Management of Callosobruchus chinensis L. (Coleoptera: Bruchidae) in Stored Chickpea Grains by using Entomopathogenic Fungi
Management of Callosobruchus chinensis L. (Coleoptera: Bruchidae) in Stored Chickpea Grains by using Entomopathogenic Fungi
Mohsin Iqbal1, Farid Asif Shaheen1*, Farah Naz2, Muhammad Usman Raja2, Muhammad Fiaz3 and Muhammad Nadeem1
1Department of Entomology, PMAS-Arid Agriculture University Rawalpindi, Pakistan, 46300; 2Department of Plant Pathology, PMAS-Arid Agriculture University Rawalpindi, Pakistan, 46300; 3Faculty of Veterinary and Animal Sciences, PMAS, Arid Agriculture University Rawalpindi, Pakistan, 46300.
Abstract | Callosobruchus chinensis is one of the most destructive insect pests of chickpea in storages and renders the grains unfit for human consumption. In current studies, entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana were used as the bio-control agents against this economic pest and proved good alternatives to chemicals. Less number of eggs (2 per grain), more number of holes (8.3 per grain), less number of F1 adults (20.3 per jar) and more number of days (7) to 100% mortality of C. chinensis adults were recorded when chickpea grains were treated with the highest concentration of B. bassiana as compared to that of M. anisopliae. These non-chemical control strategies will have significant contribution towards development of commercial formulations of bio-pesticides to be used against this grain beetle and other stored product insect pests.
Received | October 19, 2018; Accepted | December 04, 2018; Published | December 06, 2018
*Correspondence | Farid Asif Shaheen, Department of Entomology, PMAS-Arid Agriculture University Rawalpindi, Pakistan; Email: [email protected]
Citation | Iqbal, M., F.A. Shaheen, F. Naz, M.U. Raja, M. Fiaz and M. Nadeem. 2018. Management of callosobruchus chinensis l. (Coleoptera: Bruchidae) in stored chickpea grains by using entomopathogenic fungi. Pakistan Journal of Agricultural Research, 31(4): 408-418.
DOI | http://dx.doi.org/10.17582/journal.pjar/2018/31.4.408.418
Keywords | Callosobruchus chinensis, Metarhizium anisopliae, Beauveria bassiana, Insect development, Mortality and management
Introduction
Chickpea ranks 2nd in area under cultivation and 3rd in production among pulses in the world (CGIAR, 2017). In Pakistan, annual production of 0.5 million tons of dry seed is obtained from an area of around one million hectares (FAO, 2016). Under storages, severe post-harvest losses are noted due to pulse beetle, Callosobruchus chinensis attack (Zia et al., 2011) causing about 10% damage and rendering grains unfit for human consumption (Aslam, 2004). Grain protectants and fumigants are used since long for controlling stored grain insect pests but their injudicious and long-term use has resulted in development of resistance in insects. Their residual effects are further posing risks to human as well as environmental health (Rajendran and Sriranjini, 2008). Botanicals, although relatively are safe grain protectants (Bakkali et al., 2008) but they also have faced development of resistance issues inviting the other safer approaches like entomopathogens to manage insect pests (Jung and Kim, 2006).
Entomopathogenic fungi are considered safer than pesticides due to minimum risk involved and can be used directly on food items due to their entomopathogenic nature. They have also registration with Environmental Protection Agency, USA. The marketable packing of B. bassiana is now accessible like Botanigard, Boverosil and Mycotrol. These are registered against many insect pests of lab as well as field (Hidalgo, 1998). M. anisopliae previously categorized in Hyphomycetes, is previously identified as E. anisopliae and is existed in nature in all types of soils acting as parasite against insects (Correa et al., 2016).
The objective of the research encompassed investigating effectiveness of B. bassiana and M. anisopliae to manage C. chinensis in stored chickpea grains.
Materials and Methods
Infested samples of stored chickpea grains by pulse beetle were collected from different research stations including National Agriculture Research Council (NARC) Islamabad, Pakistan. C. chinensis culture was maintained in incubator at temperature of 30±2oC and 70±5 % relative humidity in the Department of Entomology, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Pakistan.
For bioassays, chickpea cultivar NOOR-2009 was obtained from NARC, Islamabad. Agtoxin was applied for fumigation to kill already existing insect pests (Shaheen et al., 2006). Entomopathogenic fungi Beauveria bassiana isolate (DEBI 005) and Metarhizium anisopliae isolate (DEMI 001) were obtained from Korean Agricultural Culture Collection (KACC), NAC, RDA, Suwon, 441-707, Korea. Initially theses cultures were developed in Potato Dextrose Agar (PDA) at 25oC at 200 rpm for two weeks and then it was multiplied in Potato Dextrose Broth medium to count the number of conidia/spores per unit volume. The conidia/spore was grown on PDA medium. Later on, then spores/conidia were counted by haemocytometer after 24 hours interval (Tuan et al., 2009). Different concentrations of spores/conidia were developed and a quantity of 0.02 percent of Tween 80 in fresh water was also added. Different concentrations of both fungi were made to conduct experiments. For propagation of fungus, petri plates were shifted to incubator at appropriate temperature and humidity.
In each jar, 50g of chickpea grains were put in plastic jars. The jar was enclosed with muslin fabric and then shifted to incubator. Ten pairs of Callosobruchus chinensis were then shifted into each jar. Pairing of pulse beetle (P.B.) was done following Halstead (1963). Different concentrations viz. 1×104, 1×105, 1×106, 1×107 and 1×108 spores/ml of both fungi were applied in this experiment.
The pathogenicity of EPF against C. chinensis was studied according to the following parameters:
Number of eggs laid
Average number of eggs laid per grain was counted to calculate effect of applications on fecundity and oviposition of P.B. For this, ten (10) grains were chosen at random from every jar and eggs were counted. Average of these laid eggs was then taken for each jar.
Number of holes made
Newly emerged F1 adults of C. chinensis: Newly emerged adults (F1) were counted in each jar.
Inhibition rate (% IR): The percent decrease in emergence of C. chinensis (F1) or inhibition rate was measured by using this formula:
% IR = (Cn – Tn) / Cn × 100
Where;
Cn = Fresh emerged adults in un-treated jar (control); Tn= Fresh emerged adults in treating jar.
Number of days to 100 percent mortality of F1 of C. chinensis: The number of days to 100 percent mortality of C. chinensis (F1) was measured to find out the effects of applications on new appeared progeny.
Loss of weight (%)
The percent weight loss was measured by this formula at the end of experiments.
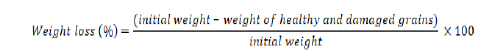
Percent damage
Percent damage of the cultivar was calculated. Healthy grains (without holes) were separated from the sieved samples and were used for percent damage calculations by using the following formula:
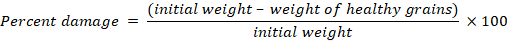
Results and Discussion
The results revealed different trends in different parameters which are explained as follows:
Number of eggs per grain
According to Table 1, eight eggs were found when chickpeas were exposed to lowest concentration (1×104) of B. bassiana. Number of eggs was higher in this concentration which was significantly different from all other concentrations. Concentrations 1 × 105 and 1 × 106 showed almost similar results with mean average of six eggs. Lowest number of eggs was observed in highest concentration (1 × 108), which was significantly different from all other concentrations. The highest number of eggs (15) was recorded in control. In case of M. anisopliae, lowest number of eggs (3) was seen in the highest concentration (1 × 108) which was significantly different to rest of concentrations. Highest number of eggs was recorded in control. However, results of concentrations i.e. 1 × 105 and 1 × 106 were not significantly different and same number of eggs was observed. On an average, nine eggs per grain were counted with application of lowest concentration of M. anisopliae.
Table 1: Number of eggs laid by C. chinensis under different concentrations of B. bassiana and M. anisopliae application.
|
Sr. No |
Concentrations (Cells/ml) | Number of eggs per grain (Mean ± SE) | |
| B. bassiana | M. anisopliae | ||
| 1 |
1×104 |
8±0.57c | 9±0.57d |
| 2 |
1×105 |
6±0.57bc | 8±0.57cd |
| 3 |
1×106 |
6±1.15bc | 8±0.57bc |
| 4 |
1×107 |
4.3±0.33b | 5.3±0.66ab |
| 5 |
1×108 |
2±0.57a | 3±0.57a |
| 6 | Control | 15±0.57d | 17.66±1.45e |
Linear regression model was applied to verify outcomes of different concentrations of fungi (B. bassiana) regarding eggs number. The modeled equation (Y= -2.1743x + 14.493) revealed that treatments had dangerous effects on fecundity. The intercept (a) value remained 14.49 while slope (b) was -2.17. With alteration in dilution of fungi, number of eggs was also minimized by -2.17. Coefficient of determination of (R2) was 0.83 which showed that treatments (independent variable) have 83% effect dependent variable. The R2 furthermore verified correctness of model to anticipate result of fungi treatments on oviposition rate. Correspondingly linear regression model was also used to confirm results of various concentration of fungus (M. anisopliae) on number of eggs. The modeled equation (Y=-2.4114 x + 16.933) indicates that treatments had harmful effect over fecundity. The intercept (a) value remained 16.93 while slope (b) was -2.41. So, with the change in concentration of fungus, number of eggs was also reduced by -2.41. Coefficient of determination of (R2) was 0.81 which displayed that treatments (independent variable) had 81% effect on the dependent variable. The R2 further confirmed accuracy of model in studying efficacy of fungal treatments against oviposition rate of the beetle (Figure 1 and 2).
Number of holes per grain
Minimum number of holes (1.3) per grain was observed with highest concentration (1 × 108) of B. bassiana which was significantly different from all other concentrations. With the lowest concentration (1×104 and 1× 105), 06 and 5.3 holes were seen respectively which was not significantly different from each other. Highest number of holes (8.3) per grain were recorded in control where no dilution of B. bassiana was used. In case of M. anisopliae, more number of holes (3.6) per grain were recorded with lowest concentration (1×104) of all other concentrations. This concentration was not significantly different from increased concentration (1 × 105) which comes up with three number of hole. Highest numbers of holes (07) were obtained in control where no dilution of M. anisopliae was used. The minimum number of holes (1) was recorded in highest concentration of M. anisopliae with application rate of 1 × 108 (Table 2).
Table 2: Number of holes made by C. chinensis under different concentrations of B. bassiana and M. anisopliae application.
| S. No | Concentrations (Cells/ml) | Number of holes per grain (Mean ± SE) | |
| B. bassiana | M. anisopliae | ||
| 1 |
1×104 |
6±0.57d | 3.6±0.33d |
| 2 |
1×105 |
5.3±0.33cd | 3±0.00cd |
| 3 |
1×106 |
4±0.00bc | 2.33±0.33bc |
| 4 |
1×107 |
3.6±0.33b | 1.67±0.33ab |
| 5 |
1×108 |
1.3±0.33a | 1±0.57a |
| 6 | Control | 8.3±0.88e | 7±0.57e |
Linear regression model was applied to verify outcomes of different concentrations of fungi (B. bassiana) regarding holes number. The modeled equation (Y= -1.2429x + 9.1) revealed that treatments had dangerous effects on fecundity. The intercept (a) value remained 9.1 while slope (b) was -1.24. So, with alteration in dilution of fungi, the number of holes also minimized @-1.24. Coefficient of determination of (R2) was 0.95 which showed that treatments (independent variable) had 95% effect on the dependent variable. The R2 furthermore verified the correctness of model to anticipate result of fungi treatments on larval development (Figure 3).
Correspondingly linear regression model was also used to confirm the results of various concentrations of fungus (M. anisopliae) on number of holes. The modeled equation (Y= -1.0417x + 6.746) showed that treatments had detrimental effects over fecundity. The intercept (a) value remained 6.74 while slope (b) was -1.0417. So, with the change in concentration of fungus, the holes number also reduced @-1.0417. Coefficient of determination of (R2) was 0.84 which displayed that treatments (independent variable) had 84% effect on the dependent variable. The R2 further confirmed the accuracy of model to expect result of fungal treatments on larval development (Figure 4).
F1 adults emerged
Highest number of F1 progeny emerged in control (42) as compared to all other treatments with B. bassiana (Table 3). Minimum difference was observed in population 24.3 and 22.3 where concentration 1 × 106 and 1 × 107 was applied respectively. There was also a difference of population in lowest concentration with 27 adults and highest concentration showing 20.33 adults. Lowest number of F1 progeny emerged in highest concentration (1×108) of M. anisopliae which was significantly different with all other treatments except control. There was no significant difference in populations 24.33 and 24 adults where concentration 1 × 105 and 1 × 106 were applied.
It was revealed in Figure 5 that the outcomes of dissimilar B. bassiana dilutions were verified by means of linear regression model. Negative outcomes of fungi dilution on new appeared adults were reported as indicated by modeled equation (Y= -3.4653x + 39.563). The intercept (a) and slope (b) values remained 39.56 and -3.46, correspondingly. Consequently, as dilution of B. bassiana enhanced numbers of F1 emerged decreased @-3.46. Determination coefficient (R2) was 0.74 that indicated that independent variable has 74% influences on the dependent variable. The R2 furthermore verifies correctness of model. Likewise results of dissimilar M. anisopliae concentrations were checked by means of linear regression model (Figure 6). Negative results for fungal concentration on the number of newly emerged adults were reported as shown by the modeled equation (Y= -3.1526x + 37.754). The intercept (a) and slope (b) value remained 37.75 and -3.15, correspondingly. Hence, as the concentration of M. anisopliae increased, number of F1 emerged decreased @-3.15. Determination coefficient (R2) was 0.59 which showed that independent variable had 59% influences on the dependent variable. The R2 further confirmed the accuracy of model.
Table 3: Number of F1 adults emerged of C. chinensis in chickpea grains applied with different concentrations of B. bassiana and M. anisopliae.
| S. No | Concentrations (Cells/ml) |
Number of F1 adults emerged per jar (Mean ± SE) |
|
| B. bassiana | M. anisopliae | ||
| 1 |
1×104 |
27±0.57d | 25.33±0.33b |
| 2 |
1×105 |
26.6±0.33cd | 24.33±0.33b |
| 3 |
1×106 |
24.3±0.33bc | 24±0.57b |
| 4 |
1×107 |
22.3±1.20ab | 23.66±0.33b |
| 5 |
1×108 |
20.33±0.33a | 21±0.57a |
| 6 | Control | 42±1.15e | 42±1.5c |
Percent inhibition rate of F1 adults
Maximum percent inhibition rate (52.45) in pulse beetle was noted with highest concentration of B. bassiana (1 × 108) which was significantly different with all other treatments. Almost similar percent inhibition i.e. 35.52 and 35.48 was recorded with B. bassiana concentration of 1 × 104 and 1 × 105 respectively. Minimum percent inhibition rate was seen in control.
Whereas percent inhibition rate of 41.32 and 42.57 was recorded with concentration of 1 × 105 and 1 × 106 of M. anisopliae that was significantly different from each other as well as from all other treatments. Lowest percent inhibition rate of C. chinensis was however recorded in control. Further, maximum percent inhibition rate (50.23) was observed with highest concentration of M. anisopliae (1 × 108) that was also significantly different from all other treatments (Figure 7).
Linear regression model was used to check effects of dissimilar dilutions of B. bassiana regarding % inhibition rate. Positive effects of fungi dilutions regarding inhibition of F1 emerged were observed as indicated by modeled equation (Y=8.6994x + 4.992). The intercept (a) value retained 4.99 while slope (b) was 8.69. So, as fungal dilutions increased, inhibition rates were also enhanced at the rate of 8.69. Coefficient of determination (R2) was 0.76 that revealed that dilutions (independent variable) had 76% effect on the dependent variable (inhibition’s rate). The value of R2 furthermore verified correctness of model to anticipate effects of fungal treatments for inhibition rate of F1 (Figure 8).
Linear regression model was also applied to find out the effect of dissimilar concentrations of M. anisopliae on the percent inhibition rate. Positive effect of fungal concentration on the inhibition of F1 emerged was observed as shown by the modeled equation (Y=7.7214x + 9.16). The intercept (a) value remained 9.16 while slope (b) was 7.72. So, as the fungal concentration was increased, inhibition rate was also increased at the rate of 7.72. Coefficient of determination (R2) was 0.63 which shows concentrations (independent variable) have 63% effect on the inhibition rate (dependent variable). The value R2 further confirmed accuracy of model to predict effect of fungal treatments on inhibition of newly emerged (Figure 9).
Number of days to 100 percent mortality of F1 adults
Minimum number of days (7.3) to 100% mortality was observed with highest concentration of B. bassiana which was significantly different to all other treatments. Yet, maximum days (18.3) to 100% mortality were seen in control. There was no difference observed in number of days (9) and (8.3) to 100% mortality between concentration of 1 × 106 and 1 × 107 respectively. The reason may be minimal difference of concentration between them. More number of days to 100% mortality was measured in highest concentration of B. bassiana i.e. 1 × 108.
There was no considerable variation observed in concentration of 1 × 107 and 1 × 106 that took 8.6 and 07 days to 100% mortality which was significantly different with all other treatments. Whereas, more number of days to 100% mortality was seen in lowest concentration of M. anisopliae (1 × 104) which was significantly different to all other treatments (Figure 10).
To determine effectiveness of diverse dilutions regarding death rate of new appeared adults, linear regression models were applied. When dilutions of fungi were used as obvious from the regression equation (Y= -2.1143x + 18.6), negative impacts on percent mortality of F1 adults were noted. The intercept (a) value remained 18.6 but slope (b) was -2.11. Consequently, as dilution of B. bassiana enhanced, numbers of days to 100% mortality of F1 were also reduced @ -2.11. Coefficient of determination (R2) was 0.87 that discovered that dilutions of fungi have 87% effects on the dependent variable. The R2 furthermore verified correctness of models to anticipate effects of fungi treatments on the days required to 100% mortality (Figure 11). Similarly, to observe the effect of diverse concentrations on the mortality rate of newly emerged adults, linear regression model was used. When the fungal concentration was applied as evident from the regression equation (Y= -2.1343x + 18.887), the negative impact on the percent mortality of F1 adults were recorded. The intercept (a) value remained 18.88 but slope (b) was -2.13. Hence as the concentration of M. anisopliae increased, days to 100% mortality of F1 was reduced @ -2.13. Coefficient of determination (R2) was 0.93 which revealed that the fungal concentrations had 93% effect on the dependent variable. The R2 further confirmed the accuracy of model to predict effect of fungal treatments on the days required to 100% mortality (Figure 12).
Likewise, more than 50% weight loss was observed in concentration of M. anisopliae 1×106 as compared to 35.03% observed in 1×107 concentration. The lowest percent weight loss (13.7%) was observed with highest concentration of M. anisopliae (1×108), while maximum weight loss was recorded (87.1%) in control. Percent weight loss in chickpea caused by C. chinensis in response to diverse dilutions of fungi was experimented by linear regression model (Figure 14). Unfavorable impacts of dilutions of fungi on the feeding of pulse beetle were noted as showed by the modeled equation (Y= -7.5503x + 93.396). The intercept (a) and slope (b) value remained 93.39 and -7.55, separately. So, as dilution of B. bassiana enhanced weight loss caused by pulse beetle lowered @ -7.55.
Percent weight loss of chickpea grains
Highest percent weight loss was measured (85.33%) in control (Figure 13). Second to it, the percent weight loss (81.16%) was lowered in lowest concentration (1×104) of B. bassiana. No significant percent weight loss was recorded in concentration of B. bassiana (1×105) and 1×106 respectively. Lowest percent weight loss was shown in highest concentration of B. bassiana i.e. (1×108).
Determination co-efficient (R2) was 0.95 which indicated that dilutions of fungi have 95% effects on the percent weight loss. The R2 furthermore verified correctness of model.
Similarly, percent weight loss caused by C. chinensis in response to diverse fungal concentrations was experimented by linear regression model (Figure 15). Unfavorable impact of fungal concentrations on the feeding of pulse beetle was observed as presented by the modeled equation (Y= -14.498x + 113.4). The intercept (a) and slope (b) value remained 113.4 and -14.49, separately. So, as the concentration of M. anisopliae increased weight loss caused by pulse beetle decreased with the rate of -14.49. Determination coefficient (R2) was 0.96 which showed that the fungal concentrations have 96% effects on the percent weight loss. The R2 further confirmed the accuracy of model.
Percent damage of chickpea grains
More than 50% damage (56.33) was recorded by C. chinensis in B. bassiana with concentration of 1 × 106 as compared to treatments at the rate of 1 × 107 and 1 × 108. Maximum percent damage (94%) was observed in control. Minimum percent damage (29%) by C. chinensis was enumerated in highest concentration of B. bassiana which was significantly different with all other treatments. Maximum damage (95.55%) was seen by C. chinensis in control. Minimum percent damage (28.5%) was calculated with maximum concentration of M. anisopliae which was significantly different with all other treatments. More damage (73.66%) was observed in lowest concentration of M. anisopliae (1×104) than all other treatments (Figure 16).
Percent damage caused by C. chinensis in response to diverse dilutions of fungi were experimented by linear regression model (Figure 17). Unfavorable impacts of dilutions of fungi on the feeding of pulse beetle was noted as showed by the modeled equation (Y= -10.734x + 101.07). The intercept (a) and slope (b) value remained 101.07 and -10.73, separately. So, as dilution of B. bassiana enhanced, percent damage caused by pulse beetle lowered with the rate of -10.73. Determination co-efficient (R2) was 0.88 which indicated that dilutions of fungi have 88% effects on the percent damage. The R2 furthermore verified correctness of model.
Similarly, percent damage caused by C. chinensis in response to diverse fungal concentrations was experimented by linear regression model (Figure 18). Unfavorable impact of fungal concentrations on the feeding of pulse beetle was observed as presented by the modeled equation (Y= -11.268x +102.37). The intercept (a) and slope (b) value remained 102.37 and -11.26, separately. So, as the concentration of M. anisopliae increased, percent damage caused by pulse beetle decreased with the rate of -11.26. Determination coefficient (R2) was 0.89 which showed that the fungal concentrations have 89% effects on the percent damage. The R2 further confirmed the accuracy of model.
Some scientists also used these entomopathogenic fungi for management of insect pests. The findings of studies are in conformity with Abdel-Raheem (2015) who evaluated efficacy of entomopathogenic fungi, Beauveria and Metarhizium spp. against some stored insect pests. In another similar study undertaken by Shaheen et al. (2016), they used entomopathogenic fungus, B. bassiana as natural management agent to control pulse beetle C. chinensis in chickpea grains at different temperatures. In this study mortality of pulse beetle was found directly proportional to concentrations of B. bassiana.
In a similar study, by Radha (2012) similar results were found when she assessed the efficiency of two Entomopathogenic fungi M. anisopliae and B. bassiana against C. maculatus. Mahdneshin et al. (2011), evaluated pathogenic viability of five isolates of B. bassiana and M. anisopliae against C. maculates by concentration assay technique at 27±1°C and 60±5 percent relative humidity under controlled conditions. The IRAN (441C) of B. bassiana proved the most detrimental against test insect because it had lesser LC50 and LT50 and it yielded maximum deaths (76 percent) in application having 1×108 conidiaml-1. The Beauveria bassiana found more virulent than M. anisopliae against test insect. In another study undertaken by Khashaveh (2013), it was observed that adults of test insects were vulnerable to whole isolates of M. anisopliae. When studied complete (03) isolates, death percentage of the two species enhanced with rise in conidial formulation and considerable variation was recorded between dilutions. The factors of probit test illustrated non-overlap of 95 percent of LC50 and LC95 and noteworthy variation was recorded in three isolates examined in opposition to test insects.
Author’s Contribution
Mohsin Iqbal: Conducted research and wrote manuscript.
Farid Asif Shaheen: Conceived and supervised research and wrote manuscript.
Farah Naz: Supervised pathological aspects of research.
Muhammad Usman Raja: Proof checking and supervised pathological aspects of research.
Muhammad Fiaz: Analyzed data and helped in manuscript writing.
Muhammad Nadeem: Data collection and collection of experimental materials.
References
Aslam, M. 2004. Pest status of stored chickpea beetle, Callosobruchus chinensis linnaeus on chickpea. J. Ento. 1(1): 28-33. https://doi.org/10.3923/je.2004.28.33
Abdel-Raheem, M.A., I.A. Ismail, R.A. Rahman, N.A. Farag and I.A. Rhman. 2015. Entomopathogenic fungi, Beauveria bassiana (Bals.) and Metarhizium anisopliae (Metsch.) As biological control agents on some stored product insects. J. Entomol. Zool. Stud. 3(6): 316-320.
Atif, H.M., N. Javed, S.A. Khan and S. Ahmed. 2012.Virulence of Xenorhabdus and Photorhabdus bacteria and their toxins against juvenile’s immobilization of Meloidogyne incognita. Pak. J. Phytopathol. 24(2): 170-174.
Bakkali, F., S. Averbeck, D. Averbeck, M. Idaomar. 2008. Biological effects of essential oils. Rev. Food Chem. Tox. Vol. 46 (2): 446-475. https://doi.org/10.1016/j.fct.2007.09.106
Consultative group on international agricultural research. 2017. (https://www.cgiar.org)
Correa, J.P., A. SáenzApontel, Rodríguez, M.X. Bocanegra. 2016. In vitro interaction of M. anisopliae Ma 9236 and B. bassiana Bb9205 with Heterorhabditis bacteriophora HNI0100 for the control of Plutella xylostella. Springer Plus. 5: 2068.
Food and agriculture organization. 2016. (www.fao.org)
Halstead, D.G.H. 1963. External sex differences in stored-products Coleoptera. Bull. Entomol. Res. 54(1): 119-134. https://doi.org/10.1017/S0007485300048665
Hidalgo, E. 1998. The effect of different formulations of Beauveria bassiana on Sitophilus zeamais in stored maize. Journal of Stored Products Research, 34(2): 171-179.
Jung, S. and Y. Kim. 2006. Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the pathogenicity of Bacillus thuringensis ssp. aizawai against Spodopteraexigua (Lepidoptera: Noctuidae). Environ. Ento. 35(6): 1584-1589. https://doi.org/10.1093/ee/35.6.1584
Khashaveh, A. 2013. Laboratory bioassay of Iranian isolates of entomopathogenic fungus, M. anisopliae (Ascomycota: Hypocreales) against two species of storage pest. Agriculturae Conspectus Scientificus, 78(1): 35-40.
Kryukov, V.Y., V.P. Khodyrev, O.N. Yaroslavtseva, A.S. Kamenova, B.A. Duisembekov and V. Glupov. 2009. Synergistic action of entomopathogenic hyphomycetes and the bacteria Bacillus thuringiensis ssp. morrisoni in the Infection of colorado potato beetle. Appl. Biochem. Microbiol. Vol. 45, (5): pp. 511–516. https://doi.org/10.1134/S000368380905010X
Mahdneshin, Z., S. Vojoudi, Y. Ghosta, M.H. Safaralizadae and M. Saber. 2011. Laboratory evaluation of the entomopathogenic fungi, Iranian isolates of B. bassiana (Balsamo) Vuillemin and M. anisopliae (Metsch) Sorokin against the control of the cowpea weevil, Callosobruchus maculatus F. (Coleoptera: Bruchidae). https://doi.org/10.5897/AJMR11.1223 Afr. J. Microbiol. Res. 5(29): 5215-5220.
Mburu, D.M., N.K. Maniania, A. Hassanali and M.W. Ndung’u. 2011. Comparison of volatile blends and gene sequences of two isolates of M. anisopliae of different virulence and repellency towards the termite Macrotermes michaelseni. J. Exp. Biol. 214 (6): 956-962. https://doi.org/10.1242/jeb.050419
Nabaei, N.A., S.M. Mehrvar and M. Bagheri. 2012. Efficacy of entomopathogenic fungi in combination with diatomaceous earth against Callosobruchus maculatus (Coleoptera: Bruchidae). Acta Entomol. Sinica. 55 (11):1282-1288.
Park, Y., J.K. Jung and Y. Kim. 2016. A mixture of B. thuringiensis sub sp israelensis with X. nematophila-cultured broth enhances toxicity against mosquitoes Aedes albopictus and Culexpipien spallens (Diptera: Culicidae). J. Econ. Entomol. 109 (3): 1086-1093. https://doi.org/10.1093/jee/tow063
Park, Y. 2015. Entomopathogenic bacterium, X. nematophila and P. luminescens, enhances B. thuringiensis Cry4Ba toxicity against yellow fever mosquito, Aedes aegypti (Diptera: Culicidae). J. Asia-Pac. Entomol. 18(3): 459-463. https://doi.org/10.1016/j.aspen.2015.05.002
Radha, R. 2012. Insecticidal efficacy of entomopathogenic fungi in stored cowpea against cowpea bruchid, Callosobruchus maculates (F) (Coleoptera:Bruchidae). Asian Acad. Res. J. Multidicip. (1): 3.
Rajendran, S. and V. Srinanjini. 2008. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 44: 126-135. https://doi.org/10.1016/j.jspr.2008.04.001
Shaheen, F.A., M.W. Akram, M.A. Rashid, M. Nadeem, M. Saeed, M. Husain and M. Khalid. 2016. Biological control of pulse beetle Callosobruchus chinensis L. (Bruchidae: Coleoptera) in stored chickpea grains using entomopathogenic fungus Beauveria bassiana Balsamo. J. Entom. Zool. 2016; 4(4): 1076-1083.
Shaheen, F.A., M.W. Akram, M.A. Rashid, M. Nadeem, M. Saeed, M. Husain and M. Khalid. 2006. Resistance of chickpea (Cicer arietinum l.) cultivars against pulse beetle. Pak. J. Bot. 38(4): 1237-1244.
Sony, S. and Y. Kim. 2010. Differential pathogenicity of two entomopathogenic bacteria, Photorhabdus temperata subsp. temperata and Xenorhabdus nematophila against the red flour beetle, Tribolium castaneum. J. Asia-Pac. Ento. (13): 209-213.
Sony and K. Yonggyun. 2010. Differential pathogenicity of two entomopathogenic bacteria, Photorhabdus temperata subsp. Temperata and Xenorhabdus nematophila against the red flour beetle, Tribolium castaneum. J. Asia-Pac. Ento.13: 209-213.
SeongchaeJ and K. Yonggyun. 2006. Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the pathogenicity of Bacillus thuringiensisssp. aizawai against Spodopteraexigua (Lepidoptera: Noctuidae). Environment. Entomol. 35(6):1584-1589. https://doi.org/10.1093/ee/35.6.1584
Tuan, A.N., J.K. Jeong, G.K. Seon and K. Keun. 2009. Production of blastospore of entomopathogenic B. bassiana in a submerged batch culture. Mycobiol. 37(3): 218-224. https://doi.org/10.4489/MYCO.2009.37.3.218
Zia, A., M. Aslam, F. Naz and M. Illyas. 2011. Bio-efficacy of some plant extracts against chickpea beetle, Callosobruchus chinensis Linnaeus (Coleoptera: Bruchidae) attacking chickpea. Pakistan J. Zool. 43(4).
To share on other social networks, click on any share button. What are these?







