.00% -75.00%).
Keywords | Forages diversity, Family richness, Species richness, Palm oil plantation, Communal free ranch
Received | January 11, 2022; Accepted | May 20, 2023; Published | July 15, 2023
*Correspondence | Deny Anjelus Iyai, Ph.D. of Animal Science Study Program. Animal Science Faculty, Universitas Hasanuddin, Jl. Tamalanrea. Km. 10. Makassar-Indonesia; Email: [email protected]
Citation | Iyai DA, Ako A, Siradjuddin SN, Nohong B (2023). Livestock forage resources in manokwari lowland valley; a case study of west papua’ abundancy, richness, and potential. Adv. Anim. Vet. Sci. 11(9): 1492-1505.
DOI | https://doi.org/10.17582/journal.aavs/2023/11.9.1492.1505
ISSN (Online) | 2307-8316

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
In the third world like Indonesia, tropical land uses are still shifting. The lands are being converted into various land uses for production (Wijka et al., 2018; Irvine et al., 1999; Abbas and Muhtarom, 2018). The need to convert land for various development purposes and uses in developing countries is unquestionable. Each space has an important meaning when other users need it. The competing land between humans and animals and the role of the landscape cannot be denied. In developing countries like Indonesia, livestock such as cows, goats, sheep and pigs can have free space to maintain their natural life activities (Kondombo 2005; Mutibvu et al., 2012). Land uses in Manokwari consist of tropical forests (64.31%), followed by oil palm plantations (23.16%), communal land (4.88%), transmigrate areas (2.12%), arable land (2, 09%), and paddy fields (0.78%) (Iyai et al., 2020) . The one is for planting oil palm fruits (Elaeis guineensis). Elaeis guineensis is the first-fifth top crops grown in Indonesia besides cereals, rice/paddy, root and tubers and sugar cane. Oil-palm plantation has been grown in four districts, i.e. Warmare, Prafi, Masni, and Sidey. Total areas of oil palm are 350.000 ha. Besides, land size where paddy is grown is 3144,83 ha, followed by mays 1334,67 ha, and taro 475,26 ha (Iyai et al., 2020) .
Indonesia’s nature is rich in plant diversity (Kusmana and Hikmat 2015; Nahlunnisa et al., 2016; Teuscher et al., 2016; Walujo et al., 1991). As one of the countries that has the largest forest in the world (9th position), has a tropical climate, is located between two continents and two oceans, and consists of islands, of course, Indonesia has a variety of endemic plants. About 40,000 species of plants live in Indonesia. This number consists of about 25,000 species of seed plants or about 10% of the world’s total seed plants, as well as 35,000 species of mosses and algae. Nearly 40% of the total plant species in Indonesia are endemic vegetation that cannot be found in other parts of the world. Plant biodiversity under palm oil plantation stands has been done in several places where plantation exists (Nahlunnisa et al., 2016; Teuscher et al., 2016). However, places like Indonesia particularly in several provinces where palm plantations exist lack complete information on plant diversity. Plant diversity will be used for many applications. The one is a plant for forages for livestock (Ouali et al., 2023; Firison and Brata 2018). Therefore, indicators of the diversity such as abundance, diversity, richness, dominance and frequency and economic benefits must become priority. Diversity can become a proper indicator for quality and environmental degradation (Asefa et al., 2020).
What factors induce abundance, richness and potentials of the plants are studied by several scholars such as Kusmana and Hikmat (2015), Raven and Wackernagel (2020), Firison and Brata (2018), and Corlett (2016). Some found that livestock movement under palm oil plantation stands can induce plant richness, abundance and distribution. Under palm oil plantation, farmers will have free choice and patterns for pasturing livestock around land uses. livestock can have free access to natural resources such as forests, scrub, water, natural shelter, waste and residues. Other distinctive natural resources are secondary forest and oil palm trees. These oil palm plantations have been defined as a major threat to biodiversity loss (Hernández-Yáñez et al., 2016; Barbault 2013; Lerman et al., 2018; Brashares et al., 2011). Malaysia and Indonesia are the two countries that have the largest oil palm plantations in the world. Under this environment, integrated livestock farming is developed intensively and extensively.
Typical areas of oil palm plantations and land-use change are open gateways to biodiversity loss (Leitner and Turner 2017; Turner et al., 2011). Therefore, disturbance can originate from human intervention, which has access to the habitat, as a result of social (Obidzinski et al., 2012) and livelihood conflicts (Rist et al., 2010). Sodhi et al. (2010) shortly concluded that bad and lack of infrastructure affects the loss of biodiversity. It can be seen that human intervention and dependence on the area is varied, high and unavoidable. Therefore, the reduction factor for biodiversity loss must be minimized.
The diversity of types of animal feed of plant origin is influenced by the occurrence and pressure of the demand for animal feed. The distribution and richness of plant species will be greatly influenced by livestock. Therefore, this study was conducted to map the richness and diversity of plant species in and around the oil palm area, including the free ranches area, former plantations, and former rice fields. The indicators measured are dominance index, species abundance index, evenness index, and species richness index (Corlett 2020; Brummitt et al., 2021; Simone et al., 2018; Gao et al., 2020; Corlett 2016). Knowing how many types of animal feed and non-fodder and their distribution will determine productivity and the carrying capacity of forage resources and animal productivity (Prihantoro et al., 2023; Ouali et al., 2023; Lüscher et al., 2020; Walujo et al., 1991; Kamau 2004). The main objective of this research is to find out the types of plants that can be a source of natural feed for livestock both inside and outside the oil palm plantations in the lowlands of Manokwari.
Materials and Methods
Research Sites
Manokwari Regency is divided into 9 districts, which have a total area of 4,650.32 km². Manokwari Regency with its 9 districts is astronomically placed below the equator, between 0”14’ S and 130”31’ E. The geographical boundaries of Manokwari Regency (BPS Manokwari 2022) are in the West bordering Tambrauw Regency, in the North it is bordered by the Pacific Ocean, while in the East is the Pacific Ocean and the South is the Arfak Mountains District and South Manokwari (Figure 1).
Table 1: Sum of sampling plot on land use types in the Warpramasi valley.
| District |
Palm oil plantation (ha,%) |
Arable land (ha,%) |
Paddy field (ha,%) |
Communal land (ha,%) |
Sample Size (ha,%) |
| Sidey | 3 (2,93) | 1 (0,98) | 1 (0,98) | 0 | 5 (4,89) |
|
Masni |
4 (3,91) | 1 (0,98) | 0 | 0 | 5 (4,89) |
| Prafi | 2 (1,95) | 0 | 0 | 2 (1,95) | 5 (4,89) |
| Warmare | 3 (2,93) | 1 (0,98) | 0 | 1 (0,98) | 5 (4,89) |
| Total (ha,%) | 12 (11,73) | 3 (2,93) | 1 (0,98) | 3 (2,93) | 20 (19,55) |
The time allocated to this research was one month and done from March to April 24th 2023. The study area of Manokwari Lowland Valley (MLV) was purposively selected from 9 districts (subdistricts) and purposively chosen 4 districts (44.44%), i.e. Sidey, Masni, Prafi and Warmare (Warpramasi). The basis for choosing these four areas is that these areas have been widely used for several types of uses, namely plantations, transmigration areas, arable land, communal land, and as livestock production centers in Manokwari. The total study area is 1,022.67 km2 (102,266.54 ha) (Iyai et al., 2020). In general, the profile of the study area consists of coastal, lowland and highland areas. Based on information from the Manokwari Regency of Meteorological office, clear precipitation conditions between wet months (rain) and dry months are from December to May (6 months) for 221 days with rainfall of 287.4 mm2. While the dry months are from June to November (6 months) every year.
Research design and sampling technique
The field research approach is carried out by means of exploratory research. In exploratory research, the approach is carried out using primary data. The primary data collected comes from field observations, measurements, and calculations at the research location. The grass sampling locations was carried out using a purposive technique (intentionally) (Kunarso and Azwar 2013; Prihantoro et al., 2023) and grass plotting applied by using transect baseline technique (Fachrul 2007). Withdrawal of grass clippings is done using a quadrant measuring 1 × 1 m2 (Figure 2). Quadrant laying is done diagonally in a land area of 100 m2 (Kartikawati et al., 2023).
The number of samples at this research location was 20 unit of the plots (Table 1), where one plot represents one hectare which is found in the land use types of oil palm plantations, garden land, rice land, and communal land. Thus, the total area of land used is 20 ha (19.55%) of 102,266.54 ha. Thus, the number of sample plots obtained is 4 districts × 5 plots × 5 clippings, which is 100 grass clippings. The fixed number of plots in each land use of district were taken using reference of (Susetyo 1980) and by considering the homogeneity of the land use, each hectare represented one plot.
Instrument of collected data
Parameters measured according to Teuscher et al. (2016), Dahal et al. (2023), Naah and Braun 2019 is the Dominance Index 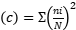 , Species abundance using the Shannon-Weiner Diversity Index index
, Species abundance using the Shannon-Weiner Diversity Index index 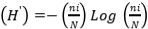 , Evenness index (Similarity index)
, Evenness index (Similarity index) 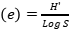 , Species richness
, Species richness 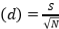 , where ni=number of individuals of species i, N=number of individuals of all samples, S=number of species of all samples. The dominance index ranges from 0 to 1, where the smaller the dominance index value indicates that no species dominates, conversely, the greater the dominance, it indicates that there is a certain species (Odum 1993). Index diversity is classified based on the criteria if H’> 3 then the species diversity is very high, if H’ is 1.6 to 3, the species diversity is high. If H’ is between 1 and 1.5, the species diversity is moderate. Meanwhile, if H’<1, the species diversity is low. The range of uniformity indices (Magurran 1988) : E = 0 – 1; E is close to 0, so the distribution of individuals between species is uneven/there are certain species that are dominant. E is close to 1, so the distribution of individuals between species is even.
, where ni=number of individuals of species i, N=number of individuals of all samples, S=number of species of all samples. The dominance index ranges from 0 to 1, where the smaller the dominance index value indicates that no species dominates, conversely, the greater the dominance, it indicates that there is a certain species (Odum 1993). Index diversity is classified based on the criteria if H’> 3 then the species diversity is very high, if H’ is 1.6 to 3, the species diversity is high. If H’ is between 1 and 1.5, the species diversity is moderate. Meanwhile, if H’<1, the species diversity is low. The range of uniformity indices (Magurran 1988) : E = 0 – 1; E is close to 0, so the distribution of individuals between species is uneven/there are certain species that are dominant. E is close to 1, so the distribution of individuals between species is even.
Data analysis
The data analysis used consisted of descriptive and inferential statistical analysis which included number, mean, proportion and ranking. To see the difference in mean by district, a comparison test was carried out k samples from Kruskal-Wallis using XLStat software (XLSTAT 2009). Identification of grass species including families was carried out by using a determination key book (identification book) entitled Weed of Rice in Indonesia editor by Soerdjani et al. (1987) and Steenis Van (2006). Data analysis results are presented in the form of maps, tables, graphs and pictures.
Results and Discussions
Typical family of plants
The general description of the plants that were identified came from 147-209 families in four districts, namely Sidey, Masni, Prafi and Warmare (Table 2). The findings in the field indicated that Masni district had the highest number of families at 209 (27.83%, ranking 1), followed by Sidey (206, 27.43%, ranking 2), Prafi 189 (25, 17%, ranking 3) and Warmare 147 (19.57%, ranking 4).
The minimum and maximum number of grass, legume and non-grass/non-legume families found in the four study location districts ranged from 147-209 families and 150-207 families respectively (Figure 3). It was found that there was no significant difference (p>0.05) in the diversity of plant families between districts in the MLV (Warpramasi) region. Family richness indicates that the existing ecosystem conditions in the Warpramasi valley can provide an abundant and varied diversity of forage, both in terms of diversity and plant growth. The land in the MLV (Warpramasi) region still provides living space for various functions and roles of forage plants which can be used as animal feed directly and also as a provider of nutrients for soil fertility and guarantees the growth of grass/other legumes.
The increase in the number of forage/non-fodder plant families will decrease and reach a flat line. The increase that occurred was not too significant. When the curve shows an almost flat line, the counting activity should be stopped because the results obtained are close to or have even reached the maximum number. If the number of families identified has reached the maximum number, a significant addition of families will be obtained in the 1 x 1 m plot included optimal plots in the Minimum Species Area Curve theory. This finding confirms some of the field researches done in several regions of Indonesia such as in Lampung, Riau and Java (Ismaini et al., 2015; Firison and Brata 2018; Kartikawati et al., 2023; Teuscher et al., 2016; Kusmana and Hikmat 2015; Kunarso and Azwar 2013).
The general description of the plant species identified was from 150-207 species in four districts of the MLV (Warpramasi) (Figure 4). The findings in the field indicated that the Sidey and Masni districts had the highest number of species, 207 (27.20%, ranking 1), followed by Prafi 197 (25.89%, ranking 2) and Warmare 150 (19.71%, ranking 3). The distribution of plant species and trend charts in each district can be seen in Figures (Figures 4-5).
The increase in the number of species of forage/non-fodder plant species will decrease and reach a flat line. The increase that occurred was not too significant. When the curve shows an almost horizontal line, the counting activity should be stopped because the results obtained are close to or have even reached the maximum number. If the number of species identified has reached the maximum number, a significant increase in species will be obtained in the 1 x 1 m plot. included optimal plots in the Minimum
Table 2: Number of family and species identified at location of Warpramasi (MLV).
|
District |
Family |
% |
Rank |
Species |
% |
Rank |
|
Sidey |
206 |
27,43 |
2 |
207 |
27,2 |
1 |
|
Masni |
209 |
27,83 |
1 |
207 |
27,2 |
1 |
|
Prafi |
189 |
25,17 |
3 |
197 |
25,89 |
2 |
|
Warmare |
147 |
19,57 |
4 |
150 |
19,71 |
3 |
|
Sum |
751 |
|
|
761 |
|
|
|
Mean |
187,75ns |
|
|
190,25ns |
|
|
|
Stdv |
28,56 |
|
|
27,24 |
|
|
|
Minum |
147 |
|
|
150 |
|
|
|
Maximum |
209 |
|
|
207 |
|
ns: not significant at p<0,05
Table 3: Number of plant families and species identified in the MLV (Warpramasi).
|
District |
|
|
Types of Plants |
|
|||||||
|
Grass |
% |
Rank |
Legume |
% |
Rank |
Non Grass/Legume |
% |
Rank |
|||
|
Sidey |
43 |
18,7 |
3 |
15 |
6,52 |
4 |
149 |
28,93 |
2 |
||
|
Masni |
64 |
27,83 |
2 |
85 |
58,62 |
1 |
180 |
34,95 |
1 |
||
|
Prafi |
84 |
36,52 |
1 |
32 |
22,07 |
2 |
77 |
14,95 |
4 |
||
|
Warmare |
39 |
16,96 |
4 |
13 |
8,97 |
3 |
109 |
21,17 |
3 |
||
|
Sum |
230 |
|
|
145 |
|
|
515 |
|
|
||
|
Mean |
57,5* |
|
|
36,25* |
|
|
128,75* |
|
|
||
|
Stdv |
20,793 |
|
|
|
|
33,599 |
|
|
45,110 |
|
|
|
Minimum |
39,000 |
|
|
|
|
13,000 |
|
|
77,000 |
|
|
|
Maximum |
84,000 |
|
|
|
|
85,000 |
|
|
180,000 |
|
|
*Significant at p<0,05
Species Area Curve theory. This finding species of the plants confirms some of the field researches done in several regions of Indonesia such as in Batturaden Java and Riau (Arisandy and Triyanti 2020; Prihantoro et al., 2023; Wardah 2005).
Botanical composition of the forages
The fodder and non-fodder plants found at the study site included 230 types of grass, 145 types of legumes and 515 types of non-grass/non-legume (Table 3).
The highest grass composition was in the Prafi district, namely 84 species (36.52%), followed by Masni 64 species (27.83%), Sidey 43 species (18.70%) and the least in Warmare, namely 39 species (16 ,96%). The largest legume composition was dominated in Masni district with 85 legume species (58.62%), followed by Prafi 32 species (22.07%), Warmare with 13 species (8.97%) and finally Sidey 15 species (10.34%). Means plot is highlighted in Figure 6. Meanwhile, non-grass/non-legume plants were dominated by the Masni district with 180 species, followed by Sidey with 149 species, Warmare with 109 species and lastly the Prafi district with 77 species. Figure 7-14 high
lights trend curves for each district at the MLV (Warpramasi).
Some researchers as well confirms similar finding such as Kusmana and Hikmat (2015), Teuscher et al. (2016), Firison and Brata (2018), Nahlunnisa et al. (2016), and Prihantoro et al. (2023). In the other sides of the world, the grass, legume and non-legumes findings confirms by
Table 4: Family plants found in the fourth districts of the MLV (Warpramasi).
| District |
No. of List |
Family |
Sum of Family |
Rank |
| Sidey | 1 | Compositae | 32 | 1 |
| 2 | Poaceae | 31 | 2 | |
| 3 | Fabaceae | 17 | 3 | |
| 4 | Rubiaceae | 10 | 4 | |
| 5 | Cyperaceae |
10 |
4 | |
| 6 | Moraceae | 10 | 4 | |
| 7 | Lamiaceae | 9 | 5 | |
| Masni | 1 | Poaceae | 28 | 1 |
| 2 | Fabaceae | 19 | 2 | |
| 3 | Rubiaceae |
16 |
3 | |
| 4 | Compositae | 16 | 3 | |
| 5 | Cyperaceae | 11 | 4 | |
| 6 | Lamiaceae | 9 | 5 | |
| Prafi | 1 | Compositae | 43 | 1 |
| 2 | Poaceae | 34 |
2 |
|
| 3 | Cyperaceae | 30 | 3 | |
| 4 | Rubiaceae | 9 | 4 | |
| 5 | Melastomataceae | 4 | 5 | |
|
Warmare |
1 | Poaceae | 41 | 1 |
| 2 | Compositae | 32 | 2 | |
| 3 | Acantaceae | 27 | 3 | |
| 4 | Fabaceae | 9 | 4 | |
| 5 | Peperomiaceae | 9 | 4 | |
| 6 | Verbenaceae | 8 |
5 |
Table 5: Species of plants found in the fourth district of the MLV (Warpramasi).
| District |
No. |
Species |
Total |
Rank |
| Sidey | 1 | Mikania micrantha Kunth | 12 | 1 |
| 2 | Ageratum conyzoides L. | 9 | 2 | |
| 3 | Nephrolepis falcata (Sw.) Schott | 7 | 3 | |
| 4 | Paspalum conjugatum P.J. Bergius | 7 | 3 | |
| 5 | Phyllanthus niruri L. | 6 | 3 | |
| 6 | Digitaria ternata (A.Rich.) Stapt | 6 | 3 | |
| 7 | Croton hirtus L. her. | 5 | 4 | |
| 8 | Lindernia ciliata (Colsm) Pennell | 5 | 4 | |
| 9 | Ludwigia octovalvis (Jacq.) | 5 | 4 | |
| 10 | Calopoginium mucunoides DESV. | 5 | 4 | |
| 11 | Sida rhombifolia L. | 5 | 4 | |
| 12 | Axonopus compressus (Sw.) P.Beau. | 5 | 4 | |
| 13 | Cyperus monocephala Endl. | 5 | 4 | |
| 14 | Oldenlandia corymbosa L. | 5 | 4 | |
| 15 | Cleome rutidosperma DC. | 4 | 5 | |
| 16 | Leucas davandulifolia SM. | 4 | 5 | |
| 17 | Mimosa pudica Linn. | 4 | 5 | |
| 18 | Ficus septica | 4 | 5 | |
| 19 | Musa akuminata Colla | 4 | 5 | |
| Masni | 1 | Cyperus monocephala Endl. | 10 | 1 |
| 2 | Borreria laevis (Lamk.) Griseb. | 9 | 2 | |
| 3 | Axonopus compressus (Sw.) P.Beauv. | 8 | 3 | |
| 4 | Hyptis capitata jacq. | 8 | 3 | |
| 5 | Selaginella willdenowii (Desv.ex.Poir.) Baker | 8 | 3 | |
| 6 | Piper aduncum L. | 8 | 3 | |
| 7 | Borreria laevis (Lamk.) Griseb. | 7 | 4 | |
| 8 | Stachytarpheta jamaicensis (L.) vahl | 7 | 4 | |
| 9 | Nephrolepis falcata (Sw.) Schott | 6 | 5 | |
| 10 | Calopoginium mucunoides DESV. | 6 | 5 | |
| 11 | Digitaria ternata (A.Rich.) Stapt | 6 | 5 | |
| 12 | Ludwigia octovalvis (Jacq.) | 6 | 5 | |
| 13 | Cynedrella nodiflora (L.) Kunth. | 6 | 5 | |
| 14 | Lindernia ciliata (Colsm) Pennell | 6 | 5 | |
| Prafi | 1 | Chromolaea odorata (L.) Rmking & H.rob | 12 | 1 |
| 2 | Cyperus rotundus L. | 11 | 2 | |
| 3 | Gynura sp. | 10 | 3 | |
| 4 | Axonopus compressus (Sw.) P.Beau. | 10 | 3 | |
| 5 | Grona triflora (L.) H.Ohashi & K.Ohashi | 10 | 3 | |
| 6 | Cyperus monocephala Endl. | 10 | 3 | |
| 7 | Cyperus distans L. | 9 | 4 | |
| 8 | Sonchus arvensis L. | 7 | 5 | |
| Warmare | 1 | Asystasia gengatica (L.) T Anderson | 20 | 1 |
| 2 | Mikania micrantha Kunth | 10 | 2 | |
| 3 | Chromolaena odorata (L.) Rmking & H. Rob. | 10 | 2 | |
| 4 | Ageratum conyzoides L. | 9 | 3 | |
| 5 | Calopoginium mucunoides DESV. | 9 | 3 | |
| 6 | Stachytarpheta jamaicensis (L.) vahl | 8 | 4 | |
| 7 | Sonchus arvensis L. | 7 | 5 | |
| 8 | Eleusina indica (L) Geartn. | 7 |
5 |
several scholars such as Naah and Braun (2019) in West Africa, Qian et al. (2020), Liu et al. (2023) in China, and Tulu et al. (2023), and Hernández-Yáñez et al. (2016) in United State of America.
Family and species dominance
At the research location in Sidey district, 7 families of grass, legume and non-grass/legume plants were found. Meanwhile, in the Masni district, 6 families were found (Table 4). In the other two districts, 5 and 6 plant families were found, respectively. Thus, in the plains of Warpramasi, 24 families of grass, legume and non-grass/non-legume plants were found (Figure 7-14).
There are 11 families found in total in the observation plots in each district, namely Compositae, Poaceae, Fabaceae, Rubiaceae, Cyperaceae, Moraceae, Lamiaceae, Melastomataceae, Acantaceae, Peperomiaceae and Verbenaceae. In the table above (Table 4), there are 4 plant families that are not found in all district plots, namely Melastomataceae, which are only found in the Prafi district. In addition, Peperomiaceae and Verbenaceae are only found in the Warmare district. Several types of grass and legume plant families spread over these four districts are Compositae, Poaceae, Fabaceae, Rubiaceae, Cyperaceae, Moraceae and Lamiaceae. Kusmana and Hikmat (2015), Teuscher et al. (2016), Firison and Brata (2018), Nahlunnisa et al. (2016), and Prihantoro et al., (2023) confirmed similar finding from some numbers of family plants. In the other sides of the world, the plant family also recorded by several schol
Table 6: Analysis of several dominant indices, abundance, equality and species richness
| District |
Code |
Dominant Index |
Species Abundance |
Similarity Index |
Species Richness |
| Sidey | A | 0,01 | 3,06 | 1,32 | 2,74 |
| Masni | B | 0,02 | 3,87 | 1,67 | 3,88 |
| Prafi | C | 0,01 | 1,92 | 0,84 | 3,09 |
| Warmare | D | 0,03 | 1,65 | 0,76 | 4,66 |
| Mean | 0,018** | 2,625** | 1,148** | 3,593** | |
| Stdv | 0,010 | 1,031 | 0,427 | 0,857 | |
| Minimum | 0,010 | 1,650 | 0,760 | 2,740 | |
| Maximum | 0,030 | 3,870 | 1,670 | 4,660 |
**significant level at p<0,01.
ars such as Naah and Braun (2019) in West Africa, Qian et al. (2020), Liu et al. (2023) in China, and Tulu et al. (2023), and Hernández-Yáñez et al. (2016) in United State of America.
At the research location in Sidey district, 19 species of grass, legume and non-grass/legume plants were found. Meanwhile, in the Masni district, 14 species were found. In the other two districts, 8 plant species were found each (Table 5).
In the table above, there are 21 plant species that were not found in all district plots, which were not evenly distributed in all four districts. The Sidey district includes 10 species viz Paspalum conjugate P.J. Bergius, Phyllanthus niruri L., Croton hirtus L. her., Sida rhombifolia L., Oldenlandia corymbosa L., Cleome rutidosperma DC., Leucas davandulifolia SM., Mimosa pudica Linn., Ficus septica, and Musa akuminata Colla.In the Masni district there are 5 species namely Borreria laevis (Lamk.) Griseb., Hyptis capitata jacq, Selaginella willdenowii (Desv.ex.Poir.) Baker, Piper aduncum L and Cynedrella nodiflora (L.) Kunth.In the Prafi district there are 4 species namely Cyperus rotundus L., Gynura sp., Grona triflora (L.) H.Ohashi & K.Ohashi, and Cyperus distans L..In the Warmare district there are 3 species namely Asystasia gengatica (L.) T Anderson , Mikania micrantha Kunth and Eleusine indica (L) Geartn.
Forages Indexes
The concept of measuring diversity is divided into 3 categories namely species richness (index of species richness), index of diversity or heterogeneity (index of Diversity), and index of evenness (index of evenness). In Table 6, the dominance of the index is higher (0,03) in the Warmare district followed by Masni, Prafi and Sidey.
Index diversity based on criteria, Masni District had the highest species abundance index (3.87) followed by Sidey (3.06), Prafi (1.92) and Warmare (1.65) with an H’ index of “medium”. The similarity index (evenness index) illustrates that Masni district has almost the same level of evenness compared to Sidey, Prafi and Warmare. The plant species richness index as measured in Table 6. Shows that the species richness is higher in the Warmare and Masni districts compared to Prafi and Sidey (Figure 15).
Several scholars studied plant diversity in Indonesia by applying indexes as well such as Arisandy and Triyanti (2020), Kusmana and Hikmat (2015), Kartikawati et al. (2023), Ismaini et al. (2015), Suarna et al. (1970), Kunarso and Azwar (2013), and Wardah (2005). Another indicator used is an Importance Value Index (Prihantoro et al., 2023). Several scholars outside Indonesia, such as from Europe, China, Africa and America as well used such indexing to record plant diversity (Qian et al., 2020; Zheng et al., 2023; Hao and Ma 2023; Kamau 2004; Lüscher et al., 2020).
Browser, Grazer and Dozer
The availability of grass and forage for livestock at the study site was dominated by plants that can be eaten by cattle, goats and pigs. Cattle can be grouped into grazer livestock and goats do more grass browsers than cows. Pigs are more dominant in dozing food on tubers such as taro, cassava and sweet potatoes. Plants available for cattle, goats and pigs can be seen in Table 7 and means plot shown in Figure 16.
Table 7: Available crops for cattle, goats and pigs in the the MLV (Warpramasi).
| District |
Browser/Grazer/Dozer |
||||||||
| Cattle |
% |
Rank |
Goat |
% |
Rank |
Pigs |
% |
Rank |
|
| Sidey | 43 | 18,69 | 3 | 43 | 18,69 | 3 | 1 | 25 | 2 |
| Masni | 64 | 27,83 | 2 | 64 | 27,83 | 2 | 3 | 75 | 1 |
| Prafi | 84 | 36,52 | 1 | 84 | 36,52 | 1 | 0 | 0 | 3 |
| Warmare | 39 | 16,96 | 4 | 39 | 16,96 | 4 | 0 | 0 | 3 |
| Sum | 230 | 230 | 4 | ||||||
| Mean | 57,5* | 57,5* | 1* | ||||||
| Stdv | 20,793 | 20,793 | 1,414 | ||||||
*Significant at p<0,05
Table 8: Status of plant availability for livestock in the MLV (Warpramasi).
| District |
Availability |
||||||||
| Scarcity |
% |
Rank |
Sufficient |
% |
Rank |
Abundant |
% |
Rank |
|
| Sidey | 29 | 60,42 | 4 | 164 | 21,84 | 3 | 12 | 66,67 | 1 |
| Masni | 14 | 29,17 | 3 | 248 | 33,02 | 1 | 4 | 22,22 | 2 |
| Prafi | 1 | 2,08 | 1 | 195 | 25,97 | 2 | 0 | 0 | 4 |
| Warmare | 4 | 8,33 | 2 | 144 | 19,17 | 4 | 2 | 11,11 | 3 |
| Sum | 48 | 751 | 18 | ||||||
| Mean | 12* | 187,75* | 4,5* | ||||||
| Stdv | 12,62 | 45,32 | 5,26 | ||||||
| Minimum | 1 | 144 | 0 | ||||||
| Maximum | 29 | 248 | 12 | ||||||
*Significant at p<0,05
Cattle and goats have the same opportunity to get grass and legumes as a source of fodder in the four districts compared to pigs (Figure 17). The results of a plant inventory in the Warpramasi area found approximately 230 plant species. The first highest composition of plant species for cattle fodder (rank 1) was in the Prafi district (84 species 36.52%), followed by Masni (64 species 27,83%), Sidey (43 species 18,69%) and ranked 4th in the Warmare district (39 species, 16 ,96%). This is the same with goats. For pigs, there were four types of fodder found and the dominant ones were in the Masni district, followed by the Sidey district.
Some researchers as well confirms similar finding such as Firison and Brata (2018) found 53 plants, i.e. 46 genus and 29 family, Nahlunnisa et al. (2016), and Prihantoro et al. (2023). Kunarso and Azwar (2013) found 98 species, Arisandy and Triyanti (2020) found 17 species, and Wardah (2005) found 145 species. In the other sides of the world, the grass, legume and non-legumes findings confirms by several scholars such as Naah and Braun (2019) in West Africa, Qian et al. (2020), Liu et al. (2023) in China, and Tulu et al. (2023), and Hernández-Yáñez et al. (2016) in United State of America.
Availability of the forages
Availability of grass and forage at the study site was dominated by sufficient availability status, followed by very poor availability status (scare) and abundant status (abundant). The availability status of fodder plants can be observed in Table 8.
Scarcity (Scarcity) status was obtained in the Prafi district (2.08%), followed by Warmare (8.33%), Masni (29.17%) and Sidey 60.47%). Prafi district experienced the first sequence of scarcity of forage, followed by Warmare, Masni and Sidey. Sufficient status was obtained in Masni (33.02%), Prafi (25.97%), Sidey (21.84%) and Warmare (19.17%) districts. The order of adequacy was obtained for Masni district, followed by Prafi district, Sidey district and Warmare district. The most abundant status of plant species (plants) was obtained in Sidey district 66.67%), followed by Masni 22.22%), Warmare (11.11%) and Prafi (0.00%). The first rank was obtained by the Sidey district, followed by the Masni district, the Warmare district and the Prafi district. Figure 18-23 shown habitats, ecosystems and livestock grassed under the MLV (Warpramasi).
Some researchers as well confirms similar finding of availability of the grass, legume and non-grass/non legume such as Kartikawati et al. (2023), Arisandy and Triyanti (2020) and Suarna et al. (1970). Kusmana and Hikmat (2015) reported plant scarcity even the endangered plants. Firison and Brata (2018) as well is reported similar finding of the plant scarcity and endangered status. In the other sides of the world, the status of plant availability of the grass, legume and non-legumes also confirms by several scholars i.e. Naah and Braun (2019) in West Africa; Qian et al., (2020), Liu et al. (2023) in China, and Tulu et al. (2023), and Hernández-Yáñez et al. (2016) in United State of America.
The length sizes of land use in study areas are dominated by tropical forest (64.31%), followed by oil palm plantation (23.16%), communal land (4.88%), transmigrate areas (2.12%), arable land (2.09%), and river (1.54%). The rest of less than 1.00% is occupied by ponds (0.11%), grasslands (0.0016%), terrestrial empty land (0.85%), coastal empty land, paddy field (0.78%) (Iyai et al., 2020). Ponds are located inside forest covers areas. Besides, ponds and small lake can be seen closed and around the main road and communal land. Ponds can support water temporary for pigs and other livestock as well as wild animals. In one hand, ponds can be used by human to catch fishes. There are rivers that flow through the areas. The rivers are named Warmare-, Prafi-, Masni- and Sidey rivers. The rivers as well are used by animal as sources for drinking water, seeking food and nesting sites near water sheet areas.
Conclusions
The number of plant families identified was 751 families spread across 4 districts, with 890 species of grass, legume and non-grass/non-legume plants. There were 11 families found in total in the observation plots in each district, namely Compositae, Poaceae, Fabaceae, Rubiaceae, Cyperaceae, Moraceae, Lamiaceae, Melastomataceae, Acantaceae, Peperomiaceae and Verbenaceae. Dominant plant species are in the range of 0.01-0.03, abundance is in the range of 1.65-3.87, evenness is in the range of 0.76-1.67 and species richness is in range of numbers 2.74-4.66. The range of scarcity numbers is in the numbers 1-29 (2.08-60.42%), followed by the adequacy status of animal feed is in the range 144-248 (19.17% -33.02%), while the abundance of animal feed livestock is in the range of 0.00% -66.67%. Cattle and goat feed is more available with a range of 39-84 (16.96% -36.52%). As for pigs, it is low enough which is in the range of 0-3 (0.00% -75.00%).
There are not many areas in Indonesia that have varied ecological conditions and relatively diverse types of livestock kept. This study is very strategic because it can provide an overview of the effects of productivity, productivity vegetation and other ecological factors where livestock interact and of course the economic impact for farmers who keep livestock. Limitation of the study consist of variation of land uses in Warpramasi valley, number of plots measured, and seasons of the year. It is hoped that if there is a positive interaction relationship, this study can recommend an intensified land use for productivity of livestock and their interactions in the aspect of sustainable livestock development.
Acknowledgements
We thanked all three field interviewers from Research Centre for Livestock and Forages, Papua Barat province, namely Mbah Kucit, Mr. Rahman and Mr. Alimin. Laboratory staffs from Faculty of Animal Science namely Mr. Alberth Manyamboi, Ms. Kendi, from Forestry Laboratory namely Ms. Rose, from Soil Laboratory of Agriculture Faculty namely Dr. Mashudi, Mr. Samsul, Mr. Daud Wambrauw, Mrs. Ferawati Runtuboi, Mr. Markus, Mr. Abraham, Mr. Hendrik. We also thanked field surveyors namely Mr. Aditya Lestaluhu, Mr. Jeki Merani, Mr. Lasmit, Mr. Ahmad Masduki, and the Driver Mr. Melvin Ui from Papua University. We thanked Statisticians from Papua University for consulting the statistical analysis. We also thanked all blind reviewers for improving this manuscript to be readable, understandable and publishable.
Ethical Statement
Ethical approval
Consent has been obtained from all the participants for this research and the Animal Ethics Committee of Animal Science Faculty, The University of Papua coordinated by Budi Santoso (No. of Reference letter: SP-004/UN42.3/PP/2022).
COnflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Novelty statement
Plants diversity shown significant number of records found around arable land, communal land, paddy field, and inside palm oil plantation in the Manokwari Lowland Valley (Warpramasi).
Authors’ contribution
DAI, AA, SNS, and BN composted the concept, analysed field data, and write the final manuscript.
References
Abbas W., A. Muhtarom. (2018). “Development of Agriculture Sector in Poverty Reduction In East Java.” Int. J. Econ. Mang. Social Sci. 1 (1): 1–8. http://agripb.gov.in/districts.
Arisandy D.A., M.. Triyanti. (2020). “Keanekaragaman Jenis Vegetasi Di Bukit Cogong Kabupaten Musi Rawas.” BIOEDUSAINS: J. Pendidikan Biologi Dan Sains. 3 (1): 40–49.
Asefa M., M. Cao, Y. He, E. Mekonnen, X. Song, J. Yang. (2020). “Ethiopian Vegetation Types, Climate and Topography.” Plant Divers. 42 (4): 302–11. https://doi.org/10.1016/j.pld.2020.04.004.
Barbault R. (2013). “Loss of Biodiversity, Overview.” Encyclopedia of Biodiversity: Second Edition 4 (1995): 656–66. https://doi.org/10.1016/B978-0-12-384719-5.00298-7.
BPS Manokwari, Kabupaten. (2022). Kabupaten Manokwari Dalam Angka (Manokwari Regency In Figures).
Brashares J. S., C. D. Golden, K. Z. Weinbaum, C. B. Barrett, G. V. Okello. (2011). “Economic and Geographic Drivers of Wildlife Consumption in Rural Africa.” Proceed. Nat. Acad. Sci. 108 (34): 13931–36. https://doi.org/10.1073/pnas.1011526108.
Brummitt N, A. Claudia, A. Timothy. (2021). “Areas of Plant Diversity — What Do We Know ?” Plants People Planet 3 (3): 33–44. https://doi.org/10.1002/ppp3.10110.
Corlett R. T. (2016). “Plant Diversity in a Changing World: Status, Trends, and Conservation Needs.” Plant Divers. 38 (1): 10–16. https://doi.org/10.1016/j.pld.2016.01.001.
Corlett R. T. (2020). “Plant Diversity Safeguarding Our Future by Protecting Biodiversity.” Plant Divers. 42 (4): 221–28. https://doi.org/10.1016/j.pld.2020.04.002.
Dahal U., N. Raut, A. Dhital, S. Mainali, D. Gautam, S. Tripathi. (2023). “Impacts of Livestock Grazing on Wild Ungulate Habitat in the Khata Corridor , Bardiya , Nepal.” Hindawi Int. J. Forest. Res. 2023.
Fachrul M. F. (2007). Metode Sampling Bioekologi. Jakarta: Bumi Aksara.
Firison J., B. Brata. (2018). “Keragaman Jenis Tumbuhan Bawah Pada Tegakan Kelapa Sawit Dan Potensinya Sebagai Pakan Ternak Sapi Potong (Kasus Di Desa Kungkai Baru Kabupaten Seluma).” Naturalis-Jurnal Penelitian Pengelolaan Sumberdaya Alam Dan Lingkungan. 8 (1): 68–76.
Gao J.G., H. Liu, N. Wang, J. Yang, X.l. Zhang. (2020). “Plant Extinction Excels Plant Speciation in the Anthropocene.” BMC Plant Biol. 20 (430): 1–11.
Hao Q, J-S Ma. (2023). “Plant Diversity Invasive Alien Plants in China : An Update.” Plant Divers. 45 (1): 117–21. https://doi.org/10.1016/j.pld.2022.11.004.
Hernández-Yáñez H, J. T. Kos, M D. Bast, J. L. Griggs, P. A. Hage, A. Killian, M. I. Loza, M. B. Whitmore, A. B. Smith. 2016. “A Systematic Assessment of Threats Affecting the Rare Plants of the United States.” Biolog. Conserv. 203: 260–67. https://doi.org/10.1016/j.biocon.2016.10.009.
Irvine S., L. Johnson, K. Peters. (1999). “Community Gardens and Sustainable Land Use Planning: A Case‐study of the Alex Wilson Community Garden.” Local Environ. 4 (1): 33–46. https://doi.org/10.1080/13549839908725579.
Ismaini L., M. Lailati, R. Rustandi, D. Sunandar. (2015). “Analisis Komposisi Dan Keanekaragaman Tumbuhan Di Gunung Dempo, Sumatera Selatan.” In Pros Sem Nas Masy Biodiv. Indon., 1:1397–1402. https://doi.org/10.13057/psnmbi/m010623.
Iyai D.A., A. Ako, S.N. Sirajuddin, Budiman. (2020). “Interaction of Integrating Land Use Systems on Pig Farming Systems, West Papua-Indonesia; Worth or Worse?” IOP Conference Series: Earth Environm. Sci. 492 (1). https://doi.org/10.1088/1755-1315/492/1/012165.
Kamau P. (2004). “Forage Diversity and Impact of Grazing Management on Rangeland Ecosystems in Mbeere District, Kenya.” Kenya.
Kartikawati P., D.P. Indriani, J. Juswardi. (2023). “Keragaman Dan Potensi Tumbuhan Pakan Kerbau Rawa (Bubalus bubalis L.) Di Tanjung Senai Ogan Ilir Sumatera Selatan.” Spizaetus: J. Biologi Dan Pendidikan Biologi., no. February 2021.
Kondombo S. R. (2005). Improvement of Village Chicken Production in a Mixed (Chicken-Ram) Farming System in Burkina Faso. Wageningen Instit. Anim. Sci. Vol. PhD.
Kunarso A., F. Azwar. (2013). “Keragaman Jenis Tumbuhan Bawah Pada Berbagai Tegakan Hutan Tanaman Di Benakat, Sumatera Selatan.” Jurnal Penelitian Hutan Tanaman 10 (2): 85–98.
Kusmana C., A. Hikmat. (2015). “Keanekaragaman Hayati Flora Di Indonesia.” Jurnal Pengelolaan Sumberdaya Alam Dan Lingkungan 5 (Desember): 187–98. https://doi.org/10.19081/jpsl.5.2.187.
Leitner W., W.R. Turner. (2017). “Measurement and Analysis of Biodiversity.” Reference Mod. Life Sci., no. June 2016. https://doi.org/10.1016/b978-0-12-809633-8.02385-2.
Lerman S. B., A. R. Contosta, J Milam, C. Bang. (2018). “To Mow or to Mow Less: Lawn Mowing Frequency Affects Bee Abundance and Diversity in Suburban Yards.” Biolog. Conserv. 221 (April 2017): 160–74. https://doi.org/10.1016/j.biocon.2018.01.025.
Liu Q, Tian-Tian Xue, Xiao-Xia Zhang, Xu-Dong Yang, F. Qin, Wen-Di Zhang, L. Wu, R. W. Bussmann, Sheng-Xiang Yu. (2023). “Distribution and Conservation of near Threatened Plants in China.” Plant Divers., no. xxxx. https://doi.org/10.1016/j.pld.2023.02.005.
Lüscher A., I. M.-Harvey, J.F. Soussana, R.M. Rees, J.L. Peyraud. (2020). “Potential of Legume-Based Grassland-Livestock Systems in Europe.” In 17th Symposium of the European Grassland Federation. (EGF), 3–29.
Magurran A.E. (1988). Ecologycal Diversity and Its Measurement. London, United Kingdom: Croom Helm Ltd.
Mutibvu T., B. E. Maburutse, D. T. Mbiriri, M. T. Kashangura. (2012). “Constraints and Opportunities for Increased Livestock Production in Communal Areas: A Case Study of Simbe, Zimbabwe.” Livest. Res. Rural Develop. 24 (9): 14.
Naah J-B S. N., B. Braun. (2019). “Local Agro-Pastoralists ’ Perspectives on Forage Species Diversity , Habitat Distributions , Abundance Trends and Ecological Drivers for Sustainable Livestock Production in West Africa.” Scient. Rep., no. January: 1–11. https://doi.org/10.1038/s41598-019-38636-1.
Nahlunnisa H., E.A.M. Zuhud, Y. Santosa. (2016). “Keanekaragaman Spesies Tumbuhan Di Areal Nilai Konservasi Tinggi (NKT) Perkebunan Kelapa Sawit Provinsi Riau.” Media Konservasi. 21 (1): 91–98.
Obidzinski K., R. Andriani, H. Komarudin, A. Andrianto. (2012). “Environmental and Social Impacts of Oil Palm Plantations and Their Implications for Biofuel Production in Indonesia.” Ecol. Soci. 17 (1). https://doi.org/10.5751/ES-04775-170125.
Odum E.P. (1993). Dasar-Dasar Ekologi. Edited by Terjemahan Tjahjono Samingan. 3rd ed. Yogyakarta: Gadjah Mada University Press.
Ouali M., F.A. Belhouadjeb, W. Soufan, H.Z. Rihan. (2023). “Sustainability Evaluation of Pastoral Livestock Systems.” Animals. 13 (April): 1–16. https://doi.org/10.3390/ani13081335.
Prihantoro I., P.D.M.H. Karti, A.T. Permana, E.L. Aditia, S.D. Putri. (2023). “Tingkat Produksi Dan Keragaman Vegetasi Hijauan Pakan Di Padang Penggembalaan Berdasarkan Sistem Penanaman Berbeda.” J. Agripet. 23 (April): 46–53.
Qian Li-Shen, J-H Chen, T. Deng, H. Sun. (2020). “Plant Diversity Plant Diversity in Yunnan : Current Status and Future Directions.” Plant Divers. 42 (4): 281–91. https://doi.org/10.1016/j.pld.2020.07.006.
Raven P., M. Wackernagel. (2020). “Plant Diversity Maintaining Biodiversity Will de Fine Our Long-Term Success.” Plant Divers. 42 (4): 211–20. https://doi.org/10.1016/j.pld.2020.06.002.
Rist L., L. Feintrenie, P. Levang. (2010). “The Livelihood Impacts of Oil Palm: Smallholders in Indonesia.” Biodivers. Conserv. 19 (4): 1009–24. https://doi.org/10.1007/s10531-010-9815-z.
Simone O., M. Chiara, F. Giuseppe, G. Domenico, P. Lorenzo, A. Thomas, A. Alessandro. (2018). “Red Listing Plants under Full National Responsibility : Extinction Risk and Threats in the Vascular Fl Ora Endemic to Italy.” Biolog. Conserv. 224 (May): 213–22. https://doi.org/10.1016/j.biocon.2018.05.030.
Sodhi N. S., L. Pin, R. Clements, T. C. Wanger, J. K. Hill, K. C. Hamer, Y. Clough. (2010). “Conserving Southeast Asian Forest Biodiversity in Human-Modified Landscapes.” Biolog. Conserv. 143 (10): 2375–84. https://doi.org/10.1016/j.biocon.2009.12.029.
Soerdjani M., A.J.G.H. Kostermans, G. Tjirosoepomo. (1987). Weeds of Rice in Indonesia. Seri Balai. Jakarta: Balai Pustaka, 1987.
Steenis Van C.G.G.J. (2006). Flora. Jakarta: Pradnya Paramita.
Suarna I. W., M. A.P. Duarsa, N. P. Mariani, L. G. Sumardani, S. A. Lindawati. (1970). “Daya Dukung Hijauan Pakan Dalam Konservasi Sapi Putih Taro.” Bumi Lestari J. Environ. 16 (1): 38–43. https://doi.org/10.24843/blje.2016.v16.i01.p06.
Susetyo S. (1980). Padang Penggembalaan. Bogor: Fakultas Peternakan Institut Pertanian Bogor.
Teuscher M., A. Gérard, U. Brose, D. Buchori, Y. Clough, M. Ehbrecht, D. Holsher. (2016). “Experimental Biodiversity Enrichment in Oil-Palm-Dominated Landscapes in Indonesia.” Front. Plant Sci. 7 (October): 1–15. https://doi.org/10.3389/fpls.2016.01538.
Tulu D., S. Gadissa, F. Hundessa, E. Kebede. (2023). “Contribution of Climate-Smart Forage and Fodder Production for Sustainable Livestock Production and Environment: Lessons and Challenges from Ethiopia.” Adv. Agricult. 2023. https://doi.org/10.1155/2023/8067776.
Turner B, J. Henryks, D. Pearson. (2011). “Community Gardens: Sustainability, Health and Inclusion in the City.” Local Environ. 16 (6): 489–92. https://doi.org/10.1080/13549839.2011.595901.
Walujo E.B., H. Soedjito, E.A. Widjaja, M.A. Rifai. (1991). “Keanekaragaman Hayati Untuk Pangan.” Jakarta.
Wardah W. (2005). “Keanekaragaman Jenis Tumbuhan Di Kawasan Hutan Krui, Taman Nasional Bukit Barisan Selatan Lampung Barat.” J.Tek.Ling. 6 (3): 477–84.
Wijka M. T.van, J. Hammond, R. Frelat, S. Fraval. (2018). “Unequal Access to Land: Consequences for the Food Security of Smallholder Farmers in Sub Saharan Africa.” Encyclop. Food Secur. Sustain. 1: 556–61. https://doi.org/10.1016/B978-0-08-100596-5.22311-1.
XLSTAT. (2009). “XLSTAT Version 2009.1.02. Microsoft Excel®.” Copyright Addinsoft 1995-2009.
Zheng Y., L. Luo, X. Li, Q. Chen, Y Yang. (2023). “Plant Diversity Human Agricultural Activities in Fl Uence the Fl Owering Time of Turnip in the Qinghai-Tibet Plateau.” Plant Divers., no. xxxx. https://doi.org/10.1016/j.pld.2023.04.002.
To share on other social networks, click on any share button. What are these?





