Morpho Yield Attributes of Bread Wheat (Triticum aestivum L.) Genotypes under Various Water Regimes
Research Article
Morpho Yield Attributes of Bread Wheat (Triticum aestivum L.) Genotypes under Various Water Regimes
Adil Ali Gadahi1, Wajid Ali Jatoi1, Saima Mir Arain2, Jay Kumar Sootaher1*, Piar Ali Shar1, Sadaf Memon1, Muhammad Saleem Chang3, Zeeshan Majeed Kumbhar1 and Kirshan Kumar Menghwar1
1Department of Plant Breeding and Genetics, Sindh Agriculture University, Tandojam, Pakistan; 2Nuclear Institute of Agriculture, Tandojam, Pakistan; 3Department of Agronomy, Subcampus Umerkot, Sindh Agriculture University, Tandojam, Pakistan.
Abstract | A fast change in the climate has perplexed the world by creating drought and its related issues. In this way, plenty of hexaploid wheat is influenced in our Pakistan. For combating this situation, research was carried out on sixteen bread wheat genotypes to see the effect of water stress at the experimental field, NIA, Tandojam in an RCBD with factorial arrangement of three treatments i.e. T1 (zero irrigation), T2 (two irrigations), T3 (four irrigations) during Rabi season, 2016-2017. The ANOVA results exposed significant differences among genotypes as well as treatments for most of the attributes, however genotypes x treatment was also significant for several characters. Among the treatments, T3 (four irrigation) recorded higher mean perform then T2 (two irrigation) and T1 (zero irrigation) which showed that different irrigation regimes caused significantly impact on all the traits. Among the genotypes, V3-10-34 showed minimum reduction in spike length, spikelets per spike, grains per spike, seed index, grain yield per plant and harvest index. The genotype, V2-10-15 manifested minimum decrease for days to 75% maturity and spikelets per spike at zero irrigation and two irrigations. C7-98-4 gave minimum decrease for flag leaf area and V2-10-3 caused smaller amount of reduction for biological yield plant-1 at zero irrigation and two irrigations. The genotypes like V3-10-34, V2-10-15, C7-98-4 and V2-10-3 could be recommended for the water deficiency areas of farmers.
Received | March 15, 2020; Accepted | February 07, 2022; Published | June 20, 2022
*Correspondence | Jay Kumar Sootaher, Department of Plant Breeding and Genetics, Sindh Agriculture University, Tandojam, Pakistan; Email: [email protected]
Citation | Gadahi, A.A., W.A. Jatoi, S.M. Arain, J.K. Sootaher, P.A. Shar, S. Memon, M.S. Chang, Z.M. Kumbhar and K.K. Menghwar. 2022. Morpho yield attributes of bread wheat (Triticum aestivum L.) genotypes under various water regimes. Journal of Innovative Sciences, 8(1): 36-52.
DOI | https://dx.doi.org/10.17582/journal.jis/2022/8.1.36.52
Keywords | Morpho yield attributes, Bread wheat, Genotypes, Stress conditions, Normal conditions
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Wheat is a winter cereal crop which requires relatively low temperatures ranging from 12 to 22º C and these temperatures are considered optimum for its reproductive development (Farooq et al., 2011). This crop is considered amongst the most important crops in Pakistan because of its contribution of 1.6% and 8.9% in GDP and value added in agriculture of our homeland being cultivated on 8.74 million hectares up to 2019 yielding 25.195 million tonnes (GOP, 2019). For our country, this crop has been very vital in terms of human utilization and agriculture. Presenting plants to water pressure unfavorably influence plant development and usefulness (Talebi et al., 2009; Shirinzadeh et al., 2010; Geravandi et al., 2011). To obtain superior yield with potential resistance against biotic and biotic stresses in Pakistani, many breeding efforts had been done in the recent past for wheat cultivars; consequently, due to its bidirectional breeding approaches a number of promising cultivars with better adaptability have also been released. The assessment of potential characters and genetic variability very much contributed by breeding programs resulting in success for the future (Sanghera et al., 2014).
Nonetheless, it is grown in every country where the environment is conducive to its production (Mateo-Sagasta et al., 2018). The worldwide climatic conditions envisage problems for agriculture as well as new thoughts to how to cultivate crops in intolerant parts of the world (Reynolds et al, 2001; Sial et al; 2009). High yielding, more stable and adaptable bread wheat genotypes in different ecological conditions is the fundamental aim in breeding (Sootaher et al., 2020). The manner and frequency of genetic variability, as well as the ratio of heritable and non-heritable changes between yield and contributing qualities, are all important factors in crop genetic improvement.
Wheat production and quality improvement are influenced by genotype-environment interactions (Amanuel et al., 2018; Nehe et al., 2019; Johansson et al., 2020). Heritability not only keeps a great importance in plant breeding by aiding a plant breeder in the forecast of segregating generation performance (Kachi et al., 2020), but also informs about population and traits contribution (Sootaher et al., 2020). High heritability is very appreciable for an effective selection (Sootaher et al., 2020). The implementation of completely redesigned genotypes has led to what seems like a 35 to 50 percent increase in wheat production (Sabri et al., 2020). All of the yield supporting attributes that may be used to estimate the yield (Li et al., 2020). In favourable environmental settings, a variety’s genetic constitution is expressed; but, in stressful environments, it may alter (Li et al., 2020).
The crops in which interrelation is beneficial so as to improve and develop wheat hybrid for seed production there a good supported is made by genetic analysis. Correlation is not able to do its work well without path coefficient analysis, because it is the one and only way to manifest not only the direct, but also indirect relation of a character in its expression (Ompal et al., 2018). Linkage among a number of characters plays an essential role for producing more and more yield in plant breeding (Dawar et al., 2018).
A great choice of parent material is critical to a breeding program’s success. The goal of this research was to find out about the natural diversity in growth and yield of several wheat genotypes. The potential wheat genotypes discovered during the research will be employed in Pakistan’s advanced wheat breeding efforts to boost yield per unit area. Therefore, the current investigation was consequently put into practice to fit selection principles of wheat genotypes through the study of yield and its components under water regime conditions in order to see its effect on wheat.
2. Materials and Methods
2.1 Experimental site and crop husbandry
The seed of total sixteen genotypes with four checks such as V3-10-9, V3-10-12, Cim-04-10, V3-10-32, V3-10-34, V3-10-31, V2-10-3, C3-98-8, C4- 98-6, V2-10-15, C7-98-4, Kiran-95 (check), NIA-Sunahri (check), Chakwal (check), Bhittai (check) was planted at the Nuclear Institute of Agriculture, Tandojam. The experiment was laid-out in in factorial design with three treatments i.e. T1 (No/Zero irrigation), T2 (Two irrigations: 1st at 03 leaves stage, 2nd at tillering stage), T3 (Four irrigations: 3rd at booting, 4th at grain filling) in three replications during 2016-2017. The seed was planted by using drill method in the month of December of 2016. Plant to plant and row to row distance was 6 and 9 cm. All agronomic practices were put into practice from the germination to the crop maturity. The crop was harvested in the month of April, 2017. Ten plants were chosen at random for each replication from parents and hybrids to record the data for traits such as days to 75% maturity, flag leaf area, plant height, spike length, spikelets per spike, grains per spike, grain yield per plant, 1000 grain weight, biological yield per plant and harvest index.
Flag leaf area = (Length x Width) x b
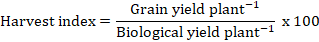
2.2 Statistical analysis
Data were statistically analyzed by using Statistix 8.1 for analysis of variance according to the methods of Gomez and Gomez (1984) and mean performance was calculated as suggested by Steel and Torrie (1960). Heritability in broad sense was estimated as developed by Gardener (1961) and correlation coefficient was analyzed by the procedure of Raghavrao (1983).
3. Results and Discussion
3.1 Analysis of variance
The ANOVA exposed significant results for genotypes, treatments and their interactions at 1% probability level for all characteristics (Table 1). While genotypes x treatments interaction was non-significant for flag leaf area and plant height which indicated that genotypes perform similarly over the treatments. Our findings were confirmed with Hannachi et al. (2013) who observed a considerable variation in the attributes of genotypes which further added that genes were very intact before the environmental conditions in which they got better chance to perform themselves. Jatoi et al. (2011) also reported about significant differences between treatments and among the cultivars in which significant interaction between treatments and genotypes performed differentially over the water stress situations and consistently for grain yield. On the other hand, Ram et al. (2017) showed reduction in the grain yield due to water stress when interaction genotypes and environmental circumstances took place. These discoveries were also in conformity with Khakwani et al. (2012) and Kachi et al. (2020).
3.2 Mean performance
3.2.1 Days to 75% maturity
The number of days it takes for a plant to begin heading is an essential plant characteristic for determining if a crop is early maturing. The data regarding days to 75% maturity which indicated that maximum average reduction of -8.29 days was noted in T1 (zero irrigation) followed by (-2.73 days) in T2 (two irrigation) over the T3 (four irrigation) for days to 75% maturity (Table 2). Among the genotypes, V-2-10-15 recorded minimum relative decrease (-4.33 and -1.67) for days to 75% maturity in T1 (zero irrigation) and T2 (two irrigations) respectively as compare to T3 (four irrigations) followed by V3-10-32 which ranked the second in reduction (-6.67 and -2.00) for days in 75% maturity in T1 and T2 respectively as compare to T3. The variety Bhittai took maximum days to 75% maturity (121.33 days) and V3-10-32 took minimum days to 75% maturity (117.33 days) in T3 (four irrigations). The overall average of genotypes in T1 (zero irrigation), T2 (two irrigations) and T3 (four irrigations) were 111.60, 117.17, and 119.90 days in 75% maturity respectively which directed that different irrigation regimes caused significantly impact on the days to 75% maturity. Correspondingly, Jatoi et al. (2011) discovered that the types TD-1, SKD-1, and Sarsabz revealed little losses in physiological and yield characteristics in stress during anthesis. Ngwako and Mashiqa (2013) also reported the same results for this character. According to a previous study, wheat varieties that take fewer days to mature are classified as early maturing (Siyal et al., 2020; Takumi et al., 2020).
Table 1: Mean squares from analysis of variances for various traits of wheat genotypes grown under different irrigation regimes.
|
Traits |
Mean squares |
||||
|
Replication (D.F. 2) |
Genotypes (D.F. 15) |
Treatment (D.F. 2) |
G x T (D.F. 30) |
Error (D.F. 94) |
|
|
Days to75% maturity |
35.63 |
15.89** |
857.13** |
7.25** |
2.48 |
|
Flag leaf area |
1.58 |
135.38** |
6485.14** |
25.10ns |
18.40 |
|
Plant height |
50.96 |
212.81** |
6521.23** |
18.11ns |
21.94 |
|
Spike length |
1.81 |
8.82** |
303.91** |
2.25** |
0.70 |
|
Spikelet’s spike-1 |
0.36 |
11.99** |
795.75** |
5.56** |
1.23 |
|
Grains spike-1 |
5.77 |
109.11** |
9400.51** |
74.36** |
2.54 |
|
1000-grain weight |
14.33 |
7.80** |
1961.58** |
6.98** |
1.05 |
|
Grain yield plant-1 |
8.66 |
4.63** |
2767.05** |
5.50** |
0.78 |
|
Biological yield plant-1 |
2.63 |
64.74** |
8689.11** |
27.88** |
1.85 |
|
Harvest index |
0.39 |
16.61** |
2286.78** |
10.83** |
1.44 |
** = Significant at 1% probability level; n.s = non-significant.
Table 2: Mean performance of wheat genotypes for days to 75% maturity grown under different water regimes.
|
Genotypes |
Days to 75% maturity |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D. (T1) |
R.D.(T2) |
|
|
V3-10-9 |
111.67 |
115.00 |
118.66 |
-7.00 |
-3.67 |
|
V3-10-12 |
110.00 |
112.00 |
120.66 |
-10.67 |
-8.67 |
|
CIM-04-10 |
107.00 |
118.67 |
120.66 |
-13.67 |
-2.00 |
|
V3-10-32 |
110.67 |
115.33 |
117.33 |
-6.67 |
-2.00 |
|
V3-10-34 |
112.67 |
116.00 |
119.33 |
-6.67 |
-3.33 |
|
V3-10-31 |
113.00 |
116.67 |
119.33 |
-6.33 |
-2.67 |
|
Kiran-95 |
112.33 |
117.00 |
119.33 |
-7.00 |
-2.33 |
|
NIA-Sunahri |
111.33 |
117.33 |
121.00 |
-9.67 |
-3.67 |
|
Chakwal |
110.00 |
117.33 |
120.00 |
-10.00 |
-2.67 |
|
Bhittai |
114.67 |
119.33 |
121.33 |
-6.67 |
-2.00 |
|
V2-10-3 |
110.00 |
118.67 |
120.33 |
-10.33 |
-1.67 |
|
C3-98-8 |
112.67 |
119.33 |
121.00 |
-8.33 |
-1.67 |
|
C4-98-8 |
113.67 |
119.33 |
121.00 |
-7.33 |
-1.67 |
|
V2-10-15 |
116.00 |
118.67 |
120.33 |
-4.33 |
-1.67 |
|
C7-98-4 |
108.67 |
116.67 |
118.66 |
-10.00 |
-2.00 |
|
V2-10-5 |
111.33 |
117.33 |
119.33 |
-8.00 |
-2.00 |
|
Mean |
111.60 |
117.17 |
119.90 |
-8.29 |
-2.73 |
|
LSD at 5% (G) |
1.47 |
||||
|
LSD at 5% (T) |
0.63 |
||||
|
LSD at 5% (G x T) |
2.55 |
||||
*R.D. = Relative decrease.
Table 3: Mean performance of wheat genotypes for flag leaf area grown under different water regimes.
|
Genotypes |
Flag leaf area (cm2) |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D. (T1) |
R.D. (T2) |
|
|
V3-10-9 |
10.92 |
31.56 |
39.41 |
-28.50 |
-7.85 |
|
V3-10-12 |
11.50 |
24.66 |
32.25 |
-20.75 |
-7.59 |
|
CIM-04-10 |
14.33 |
28.24 |
30.16 |
-15.83 |
-1.92 |
|
V3-10-32 |
11.91 |
28.37 |
32.58 |
-20.67 |
-4.21 |
|
V3-10-34 |
11.96 |
25.84 |
28.78 |
-16.83 |
-2.94 |
|
V3-10-31 |
12.18 |
29.14 |
34.87 |
-22.68 |
-5.73 |
|
Kiran-95 |
11.58 |
28.92 |
32.45 |
-20.87 |
-3.53 |
|
NIA-Sunahri |
11.27 |
26.15 |
29.24 |
-17.97 |
-3.09 |
|
Chakwal |
15.47 |
26.72 |
34.49 |
-19.02 |
-7.77 |
|
Bhittai |
14.08 |
35.13 |
40.30 |
-26.22 |
-5.17 |
|
V2-10-3 |
17.18 |
41.66 |
46.27 |
-29.09 |
-4.61 |
|
C3-98-8 |
9.92 |
28.88 |
31.48 |
-21.57 |
-2.61 |
|
C4-98-8 |
16.83 |
31.26 |
37.09 |
-20.26 |
-5.83 |
|
V2-10-15 |
13.79 |
33.62 |
39.44 |
-25.66 |
-5.82 |
|
C7-98-4 |
11.35 |
22.97 |
26.27 |
-14.92 |
-3.29 |
|
V2-10-5 |
11.00 |
37.40 |
44.44 |
-33.44 |
-7.04 |
|
Mean |
12.83 |
30.03 |
34.97 |
-22.14 |
-4.94 |
|
LSD at 5 %(G) |
4.01 |
||||
|
LSD at 5% (T) |
.1.73 |
||||
|
LSD at 5% (G x T) |
1.23 |
||||
*R.D. = Relative decrease.
3.2.2 Flag leaf area (cm2)
Larger leaf area in wheat is desired because it plays a vital role in photosynthesis. The mean performance of flag leaf area which exhibited that flag leaf area varied from 9.92-17.18cm in T1 (zero irrigation), 22.97-41.66 in T2 (two irrigations) and 26.27-46.27 in T3 (four irrigations). Among the genotypes C7-98-4 showed minimum relative decrease (14.92 cm2) for the trait, secondly Cim-04-10 recorded relative decrease of -15.83 in T1 (zero irrigation) over the T3 (four irrigations), while the same genotypes also showed minimum reduction in flag leaf area in T2 (two irrigations) but their ranked order was changed i.e. Cim 04-10 (-1.92 cm2) and C7-98-4 (-3.29 cm2) over the T3 (four irrigations) (Table 3). The overall mean reductions in flag leaf area were -22.14 and -4.94 cm2 for genotypes in T1 and T2 respectively. Asif et al. (2012) told that he improved genotypes by applying more and more water to the crop for seeing the effect of water on the crop. Thus, he saw betterments in many physiological as well as morphological characteristics. Some researchers studied the area of wheat flag leaves and discovered that this region was important in increasing wheat grain production (Luo et al., 2018; Zhao et al., 2018; Ma et al., 2020).
3.2.3 Plant height (cm)
Wheat with a semi-dwarf height is a desirable characteristic. Among the genotypes, V3-10-12 displayed minimum reduction (-15.50 and -3.56 cm) for plant height in T1 (zero irrigation) and T2 (two irrigations) as compare to T3 (four irrigations), genotype C4-98-8 ranked second order in reduction for plant height in T1 (zero irrigation) which is -17.00 cm followed by V3-10-34 (-17.75) in the same treatment (Table 4). Where as in T2 (two irrigations), C-3-98-8 showed next order in case of minimum reduction (-4.06 cm) for plant height followed by V3-10-32 (-4.44cm) in T2 (two irrigations) over the T3 (four irrigations). The average mean plant height was 71.07, 87.50 and 93.60 cm in T1 (zero irrigation), T2 (two irrigations) and T3 (four irrigations), respectively with relative decrease of -22.54 and -6.11 cm in T1 (zero irrigation) and T2 (two irrigations) respectively as compared with T3 (four irrigations). The same results were in paradox with Johari et al. (2011). Our findings were also confirmed by Bazai et al. (2020). Medium statured genotypes yielded more grain than tall statured genotypes, according to Zhao et al. (2018) and Siyal et al. (2020).
3.2.4 Spike length (cm)
The mean performance for spike length of wheat genotypes under different irrigation regimes in Table 5 which exposed that smaller spikes were recorded in T1 (zero irrigation) which was varied from 5.35-8.31 cm in length and medium size of spikes were recorded in T2 (two irrigations) which was ranked from 7.56-10.42 cm and larger spikes were measured in T3 (four irrigations) Among the genotypes, V3-10-32 gave the minimum reduction (-2.99 and-101 cm) for spike length in T1 and T2 respectively followed by V2-10-5 (-3.43 and 1.76 cm) in T1 and T2 respectively (Table 5). Whereas maximum relative decrease (-9.68 and -6.94 cm) was presented by the genotype Cim-04-10 in T1 (zero irrigation) and T2 (two irrigations) respectively over the T3 (four irrigation) for spike length. Similar results were also reported by Ahmad (2022). Similar findings were not only published by Mahpara et al. (2017), but also by Sootaher et al. (2020) who found that increased spike length contributed to increased grain production in wheat.
3.2.5 Spikelets spike-1
The average number of spikelets spike-1 were 12.77, 16.34 and 20.12 in T1 (zero irrigation), T2 (two irrigations) and T3 (four irrigations) respectively with average reduction of -8.12 and -4.55 for number of spikelets spike-1 in T1 (zero irrigation) and T2 (two irrigations) respectively as compare to T3 (four irrigations). Among the genotypes, C4-98-8, V3-10-32 and V2-10-15 with an addition of Bhittai also gave minimum reduction (-2.17, -2.44 and -2.39) for number of spikelets spike-1 in T2 (two irrigations) against T3 (four irrigations) respectively (Table 6). The results of Asif et al. (2012) suggested that the number of grains in a single spike can be enhances by increasing the number of irrigation frequencies. Several studies have found that increasing the number of spikelets increases grain yield (Philipp et al., 2018; Würschum et al., 2018).
3.2.6 Grains spike-1
The information regarding number of grains spike-1 showed that number of grain spike-1 was significantly reduced in T1 (zero irrigation) and T2 (two irrigations) due to less number of irrigations, the average reduction in number of grains spike-1 were -27.98 and -14.52 in T1 (zero irrigation) and T2 (two irrigations) respectively as compare to T3 (four irrigations). However, the number of grains spike-1 in T1 which was varied from 22.39 to 39.39 and in T2 was 39.20 to 48.72 and in T3 was 51.70
Table 4: Mean performance of wheat genotypes for plant height grown under different water regimes.
|
Genotypes |
Plant height (cm) |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D. (T1) |
R.D. (T2) |
|
|
V3-10-9 |
72.71 |
89.83 |
94.61 |
-21.90 |
-4.78 |
|
V3-10-12 |
68.72 |
80.67 |
84.22 |
-15.50 |
-3.56 |
|
CIM-04-10 |
75.19 |
91.00 |
98.39 |
-23.20 |
-7.39 |
|
V3-10-32 |
66.16 |
84.72 |
89.17 |
-23.01 |
-4.44 |
|
V3-10-34 |
68.56 |
81.17 |
86.28 |
-17.72 |
-5.11 |
|
V3-10-31 |
73.22 |
87.72 |
92.61 |
-19.39 |
-4.89 |
|
Kiran-95 |
74.44 |
88.82 |
93.56 |
-19.11 |
-4.73 |
|
NIA-Sunahri |
65.44 |
82.38 |
88.11 |
-22.67 |
-5.73 |
|
Chakwal |
75.23 |
95.39 |
104.33 |
-29.10 |
-8.94 |
|
Bhittai |
71.89 |
86.63 |
93.44 |
-21.56 |
-6.81 |
|
V2-10-3 |
73.44 |
98.72 |
104.22 |
-30.78 |
-5.50 |
|
C3-98-8 |
65.56 |
85.67 |
89.72 |
-24.17 |
-4.06 |
|
C4-98-8 |
72.00 |
83.00 |
89.00 |
-17.00 |
-6.00 |
|
V2-10-15 |
73.67 |
86.66 |
98.44 |
-24.78 |
-11.78 |
|
C7-98-4 |
65.67 |
82.06 |
89.70 |
-24.03 |
-7.64 |
|
V2-10-5 |
75.17 |
95.50 |
101.83 |
-26.67 |
-6.33 |
|
Mean |
71.07 |
87.50 |
93.60 |
-22.54 |
-6.11 |
|
LSD at 5 %(G) |
4.38 |
||||
|
LSD at5% (T) |
1.89 |
||||
|
LSD at 5% (G x T) |
1.11 |
||||
*R.D. = Relative decrease.
Table 5: Mean performance of wheat genotypes for spike length grown under different water regimes.
|
Genotypes |
Spike length(cm) |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D. (T1) |
R.D. (T2) |
|
|
V3-10-9 |
7.72 |
9.62 |
13.33 |
-5.62 |
-3.72 |
|
V3-10-12 |
7.96 |
9.78 |
14.33 |
-6.38 |
-4.56 |
|
CIM-04-10 |
7.62 |
10.36 |
17.30 |
-9.68 |
-6.94 |
|
V3-10-32 |
7.85 |
10.06 |
13.33 |
-5.48 |
-3.28 |
|
V3-10-34 |
7.97 |
9.94 |
10.96 |
-2.99 |
-1.01 |
|
V3-10-31 |
8.00 |
9.95 |
12.33 |
-4.33 |
-2.38 |
|
Kiran-95 |
8.21 |
10.28 |
13.83 |
-5.63 |
-3.56 |
|
NIA-Sunahri |
6.99 |
8.33 |
11.70 |
-4.71 |
-3.37 |
|
Chakwal |
7.47 |
9.86 |
12.06 |
-4.58 |
-2.19 |
|
Bhittai |
8.06 |
9.44 |
12.47 |
-4.41 |
-3.02 |
|
V2-10-3 |
7.95 |
10.42 |
12.23 |
-4.29 |
-1.82 |
|
C3-98-8 |
5.35 |
7.56 |
10.27 |
-4.92 |
-2.71 |
|
C4-98-8 |
7.95 |
8.14 |
12.37 |
-4.42 |
-4.22 |
|
V2-10-15 |
8.31 |
10.19 |
13.03 |
-4.73 |
-2.84 |
|
C7-98-4 |
6.22 |
7.89 |
10.33 |
-4.11 |
-2.44 |
|
V2-10-5 |
7.83 |
9.50 |
11.26 |
-3.43 |
-1.76 |
|
Mean |
7.59 |
9.46 |
12.57 |
-4.98 |
-3.11 |
|
LSD at 5% (G) |
0.78 |
||||
|
LSD at 5% (T) |
0.34 |
||||
|
LSD at 5% (G x T) |
1.36 |
||||
*R.D. = Relative decrease
Table 6: Mean performance of wheat genotypes for spikelets spike-1 grown under different water regimes.
|
Genotypes |
Spikelets spike-1 |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D (T1) |
R.D (T2) |
|
|
V3-10-9 |
13.50 |
16.56 |
28.50 |
-15.00 |
-11.94 |
|
V3-10-12 |
11.67 |
16.17 |
21.67 |
-10.00 |
-5.50 |
|
CIM-04-10 |
14.83 |
17.56 |
21.83 |
-7.00 |
-4.28 |
|
V3-10-32 |
12.33 |
16.44 |
18.61 |
-6.28 |
-2.17 |
|
V3-10-34 |
12.07 |
15.33 |
18.61 |
-6.54 |
-3.28 |
|
V3-10-31 |
11.94 |
16.06 |
20.33 |
-8.39 |
-4.28 |
|
Kiran-95 |
13.28 |
16.06 |
19.90 |
-6.62 |
-3.84 |
|
NIA-Sunahri |
12.00 |
14.50 |
20.53 |
-8.53 |
-6.03 |
|
Chakwal |
13.11 |
17.28 |
21.67 |
-8.56 |
-4.39 |
|
Bhittai |
13.28 |
17.67 |
20.06 |
-6.78 |
-2.39 |
|
V2-10-3 |
12.17 |
18.00 |
21.83 |
-9.67 |
-3.83 |
|
C3-98-8 |
11.33 |
14.06 |
20.33 |
-9.00 |
-6.28 |
|
C4-98-8 |
13.11 |
15.32 |
19.00 |
-5.89 |
-3.68 |
|
V2-10-15 |
14.22 |
18.06 |
20.50 |
-6.28 |
-2.44 |
|
C7-98-4 |
12.00 |
15.28 |
19.78 |
-7.78 |
-4.50 |
|
V2-10-5 |
13.50 |
17.17 |
21.17 |
-7.67 |
-4.00 |
|
Mean |
12.77 |
16.34 |
20.90 |
-8.12 |
-4.55 |
|
LSD at 5% (G) |
1.03 |
||||
|
LSD at 5% (T) |
0.45 |
||||
|
LSD at 5% (G x T) |
1.80 |
||||
*R.D. = Relative decrease.
Table 7: Mean performance of wheat genotypes for grains spike-1 grown under different water regimes.
|
Genotypes |
Grains spike-1 |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D.(T1) |
R.D.(T2) |
|
|
V3-10-9 |
26.11 |
45.67 |
80.33 |
-54.22 |
-34.67 |
|
V3-10-12 |
33.33 |
45.31 |
69.00 |
-35.67 |
-23.69 |
|
CIM-04-10 |
37.98 |
46.70 |
59.67 |
-21.69 |
-12.96 |
|
V3-10-32 |
34.83 |
44.00 |
53.50 |
-18.67 |
-9.50 |
|
V3-10-34 |
31.06 |
41.11 |
54.44 |
-23.39 |
-13.33 |
|
V3-10-31 |
22.39 |
47.22 |
51.44 |
-29.06 |
-4.22 |
|
Kiran-95 |
30.72 |
48.13 |
60.47 |
-29.74 |
-12.33 |
|
NIA-Sunahri |
31.74 |
44.00 |
60.03 |
-28.29 |
-16.03 |
|
Chakwal |
27.83 |
44.91 |
59.33 |
-31.50 |
-14.43 |
|
Bhittai |
39.39 |
48.28 |
57.00 |
-17.61 |
-8.72 |
|
V2-10-3 |
30.00 |
47.17 |
61.44 |
-31.44 |
-14.28 |
|
C3-98-8 |
30.50 |
44.00 |
51.07 |
-20.57 |
-7.07 |
|
C4-98-8 |
37.72 |
48.72 |
62.37 |
-24.64 |
-13.64 |
|
V2-10-15 |
32.97 |
45.28 |
66.06 |
-33.09 |
-20.78 |
|
C7-98-4 |
27.72 |
39.20 |
51.28 |
-23.56 |
-12.08 |
|
V2-10-5 |
37.28 |
47.33 |
61.86 |
-24.58 |
-14.52 |
|
Mean |
31.97 |
45.44 |
59.96 |
-27.98 |
-14.52 |
|
LSD at 5% (G) |
1.49 |
||||
|
LSD at 5% (T) |
0.64 |
||||
|
LSD at 5% (G x T) |
2.58 |
||||
*R.D. = Relative decrease.
Table 8: Mean performance of wheat genotypes for 1000-grain weight grown under different water regimes.
|
Genotypes |
1000-grain weight (g) |
Relative decrease over T3 (four irrigation |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D(T1) |
R.D.(T2) |
|
|
V3-10-9 |
32.00 |
40.41 |
44.68 |
-12.68 |
-4.27 |
|
V3-10-12 |
30.00 |
40.02 |
45.12 |
-15.12 |
-5.09 |
|
CIM-04-10 |
32.00 |
39.99 |
43.97 |
-11.97 |
-3.98 |
|
V3-10-32 |
34.33 |
40.45 |
43.33 |
-9.00 |
-2.88 |
|
V3-10-34 |
35.30 |
40.11 |
42.53 |
-7.23 |
-2.42 |
|
V3-10-31 |
30.00 |
40.78 |
45.37 |
-15.37 |
-4.58 |
|
Kiran-95 |
31.00 |
42.87 |
46.24 |
-15.24 |
-3.37 |
|
NIA-Sunahri |
35.63 |
39.59 |
43.98 |
-8.35 |
-4.39 |
|
Chakwal |
34.33 |
40.86 |
45.49 |
-11.15 |
-4.63 |
|
Bhittai |
34.00 |
41.23 |
44.39 |
-10.39 |
-3.17 |
|
V2-10-3 |
32.17 |
40.52 |
45.57 |
-13.40 |
-5.05 |
|
C3-98-8 |
30.67 |
40.11 |
44.68 |
-14.01 |
-4.57 |
|
C4-98-8 |
32.00 |
40.50 |
46.17 |
-14.17 |
-5.68 |
|
V2-10-15 |
32.33 |
41.22 |
44.61 |
-12.27 |
-3.38 |
|
C7-98-4 |
32.17 |
43.78 |
49.33 |
-17.17 |
-5.55 |
|
V2-10-5 |
34.17 |
41.57 |
48.00 |
-13.83 |
-6.43 |
|
Mean |
32.63 |
40.88 |
45.22 |
-12.59 |
-4.34 |
|
LSD at 5% (G) |
0.95 |
||||
|
LSD at 5% (T) |
0.41 |
||||
|
LSD at 5% (G x T) |
1.65 |
||||
*R.D. = Relative decrease.
to 80.33 (Table 7). Among the genotypes, Bhittai and V3-10-34 gave minimum reduction (-17.61 and -4.33) in T1 (zero irrigation) and T2 (two irrigations) respectively for the trait while Bhittai ranked second order also in T2 (two irrigations) which was -8.72 grains spike-1 reduced as compare to T3 (four irrigations). Such results were also notified by Khavarinejed and Karmov (2012). These findings matched those of other studies who found that having a lot of grains per spike enhances wheat crop grain yield (Wolde et al., 2019; Sakuma and Schnurbusch, 2020).
3.2.7 1000 grain weight (g)
The mean performance for 1000- grain weight (Table 8) of ten wheat genotypes disclosed that maximum 1000- grain weight was measured in T3 (four irrigation) which ranked from 42.53 g 49.33 g, secondly in T2 (two irrigation) which was varied from 39.59-43.78 g and minimum 1000-seed weight was weighted in T1 (zero irrigation) which was ranked from 30.67- 35.63 g (Table 8). Among the genotypes, NIA Sunahri, V3-10-34 and V310-32 showed minimum losses in 1000-seed weight i.e. -8.35, -7.23 and -9.00 g in T1 (zero irrigation) and -4.39, -2.42 and -2.88 g in T2 (two irrigations) against the T3 (four irrigations). Whereas highest 1000-seed weight (49.33g) was identified in C7-98-4 followed by in V2-10-5 (48.00g) in T3 (four irrigations). Laghari et al. (2012) also found the same results in their final results. Sial et al. (2012) experimented with wheat genotypes in which high seed index values were found out under water stress conditions concluding relative tolerance to moisture content. Higher 1000-grain weight led directly to increased grain yield, according to Bilgrami et al. (2018) and Kamaran et al. (2019).
3.2.8 Grain yield plant-1 (g)
The information for grain yield plant-1 exhibited that the overall mean performance of genotypes in three treatments are 5.60, 9.32 and 20.21 g (T1, zero irrigation, T2, two irrigations and T3, four irrigations). Nevertheless, maximum reduction of grain yield plant-1 was detected in T1 (zero irrigation) which was -14.61 and secondly in T2 (two irrigations) which was -10.88 g against the T3 (four irrigations). Among the genotypes, V3-10-9 and V2-10-5 showed highest grain yield plant-1 with same weight (23.33 g) in T3 (Table 9). Whereas V3-10-34 and V2-10-3 gave minimum reduction (-11.17 and -11.02 g) in T1 (zero irrigation) and -7.17 and -7.64 g in T2 (two irrigations) against T3 (four irrigations) for grain yield plant-1. These final results were in combination with Noorifarjam et al. (2013) and Mujtaba et al. (2016). Mahpara et al. (2018) also reported the same findings.
3.2.9 Biological yield plant-1 (g)
The results of this character showed that the average performance of genotypes in three treatments were 15.97, 22.15, and 41.74 g (T1, zero, T2, two irrigations and T3 four irrigation), however the maximum reduction (-25.77g) for biological yield plant-1 was observed in T1 (zero irrigation) and secondly (-19.59 g) in T2 (two irrigations) against the T3 (four irrigations). Among the genotypes, V-10-15 showed the highest (48.33 g) biological yield plant-1 in T3 (four irrigations) whereas V2-10-5 recorded minimum reduction of -18.83g in T1 (zero irrigation) and -18.83 g in T2 (two irrigations) against T3 (four irrigation) for biological yield plant-1 (Table 10). In the study of Salehi et al. (2016), reduction was seen in the performance of different yield characters of hexaploid wheat.
3.2.10 Harvest index (%)
The information regarding the mean performance of wheat genotypes for harvest index under different irrigation regimes was presented in Table 11 which exhibited that average mean performance of all the genotypes in three treatments were 29.03, 36.89 and 42.79 % (T1= zero irrigation, T2 = two irrigations and T3 (Table 11). However maximum reduction for harvest index was observed in T1 (zero irrigation) which was -20.40 and secondly in T2 (two irrigations) which was -10.09% against the T3 (four irrigations). The genotype, V3-10-34 recorded the highest harvest index (46.98%) in T3 (four irrigations). The same genotype (V3-10-34) produced the lowest reduction (-10.20%) for the trait in T1 (zero irrigation) and -2.98 % in T2 (two irrigations) against T3 (four irrigations). Kumar et al. (2014) carried out studies for breeding purpose through selection from the germplasm and used different traits of economic
Table 9: Mean performance of wheat genotypes for grain yield plant-1 grown under different water regimes.
|
Genotypes |
Grain yield plant-1 (g) |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D (T1) |
R.D(T2) |
|
|
V3-10-9 |
6.10 |
9.53 |
23.33 |
-17.23 |
-13.80 |
|
V3-10-12 |
4.74 |
8.78 |
22.33 |
-17.60 |
-13.55 |
|
CIM-04-10 |
6.00 |
9.50 |
21.00 |
-15.00 |
-11.50 |
|
V3-10-32 |
5.69 |
9.00 |
19.00 |
-13.31 |
-10.00 |
|
V3-10-34 |
5.83 |
9.83 |
17.00 |
-11.17 |
-7.17 |
|
V3-10-31 |
5.68 |
9.39 |
21.00 |
-15.32 |
-11.61 |
|
Kiran-95 |
4.75 |
9.87 |
19.00 |
-14.25 |
-9.13 |
|
NIA-Sunahri |
6.36 |
8.97 |
21.00 |
-14.64 |
-12.03 |
|
Chakwal |
5.50 |
9.00 |
22.00 |
-16.50 |
-13.00 |
|
Bhittai |
4.53 |
10.06 |
19.33 |
-14.81 |
-9.27 |
|
V2-10-3 |
5.98 |
9.36 |
17.00 |
-11.02 |
-7.64 |
|
C3-98-8 |
4.36 |
9.63 |
17.33 |
-12.97 |
-7.70 |
|
C4-98-8 |
5.93 |
9.47 |
21.33 |
-15.41 |
-11.87 |
|
V2-10-15 |
5.59 |
9.13 |
23.33 |
-17.75 |
-14.20 |
|
C7-98-4 |
6.08 |
8.48 |
21.33 |
-15.25 |
-12.85 |
|
V2-10-5 |
6.43 |
9.17 |
18.00 |
-11.57 |
-8.83 |
|
Mean |
5.60 |
9.32 |
20.21 |
-14.61 |
-10.88 |
|
LSD at 5% (G) |
0.82 |
||||
|
LSD at 5% (T) |
0.35 |
||||
|
LSD at 5% (G x T) |
1.43 |
||||
*R.D. = Relative decrease.
Table 10: Mean performance of wheat genotypes for biological yield plant-1 grown under different water regimes.
|
Genotypes |
Biological yield plant-1 (g) |
Relative decrease over T3 (four irrigation) |
|||
|
Treatments |
|||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D.(T1) |
R.D.(T2) |
|
|
V3-10-9 |
18.82 |
25.33 |
44.67 |
-25.85 |
-19.33 |
|
V3-10-12 |
14.11 |
18.44 |
45.83 |
-31.72 |
-27.39 |
|
CIM-04-10 |
15.19 |
19.17 |
39.00 |
-23.81 |
-19.83 |
|
V3-10-32 |
10.28 |
15.61 |
40.00 |
-29.72 |
-24.39 |
|
V3-10-34 |
9.56 |
15.59 |
37.00 |
-27.44 |
-21.41 |
|
V3-10-31 |
16.39 |
21.78 |
41.00 |
-24.61 |
-19.22 |
|
Kiran-95 |
11.00 |
24.78 |
39.67 |
-28.67 |
-14.89 |
|
NIA-Sunahri |
21.17 |
26.39 |
42.33 |
-21.17 |
-15.94 |
|
Chakwal |
18.39 |
20.89 |
44.33 |
-25.94 |
-23.44 |
|
Bhittai |
18.31 |
21.00 |
41.00 |
-22.69 |
-20.00 |
|
V2-10-3 |
17.44 |
33.46 |
39.67 |
-22.22 |
-6.21 |
|
C3-98-8 |
15.09 |
22.00 |
39.33 |
-24.24 |
-17.33 |
|
C4-98-8 |
19.11 |
21.33 |
44.00 |
-24.89 |
-22.67 |
|
V2-10-15 |
14.11 |
23.11 |
48.33 |
-34.22 |
-25.22 |
|
C7-98-4 |
17.33 |
23.56 |
43.67 |
-26.33 |
-20.11 |
|
V2-10-5 |
19.17 |
22.00 |
38.00 |
-18.83 |
-16.00 |
|
Mean |
15.97 |
22.15 |
41.74 |
-25.77 |
-19.59 |
|
LSD at 5% (G) |
1.27 |
||||
|
LSD at 5% (T) |
0.55 |
||||
|
LSD at 5% (G x T) |
2.20 |
||||
*R.D. = Relative decrease
Table 11: Mean performance of wheat genotypes for harvest index grown under different water regimes.
|
Genotypes |
Harvest index (%) |
Relative decrease over T3 (four irrigation) |
||||
|
Treatments |
||||||
|
Zero irrigation |
Two irrigation |
Four irrigation |
R.D. (T1) |
R.D.(T2) |
||
|
V3-10-9 |
29.32 |
39.03 |
42.17 |
-12.84 |
-3.13 |
|
|
V3-10-12 |
28.23 |
35.21 |
42.03 |
-13.80 |
-6.82 |
|
|
CIM-04-10 |
29.53 |
35.37 |
41.20 |
-11.67 |
-5.83 |
|
|
V3-10-32 |
29.67 |
35.05 |
41.46 |
-11.79 |
-6.41 |
|
|
V3-10-34 |
32.20 |
38.10 |
42.40 |
-10.20 |
-4.30 |
|
|
V3-10-31 |
33.74 |
37.01 |
46.98 |
-13.24 |
-9.97 |
|
|
Kiran-95 |
27.06 |
37.67 |
40.45 |
-13.39 |
-2.79 |
|
|
NIA-Sunahri |
31.00 |
35.90 |
44.07 |
-13.07 |
-8.17 |
|
|
Chakwal |
27.75 |
34.40 |
39.20 |
-11.45 |
-4.80 |
|
|
Bhittai |
26.00 |
38.17 |
46.40 |
-20.40 |
-8.23 |
|
|
V2-10-3 |
27.74 |
40.00 |
44.53 |
-16.79 |
-4.53 |
|
|
C3-98-8 |
28.99 |
38.02 |
41.00 |
-12.01 |
-2.98 |
|
|
C4-98-8 |
30.80 |
37.76 |
42.40 |
-11.60 |
-4.64 |
|
|
V2-10-15 |
25.00 |
35.27 |
45.36 |
-20.36 |
-10.09 |
|
|
C7-98-4 |
28.00 |
36.30 |
40.33 |
-12.33 |
-4.03 |
|
|
V2-10-5 |
29.41 |
37.00 |
44.58 |
-15.17 |
-7.58 |
|
|
Mean |
29.03 |
36.89 |
42.79 |
-13.76 |
-5.90 |
|
|
LSD at 5% (G) |
1.12 |
|||||
|
LSD at 5% (T) |
0.48 |
|||||
|
LSD at 5% (G x T) |
1.94 |
|||||
*R.D. = Relative decrease
importance from breeding point of view. These traits mainly included area of flag leaf, number of spikes plant-1 and seed index on the basis of thousand grain weight. The findings showed that the minimum values for drought susceptibility index, drought tolerance values and yield reduction were recorded with most drought tolerance with yield stable genotypes. According to the study of Yildrim (2013), these genotypes could keep a good importance for the water stress breeding.
3.3. Heritability (h2% b.s)
The heritability was calculated from variance components for all the traits. All the traits recorded moderate to high heritability due to environment factors (Table 12). The phenotypic variance ranged from 6.43 to 220.12) and genotypic variance varied from 3.85 to 190.87. The variance of phenotype was greater than the variance of genotype which showed that most of traits were influenced by environment. The high heritability was recorded for plant height (h2 = 86.71%), spike length (h2 = 67.72%), grains spike, (h2 = 73.94%), biological yield plant-1 (h2= 72.34%) and harvest index (h2 = 67.06%) while the moderate heritability was noted for days to 75% maturity (h2 = 60.92%), flag leaf area (h2 = 62.67%), spikelets spike-1 (h2=65.64%), 1000- grain weight (h2 = 62.79%) and grain yield plant-1 (h2 = 59.87%) (Table 12). Other researchers like Rehman et al. (2016) and Sootaher et al. (2020) showed maximum heritability in plant height, grains in a single spike and seed index. Ahmed et al. (2016) reported that maximum heritability was estimated in grains spike-1, seed production of single plant, fertile tillers of single plant, grains of single spike, seed yield of single plant, plant tallness, and leaf area and Khan and Hassan (2017) reported that heritability estimates were observed high (h2 ˃ 0.60) for all the traits. Azimi et al. (2017) observed that maximum genotypically variability and phenotypically variation was noted for seed yield plant-1, followed by biological yield, seed index and plant tallness. Conversely, Bartaula et al. (2019) and Mofokeng et al. (2020) discovered that most yield-related characteristics exhibited modest phenotypic and genotypic variances and considerable heritability with increasing seed vigour, which corresponded to the findings in our study.
Table 12: Heritability estimates for various traits of wheat genotypes grown under different irrigation regimes.
|
Traits |
Phenotypic variance (σ2p) |
Genotypic variance (σ2g) |
Heritability (h2% b.s) |
|
Days to75% maturity |
22.01 |
13.41 |
60.92 |
|
Flag leaf area |
186.64 |
116.98 |
62.67 |
|
Plant height |
220.12 |
190.87 |
86.71 |
|
Spike length |
11.99 |
8.12 |
67.72 |
|
Spikelets spike-1 |
16.39 |
10.76 |
65.64 |
|
Grains spike-1 |
145.89 |
107.88 |
73.94 |
|
1000-grain weight |
10.75 |
6.75 |
62.79 |
|
Grain yield plant-1 |
6.43 |
3.85 |
59.87 |
|
Biological yield plant-1 |
86.92 |
62.88 |
72.34 |
|
Harvest index |
22.62 |
15.17 |
67.06 |
3.4 Correlation coefficient (r)
The correlation results indicated the significant and positive association between days to 75% maturity and grain yield (r=0.71**) and its other attributes. Seher et al. (2015) determined promising role of wheat genotypes for multiplication. Flag leaf area was positively and significantly associated with grain yield
Table 13: Correlation coefficient (r) for various quantitative traits wheat genotypes grown under different irrigation regimes.
|
Characters |
Days to 75% maturity |
Flag leaf area |
Plant height |
Spike length |
Spikelets spike-1 |
1000-grain weight |
Grains spike-1 |
Grain yield plant-1 |
Biological yield plant-1 |
|
Flag leaf area |
0.75** |
- |
|||||||
|
Plant height |
0.72** |
0.84** |
- |
||||||
|
Spike length |
0.65** |
0.68** |
0.72** |
- |
|||||
|
Spikelets spike-1 |
0.71** |
0.79** |
0.79** |
0.82** |
- |
||||
|
1000-grain weight |
0.75** |
0.80** |
0.75** |
0.81** |
0.91** |
- |
|||
|
Grains spike-1 |
0.81** |
0.82** |
0.79** |
0.72** |
0.81** |
0.85** |
- |
||
|
Grain yield plant-1 |
0.71** |
0.70** |
0.69** |
0.83** |
0.87** |
0.88** |
0.83** |
- |
|
|
Biological yield plant-1 |
0.71** |
0.70** |
0.69** |
0.78** |
0.83** |
0.85** |
0.81** |
0.95** |
- |
|
Harvest index |
0.77** |
0.82** |
0.76** |
0.74** |
0.79** |
0.82** |
0.87** |
0.85** |
0.83** |
** = Significant at 1% probability level.
per plant, (r=0.70**), biomass per plant (r=0.70**) and harvest index (r=0.82**) and the rest of the traits (Table 13). Laghari et al. (2012) experimented with bread wheat genotypes and found the similar results of this trait with other attributes. Plant height also expressed highly significant and positive relations with all of the characters. Such results were in paradox with Bagrei and Bybordi (2015) who studied water shortage for wheat attributes. Similarly, spike length also had positive and significant associations with all described attributes (r=0.82**, 0.81**, 0.72**, 0.83**, 0.78** and 0.74**). Salehi et al. (2016) showed positive significant relationshipfor seed production and its contributing traits. Shahryari et al. (2013) displayed similar results for spike length. In case of spikelets per spike, the trait also articulated positive and significant connections with grain yield per plant (r=0.87**) and the rest of the characters (Table 13). Golparvar et al. (2017) reported that the yield per spike and grain yield was positive and significantly intercorrelated. Seed index was also positively and significantly related with yield and yield related characters. Ahmad (2022) who reported the like interrelationship outcomes of seed index with all the mentioned attributes. The grains spike sustained positive and significant relationship with grain yield, biological yield and harvest index with the correlation coefficient values of r = 0.83**, 0.81** and 0.87**, respectively (Table 13). Such findings were also contributed by Bhutto et al. (2016). Thus, above traits could be utilized for improving yield through simple selection. Grain yield per plant was in a positive as well as significant way interrelated with biomass per plant (r=0.95**) and harvest index (r =0.88**). Bagrei and Bybordi (2015) demonstrated that in drought stress condition, grain yield had the same links with harvest index. These results were the same as (Fellahi et al., 2013; Peymaninia et al., 2012) under water regime conditions.
Conclusions and Recommendations
Among the treatments, T3 (four irrigations) recorded higher mean performance than T2 (two irrigations) and T1 (zero irrigation) which disclosed that different irrigation regimes triggered significantly impact on all the traits. Among the genotypes, V3-10-34 presented minimum reductions in most of the yield and yield related characters. V2-10-15 displayed minimum decrease for days to 75% maturity, spikelets spike-1 at zero irrigation and two irrigations. C7-98-4 contributed minimum decrease for flag leaf area and V2-10-3 demonstrated smaller amount of reduction for biological yield plant-1 at zero irrigation and two irrigations. The genotypes like V3-10-34, V2-10-15, C7-98-4 and V2-10-3 could be recommended in the absence of water for drought areas.
Acknowledgments
This study was financially supported by Nuclear Institute of Agriculture (NIA), Tandojam. We are extremely grateful to its all team for a kind guidance and behavior.
Novelty statement
Drought stress administered at different phases of growth has been demonstrated to have diverse impacts on cultivated plants. Water stress given during different stages has a considerable influence on physiological and yield parameters, as well as cultivars.
Author’s Contribution
Adil Ali Gadahi: Conducted this study and collected the data.
Wajid Ali Jatoi: Designed the study and supervised it.
Piar Ali Shar: Analyzed the data.
Jay Kumar Sootaher: Wrote and revised the manuscript.
Saima Mir Arain: Designed the study and supervised.
Sadaf Memon: Helped in collecting the data.
Muhammad Saleem Chang: Formatted the manuscript.
Zeeshan Majeed Kumbhar: Contributed tools.
Kirshan Kumar Menghwar: Collected the data.
Conflict of interest
The authors have declared no conflict of interest.
References
Ahmad, W.G., Murad, H.A., Naseem, K., Muhammad, I., Khilwat, A., Irfan, A.S., Muhammad, A., Raja, T., Lesen, U., Attiqur and Babar, H., 2016. Estimates of heritability, genetic advance and correlation in F3 populations of Wheat. Pure and Applied Biology, 50(13): 7-13. https://doi.org/10.19045/bspab.2016.50137
Ahmad, A., Aslam, Z., Javed, T., Hussain, S., Raza, A., Shabbir, R., Mora-Poblete, F., Saeed, T., Zulfiqar, F., Ali, M.M., et al., 2022. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy, 12, 287. https://doi.org/ 10.3390/agronomy12020287
Ali, A., Ali, N., Ullah, N., Adnan, M. and Swati, Z.A., 2013. Effect of drought stress on the physiology and yield of the Pakistani wheat germplasm. International Journal of Advanced Research and Technology, 2(7): 419-430.
Amanuel, M., Gebre, D., and Debele, T., 2018. Performance of bread wheat genotypes under different environment in lowland irrigated areas of Afar Region, Ethiopia. African Journal of Agricultural Research, 13: 927–933. https://doi.org/10.5897/AJAR2017.12669
Asif, M., Maqsood, M., Ali, A., Hassan, S.W., Ahmed, S. and Javed, M.A., 2012. Growth, yield components and harvest index of wheat (Triticum aestivum L.) affected by different irrigation regimes and nitrogen management strategy. Science International Lahore, 24: 215-218.
Azimi, A., Matin, S., Marker and Indranil, B., 2017. Genotypic and phenotypic variability and correlation analysis for yield and its components in late sown wheat (Triticum aestivum L.). Journal of Pharmacognosy and Phytochemistry, 6(4): 167-173.
Bageri, B. and Bybordi, A., 2015. Yield and yield components in bread wheat (Tritium aestivum L.) under nonstress and drought stress condition. International Journal of Biosciences, 6(3): 338-348. https://doi.org/10.12692/ijb/6.3.338-348
Bartaula, S., Panthi, U., Timilsena, K., Acharya, S.S., and Shrestha, J., 2019. Variability, heritability and genetic advance of maize (Zea mays L.) genotypes. Research in Agriculture, Livestock and Fisheries, 6: 163–169. https://doi.org/10.3329/ralf.v6i2.42962
Bazai, K.K., Baloch, M., Sootaher, J.K., Baloch, T.A., Naeem, M., Abro, T.A., Chang, M.S., and Menghwar, K.K., 2020. Correlation, heritability and genetic distance analysis in bread wheat (Triticum aestivum L.) genotypes. Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences, 57(1): 75-83.
Bhutto, A.H., Asghar, A.R., Shahmir, A.K., Amjad, A., Fahad, A.K., Muneer, A., Sajjad, A. and Niaz, A.K., 2016. Correlation and regression analysis for yield traits in wheat (Triticum aestivum L.). Natural Science, 8: 96-104. https://doi.org/10.4236/ns.2016.83013
Bilgrami, S.S., Fakheri, B.A., Razavi, K., Mahdinezhad, N., Tavakol, E., Ramandi, H.D., Ghaderian, M., and Shariati, J.V., 2018. Evaluation of agro-morphological traits related to grain yield of Iranian wheat genotypes in drought-stress and normal irrigation conditions. Australian Journal of Crop Sciences, 12: 738–748. https://doi.org/10.21475/ajcs.18.12.05.PNE878
Chachar, Z., Chachar, N.A., Chachar, Q.I., Mujtaba, S.M., Chachar, G.A. and Chachar, S., 2016. Identification of drought tolerant wheat genotypes under water deficit conditions. International Journal of Research - Granthaalayah, 4(2): 206-214. https://doi.org/10.29121/granthaalayah.v4.i2.2016.2830
Dawar, S., Kumar, N. and Mishra, S.P., 2018. Genetic variability, correlation and path coefficient analysis in the indian mustard Brassica juncea varieties grown in Chitrakoot, India. International Journal of Current Microbiology and Applied Sciences, 7(3): 883-890. https://doi.org/10.20546/ijcmas.2018.703.103
Eftekhari, A., Baghizadeh, A., Yaghoobi, M.M. and Abdolshahi, R., 2017. Difference in the drought stress response of DREBS and catigenes and evolution of related physiological parameters in some bread wheat cultivars. Biotechnology and Biotechnological Equipment, 31(4): 709-716.
Farooq, Bramley, M.H., Palta, J.A. and Siddique, K.H.M., 2011. Heat stress in wheat during reproductive and grain filling-phases. Critical Reviews in Plant Sciences, 30: 491-507. https://doi.org/10.1080/07352689.2011.615687
Fellahi, Z., Hannachi, A., Bouzerzour, H. and Boutekrabt, A., 2013. Correlation between traits and path analysis coefficient for grain yield and other quantitative traits in bread wheat under semi-arid conditions. Journal of Agriculture and Sustainability, 3(1): 16-26. https://doi.org/10.1155/2013/201851
Gardener, C.O., 1961. An evaluation of effects of mass selection and seed irradiation with thermal neutrons on yield of corn. Crop Sciences, 1: 241-245. https://doi.org/10.2135/cropsci1961.0011183X000100040004x
Geravandi, M., Farshadfar, E. and Kahrizi, D., 2011. Evaluation of some physiological traits as indicators of drought tolerance in bread wheat genotypes. Russian Journal of Plant Physiology, 58(1): 69-75. https://doi.org/10.1134/S1021443711010067
Golparvar, A.R., Gheisari, M.M., Amin, H. and Nader, D., 2017. Relationship of morphological traits and yield components with seed and protein yields in Iranian bread wheat (Triticum aestivum L.) cultivars. Research on Crops, 18(2): 216-218. https://doi.org/10.5958/2348-7542.2017.00036.5
Gomez, K.A. and Gomez, A.A., 1984. Statistical procedures for agricultural research. John Wiley and Sons Inc. 2nd (ed.) New York U.S.A.
GOP, 2019. Pakistan Economic Survey 2018-19. Government of Pakistan, Islamabad.
Hannachi, Fellahi, A.Z., Bouzerzour, H. and Boutekrabt, A., 2013. Correlation, path analysis and stepwise regression in durum wheat (Triticum durum) under rainfed conditions. Journal of Agriculture and Sustainability, 3(2): 122-131. https://doi.org/10.19045/bspab.2021.100087
Jatoi, W.A., Baloch, M.J., Kumbhar, M.B., Khan, N.U. and Kerio, M.I., 2011. Effect of water stress on physiological and yield parameters at anthesis stage in elite spring wheat cultivars. Sarhad Journal of Agriculture, 27(1): 59-65.
Johansson, Eva, Branlard, Gérard, Cuniberti, Marta, Flagella, Zina, Hüsken, Alexandra, Nurit, Eric, Peña, Roberto Javier, Sissons, Mike, Vazquez, Daniel, 2020. In: Wheat quality for improving processing and human health. Springer International Publishing, Cham, pp. 171–204. https://doi.org/10.1007/978-3-030-34163-3_8
Johari, P., Moharram and Habib, M., 2011. Evaluation of 10 wheat cultivars under water stress at Moghan (Iran) condition. African Journal of Biotechnology, 10(53): 10900-10905. https://doi.org/10.5897/AJB11.221
Kachi, M., Abro, T.F., Sootaher, J.K., Baloch, T.A., Mastoi, M.A., Soomro, T.A., Menghwar, K.K., Jadgal, G.M., Chang, M.S., and Shah, W.J., 2020. Estimation of heritability and genetic advance in F2 populations of bread wheat (Triticum aestivum L.) genotypes. International Journal of Biosciences, 16(2): 286-295.
Kamaran, S., Khan, T.M., Bakhsh, A., Hussain, N., Mahpara, S., Manan, A., Chattha, W. S., Jilani, T.A., Sherani, J., and Iqbal, M., 2019. Assessment of morphological and molecular marker based genetic diversity among advanced upland cotton genotypes. Pakistan Journal of Agricutural Sciences, 56(3): 645-652.
Karem, F., Rihan, H. and Pfuller, M., 2017. The effect of exogenous applications of salicylic acid and molybdenum on the tolerance of drought in wheat. Agricultural Research and Technology, 9(4): 1-9. https://doi.org/10.19080/ARTOAJ.2017.09.555768
Khakwani, A.A., Dennett, M.D., Munir, M. and Abid. 2012. Growth and yield response of wheat varieties to water stress at booting and anthesis stages of development. Pakistan Journal of Botany, 44(3): 879-886.
Khan, S.H and Hassan, G., 2017. Heritability and correlation studies of yield and yield related traits in bread wheat. Sarhad Journal of Agriculture, 33(1): 1003-1007. https://doi.org/10.17582/journal.sja/2017.33.1.103.107
Khavarinejed, M.S. and Karmov, M., 2012. Study of genetic diversity among spring wheat genotypes in drought stress by advanced statistical analysis. International Journal of Agronomy and Plant Production, 3(12): 590 -598.
Kumar, S.R., Mittai, K., Dhima, R. and Gupta, D., 2014. Assessment of triticale x bread wheat genotypes for drought tolerance based on morpho-physiological, grain yield and drought tolerance indices under non-irrigated and irrigated environments. International Journal of Food Science, Nutrition and Dietetics, 23(3): 234-244.
Laghari, K.A., Sial, M.A. and Arain, M.A., 2012. Effect of high temperature stress on grain yield and yield components of wheat (Triticum aestivum L.). Science, Technology and Development, 31(2): 83-90.
Li, J., Wen, S., Fan, C., Zhang, M., Tian, S., Kang, W., Zhao, W., Bi, C., Wang, Q., and Lu, S., 2020. Characterization of a major quantitative trait locus on the short arm of chromosome 4B for spike number per unit area in common wheat (Triticum aestivum L.). Theoretical and Applied Genetics, 133: 2259–2269. https://doi.org/10.1007/s00122-020-03595-z
Luo, F., Deng, X., Liu, Y., and Yan, Y., 2018. Identification of phosphorylation proteins in response to water deficit during wheat flag leaf and grain development. Botanical Studies, 59: 1–17. https://doi.org/10.1186/s40529-018-0245-7
Ma, J., Tu, Y., Zhu, J., Luo, W., Liu, H., Li, C., Li, S., Liu, J., Ding, P., and Habib, A., 2020. Flag leaf size and posture of bread wheat: Genetic dissection, QTL validation and their relationships with yield-related traits. Theoretical and Applied Genetics, 133, 297–315. https://doi.org/10.1007/s00122-019-03458-2
Mahpara, S., Ali, Z., Rehmani, M.I.A., Iqbal, J., and Shafiq, M.R., 2017. Studies of genetic and combining ability analysis for some physio-morphological traits in spring wheat using 77 diallel crosses. International Journal of Agricultural and Applied Sciences, 9: 33–40.
Mahpara, S., Hussain, S.T., Iqbal, J., Rasool, I., and Salman, S., 2018. Analysis of generation means for some metric plant traits in two wheats (Triticum aestivum L.) hybrids. Pure and Applied Biology, 7(1): 93-102. https://doi.org/10.19045/bspab.2018.70012
Mateo-Sagasta, J., Zadeh, S.M., and Turral, H., 2018. More people, more food, worse water? A global review of water pollution from agriculture.
Mofokeng, M.A., Mashilo, J., Rantso, P., and Shimelis, H., 2020. Genetic variation and genetic advance in cowpea based on yield and yield-related traits. Acta Agriculturae Scandinavica. Section B. Soil and Plant Science, 70: 381–391. https://doi.org/10.1080/09064710.2020.1749295
Mujtaba, S.M., Faisal, S., Khan, M.A., Mumtaz, S., and Khanzada, B., 2016. Physiological studies on six wheats (Triticum aestivum L.) genotypes for drought stress tolerance at seedling stage. Agricultural Research and Technology, 1(2): 01-06. https://doi.org/10.19080/ARTOAJ.2016.01.555559
Nehe, A., Akin, B., Sanal, T., Evlice, A.K., Ünsal, R., Dinçer, N., Demir, L., Geren, H., Sevim, I., and Orhan, S., 2019. Genotype x environment interaction and genetic gain for grain yield and grain quality traits in Turkish spring wheat released between 1964 and 2010. PLoS One, pp. 14. https://doi.org/10.1371/journal.pone.0219432
Ngwako, S. and Mashiqa, P.K., 2013. The effect of irrigation on the growth and yield of winter (Triticum aestivum L.) cultivars. International Journal of Agriculture and Crop Sciences, 5: 976-982.
Noorifarjam, S.E., Farshadfar and Saeidi, M., 2013. Evaluation of drought tolerant genotypes in bread wheat using based yield screening techniques. European Journal of Experimental Biology, 3(1): 138-143.
Ompal, Kerkhi, S.A., Chand, P., Singh, S.K. and Yadav, M.K., 2018. Correlation and path coefficient analysis in Indian mustard Brassica juncea. Journal of Pharmacognosy and Phytochemistry, 7(6): 890-894.
Peymaninia, Y.M., Shahryari, V.R., Ahmadizadeh, M. and Habibpour, M., 2012. Relationship among morpho-physiological traits in bread wheat against drought stress at presence of a leonardite derived humic fertilizer under greenhouse condition. International Research Journal of Applied and Basic Sciences, 3(4): 822-830.
Philipp, N., Weichert, H., Bohra, U., Weschke, W., Schulthess, A.W., and Weber, H., 2018. Grain number and grain yield distribution along the spike remain stable despite breeding for high yield in winter wheat. PLoS One, 13. https://doi.org/10.1371/journal.pone.0205452
Raghavrao, D., 1983. Design of experiments statistical techniques in Agricultural and biological research. Oxford and IBH publishing company, New Delhi.
Rahman, A.U., Mahboob, A., Khalid, U.B., Razaq, A., Hameed, G. and Haider, Z., 2016. Heritability of yield and yield components in hexaploid wheat. Academia Journal of Agricultural Research, 4(5): 277-280.
Ram, K., Munjal, R.S. and Kumar, N., 2017. Combine effects of drought and high temperature on water relation traits in wheat genotypes under late and very late sown condition. International Journal of Current Microbiology and Applied Sciences, 6(8): 567-576. https://doi.org/10.20546/ijcmas.2017.608.074
Rashidi, V., 2011. Genetic parameters of some morphological and physiological traits in durum wheat genotypes. African Journal of Agricultural Research, 6(10): 2285-2288.
Reynold, M.P., Nagavajan, S., Razzaque, M.A., and Ageeb, O.A.A., 2001. Heat tolerance. In: Application of physiology in wheat breeding (Eds): Reynold, M.P., J.I. Ortiz-Monasterio and A. Mc. Nab. Mexico, D.F. CIMMYT, 124-135.
Sabri, R.S., Rafii, M.Y., Ismail, M.R., Yusuff, O., Chukwu, S.C., and Hasan, N., 2020. Assessment of agro-morphologic performance, genetic parameters and clustering pattern of newly developed blast resistant rice lines tested in four environments. Agronomy, 10: 1098. https://doi.org/10.3390/agronomy10081098
Sakuma, S., and Schnurbusch, T., 2020. Of floral fortune: tinkering with the grain yield potential of cereal crops. New Phytology, 225: 1873–1882. https://doi.org/10.1111/nph.16189
Salehi, S., Soleyman, G., Mahin, M., Soraya, N. and Tahereh, P., 2016. Effects of morphological traits on qualitative and quantitative yield of bread wheat (Triticum aestivum L.) cultivars. Biosciences Biotechnology Research Areas, 13(2): 711-714. https://doi.org/10.13005/bbra/2089
Sanghera, G.S., Kashyap, S.C., Rana, V. and Parray, G.A., 2014. Agro-morphological and genetic diversity among elite wheat genotypes grown under Kashmir conditions. International Journal of Current Research, 6(8): 7735-7740.
Seher, Shabbir, M.G., Rasheed, A., Kazi, A.G., Mahmood, T. and Kazi, A.M., 2015. Performance of diverse wheat genetic stocks under moisture stress condition. Pakistan Journal of Botany, 47(1): 21-26.
Shahryari, R., Eshghi, A.G., Mollasadeghi, V. and Serajamani, R., 2013. Separating correlation coefficients into direct and indirect effects of important morphological traits on grain yield in 28 bread wheat genotypes under terminal drought stress. International of Farming and Allied Sciences, 2(24): 1210-1216.
Shamsi, K.M., Petrosyan, Noor-Mohammadi, G. and Haghparast, R., 2010. The role of water deficit stress and water use efficiency on bread wheat cultivars. Journal of Biological Sciences, 35: 2331-2335.
Shirinzadeh, A., Zarghami, R., Azghandi, A.V., Shiri, M.R. and Mirabdulbaghi, M., 2010. Evaluation of drought tolerance in mid and late mature corn hybrids using stress tolerance indices. Asian. Journal of Plant Sciences, 9(2): 67-73. https://doi.org/10.3923/ajps.2010.67.73
Sial, M.A., Dahot, M.U., Arain, M.A., Markhand, G.S., Mangrio, S.M., Naqvi, M.H., Laghari, K.A. and Mirbahar, A.A., 2009. Effect of water stress on yield components of semi dwarf Bread wheat (Triticum aestivum L.). Pakistan Journal of Botany, 41(4): 1715-1728.
Sial, M.A., Laghari, K.A., Panhwari, N.A., Arian, M.A. and Baloch, G.M., 2012. Genetic improvement of drought tolerance in semi-dwarf wheat. Science, Technology and Development, 31(4): 335- 340.
Sial, M.A., Mangrio, S.M., Bux, H., Channa, A.W. and Shaikh, M., 2017. Effect of water stress on some physiological traits of bread wheat genotypes. Pakistan Journal of Agriculture, Agricultural Engineering and Veterinary Sciences, 33(1): 1-11.
Siyal, A.L., Siyal, F.K., Jatt, T., 2020. Yield from genetic variability of bread wheat (Triticum aestivum L.) genotypes under water stress condition: A case study of Tandojam, Sindh. Pure and Applied Biology, 10 (3): 841-860. https://doi.org/10.19045/bspab.2021.100087
Sootaher, J.K., Abro, T.F., Soomro, Z.A., Soothar, M.K., Baloch, T.A, Menghwar, K.K., Kachi, M., Mastoi, MA., and Soomro, T.A., 2020. Assessment of genetic variability and heritability for grain yield and its associated traits in F2 populations of bread wheat (Triticum aestivum L.). Pure and Applied Biology, 9(1): 36-45.
Steel, R., and Torie, J., 1980. Principles and Procedures of Statistics. McGraw Hill, New York, NY. pp.107-109. (Duncan): p.144.
Takumi, S., Mitta, S., Komura, S., Ikeda, T.M., Matsunaka, H., Sato, K., Yoshida, K., and Murai, K., 2020. Introgression of chromosomal segments conferring early heading date from wheat diploid progenitor, Aegilops tauschii Coss., into Japanese elite wheat cultivars. PLoS One, 15. https://doi.org/10.1371/journal.pone.0228397
Talebi, R., Fayaz, F. and Naji, A.M., 2009. Effective selection criteria for assessing drought stress tolerance in durum wheat. General and Applied Plant Physiology, 35: 64-74.
Wolde, G.M., Mascher, M., Schnurbusch, T., 2019. Genetic modification of spikelet arrangement in wheat increases grain number without significantly affecting grain weight. Molecular Genetics and Genomics, 294: 457–468. https://doi.org/10.1007/s00438-018-1523-5
Würschum, T., Leiser, W.L., Langer, S.M., Tucker, M.R., and Longin, C.F.H., 2018. Phenotypic and genetic analysis of spike and kernel characteristics in wheat reveals long-term genetic trends of grain yield components. Theoretical and Applied Genetics, 131: 2071–2084. https://doi.org/10.1007/s00122-018-3133-3
Yildirim, M., 2013. The effect of water stress position weight variation in wheat. Turkish Journal of Field Crops, 18(2): 144-150.
Zhao, C., Bao, Y., Wang, X., Yu, H., Ding, A., Guan, C., Cui, J., Wu, Y., Sun, H., and Li, X., 2018. QTL for flag leaf size and their influence on yield-related traits in wheat. Euphytica, 214: 1–15. https://doi.org/10.1007/s10681-018-2288-y
To share on other social networks, click on any share button. What are these?




