Nest size and its Contributing Factors for Black-Necked Cranes Grus nigricollis
Nest size and its Contributing Factors for Black-Necked Cranes Grus nigricollis
Zheng Quan Jiang1,2, Feng Shan Li3, Jiang Hong Ran1,*, Chen Hao Zhao1, Man Zhang1 and Hua Li4
1College of Life Sciences, Sichuan University, Key Laboratory of Bio-resources and Eco-environment of Ministry of Education, Chengdu 610064, China
2College of History, Culture and Tourism, Guangxi Normal University, Guilin 541001, China
3International Crane Foundation, Baraboo, Wisconsin 53913, USA
4Ruoergai National Nature Reserve, Ruoergai, Sichuan Province 624500, China
ABSTRACT
The black-necked crane Grus nigricollis is the only alpine crane species, whose distribution is restricted to the Tibetan-Qinghai Plateau and the adjacent high altitude areas of China, Bhutan, Pakistan, and India. Study on nest type and size is useful for understanding the life history, evolution and adaptation of birds. A survey was conducted on nest size and the underlying regulatory factors of Black-necked Cranes from 25 March to 31 July in 2013 and 2014 at Ruoergai Internationally Important Wetland and its surrounding area, covering 83 nests. Four types of nests were found in the area, i.e., natural island nest, grass mound nest, dirt mound nest and floating grass nest. The nest length, nest width, nest height and nest volume among the four types of nests all were significantly different in the order: floating grass nests >dirt mound nests >grass mound nests >natural island nests, indicating that nest size was related to nest type. Among the three kinds of nest-site habitats, there were no significant differences in nest length and nest width, but differences were found in nest height and nest volume in the order: lake nests >river nests >swamp nests, indicating that nest size of Black-necked Cranes was also related to nest-site habitat. Nest length, nest width, nest height and nest volume were all found to be the greatest in April while the smallest in June with medium size in May, implying that nest size tended to decrease with time over a year.
Article Information
Received 01 June 2016
Revised 24 November 2016
Accepted 16 February 2017
Available online 11 April 2018
Authors’ Contribution
ZQJ designed and executed field studies, and wrote the article. JHR and FSL helped in manuscript writing. FSL, CHZ and HL helped in field work. MZ helped in data analysis.
Key words
Nest size, Nest type, Breeding biology, Habitat, Date of nest-building, Black-necked Cranes, Grus nigricollis.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.3.877.884
* Corresponding author: rjhong-01@163.com
0030-9923/2018/0003-0877 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
INTRODUCTION
Bird nest is a special breeding construction for egg-laying and hatching (Palomino et al., 1998; Zheng, 2012). Nests are species specific (Vergara et al., 2010). The type and size of nests are not only a result of natural selection in the evolution of birds (Collias, 1997; Slagsvold, 1989; Hansell and Overhill, 2000), but also a result of combined effects of birds themselves, i.e., body size and experience (Hoi et al., 1996) and environmental factors (predation pressure, nest-site quality, territory quality, nesting material) (Martin et al., 2000; Broggi and Senar, 2009). Nest size is mainly determined by the type of nest, body size of parent birds and clutch size (Slagsvold, 1982, 1989). Nest size is also related to many other factors, including the type and quality of nest-site habitat (De Neve et al., 2004; Vergara et al., 2010), the temperature during nest-building period (Nager and van Noordwijk, 1992; Britt and Deeming, 2011), altitude and latitude of nest-site (Kern and Riper, 1984), the quality of breeding pairs (De Neve and Soler, 2002; Tomás et al., 2006; Soler et al., 2007), and the reproductive investment will of breeding pairs (Soler et al., 1998, 2001). Research on nests is one of the important steps to understand the reproductive strategies of birds (Martin, 1995; Fontaine and Martin, 2006), and is necessary for uncovering bird life history (Martin, 1995). In addition, it can provide important information on bird evolution and adaptation and the relationship of birds with their environments (Collias, 1997; Coleman et al., 1985; Wang et al., 2012).
Nest size is associated with the breeding strategy of birds (Geupel and DeSante, 1990). To build a smaller nest is one of the strategies to reduce the breeding cycle (De Neve and Soler, 2002), and is associated with later breeding (Soler et al., 1995). Only a limited number of studies have been conducted to relate nest size with the time of birds building nests (Jiao et al., 2010), and the relationship between nest size and the dates of cranes building the nests is still unknown. The Black-necked Crane (Grus nigricollis), a threatened (vulnerable) species, is the only crane that inhabits and reproduces in high plateau (2500–5000 m ASL) wetlands and breeds every year (Li and Li, 2005). In Ruoergai, the breeding season of Black-necked Cranes starts from early April and ends in late July (Li and Li, 2005); the time of nest-building starts from early April and ends in late June (Li and Li, 2005; Jiang et al., 2014). The dates of Black-necked Cranes initiating building of nests, however, are quite varied (Dou et al., 2013; Jiang et al., 2014). Nevertheless, the relationship between nest size and the dates of birds’ nest building has not been investigated.
As per life history theory, the nest size is affected by the quality of breeding habitat (such as the predation risk in nest-site habitat) (Soler et al., 1998; Wang et al., 2012). Cranes can nest in different wetland habitats (Leito et al., 2005). Building a smaller nest is one of the strategies of the bird to reduce the current reproduction investment in a low-quality breeding habitat (Bosque and Bosque, 1995; Julliard et al., 1997; Fontaine and Martin, 2006). For the same species of birds, a bigger nest is associated with a lower predation risk and a better breeding habitat. Black-necked Cranes can nest in lakes, rivers and marshes (Wu et al., 2009; Jiang et al., 2014). Their nest predation risk mainly comes from carnivorous mammals such as dogs, Red Fox (Vulpes vulpes) and Grey Wolf (Canis lupus) (Wu et al., 2009). The risks of predation by carnivorous mammals may be different in the three habitats (Wang et al., 1989; Li and Li, 2005; Wu et al., 2009). However, it is not clear whether the nest sizes of Black-necked Cranes are different in the three habitats in response to reduce predation risk.
In order to understand the nest size and the underlying regulatory factors of Black-necked Cranes, a survey was conducted from 25 March to 31 July in 2013 and 2014. The main objectives were to: (1) describe in detail the type and size of nests of Black-necked Cranes; (2) find the relationships between nest size and nest-site habitats, and (3) relate nest size with the dates of birds building the nests.
MATERIALS AND METHODS
Study area
The study was carried out at Ruoergai Internationally Important Wetland (RIIW) and its surrounding area (33°18′-34°01′N, 102°30′-103°06′E), with an elevation of 3450 m and a relative elevation of 50-100 m above sea level. The area is located in the upper Yellow River valley on the eastern Tibetan Plateau in the province of Sichuan. Mean annual precipitation in the region ranges from 660 to 750 mm and mean annual temperatures between 0.6-1.2 °C. The area supports four types of vegetation i.e., subalpine shrub, subalpine meadow, swamp meadow and swamp vegetation; the swamp vegetation however is the only vegetation that Black-necked Cranes nest in. The swamp vegetation mainly includes: Carex muliensis, C. lasiocarpa, C. meyeriana, Glyceria maxima, Kobresia tibetica and Menyanthes trifolia (Tian, 2005; Dou et al., 2013). RIIW is the most important breeding area for the Black-necked Crane in China. The birds migrate from the wintering areas into RIIW in late March and migrate out in late October each year (Li and Li, 2005).
Nest type classification
As per the location, structure and the nesting material, nests of Black-necked Crane were classified into four types: natural island nest, grass mound nest, dirt mound nest and floating grass nest (Table I, Fig. 1). Natural island nests were located on islands in the lake or river and constructed with a layer of dry grass. The bottom of natural island nests is normally >10 cm above the water surface, and the risk of being flooded is low. Grass mound nests were located on grassy islands in the lake, river or grassy swamp and constructed with the mixture of mud, grass roots and dry grass. The bottom of grass mound nests is normally <5 cm above the water surface, therefore, there is a risk of being flooded. Dirt mound nests were located only in the lake and constructed with the mixture of mud and grass roots. Similar to grass mound nests, the bottom of dirt mound nests is normally <5 cm above the water surface, therefore, there is a risk of being flooded. Floating grass nests were also located in the lake and constructed with dry grass. Since they are floating in water, the risk of being flooded is low.
| Characteristics | Natural island nest (9) | Grass mound nest (64) | Dirt mound nest (6) | Floating grass nest (4) |
|
Location |
Natural islands | Natural grassy islands | Water | Emergent plants |
| Habitats | Lake or river | Lake, river or grassy swamp | Lake | Lake |
| Main nest materials | Dry grass | Mud, grass roots and dry grass | Mud and grass roots | Dry grass |
| Length(cm) | 45.0-90.0 | 32.0-130.0 | 60.0-134.0 | 86.0-135.0 |
| Width(cm) | 45.0-86.0 | 30.0-115.0 | 58.0-130.0 | 85.0-128.0 |
| Height(cm) | 2.0-4.0 | 1.5-14.8 | 30.0-52.6 | 38.3-44.4 |
|
Volume(cm3) |
5089.4-24315.9 | 1885.0-130061.8 | 138572.4-719653.5 | 246874.0-519794.9 |
| Nest-building period (days) | 0.5±1.5(5) | 0.5±6.0(41) | 20.0±40.0(5) |
15.0±21.0(4) |
Measurement of nest and nest-site characteristics
Following the previous investigations (Ran et al., 1999; Dou et al., 2013; Jiang et al., 2014), we first determined/demarcated the main breeding area and the distribution of paired birds. From 25 March to 31 July in 2013 and 2014, we monitored the behavior of paired birds using a Leica 10×42 mm binocular and a Leica 25-50×65 mm telescope each day. On the start of nest building, we continuously observed the bird behavior and recorded the nest-site habitat, nest-building time and material until the first egg was laid. After the birds left the nest, we approached, located and demarcated the nest by GPS, then measured nest parameters including length, width and height of the nest. After the chicks were hatched, we measured nest depth. Because most of the upper surfaces of nests on initial completion were flat, there was no obvious distinction between internal and external diameter. Anyway, diameters were measured of the external surface of nests as well. Length, width, height and diameter were measured on the spot with a steel tape on the upper surface of nests. The volume of nest (V) was calculated according to the equation:
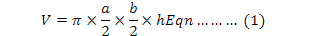
Where a, b and h denote nest length, nest width and nest height, respectively (Vergara et al., 2010).
Nesting time classification
The date and month of nest-building is the date that paired birds began to build the nest. Nest-building period is the number of days the birds had taken to finish construction of their nests.
Table II.- Size of four types of nests of Black-necked Cranes in Ruoergai (Mean±SD).
| Variables | Natural island nest (9) | Grass mound nest (64) | Dirt mound nest (6) | Floating grass nest (4) | F | P |
| Length(cm) | 68.4±14.7 | 84.1±22.1 | 99.3±28.3 | 111.5±201 | 4.587 | 0.005 |
| Width(cm) | 65.8±14.3 | 79.4±19.6 | 87.7±23.6 | 104.8±18.6 | 4.129 | 0.009 |
| Height(cm) | 3.1±0.8 | 8.6±3.5 | 40.8±10.8 | 42.0±2.6 | 46.792 | <0.001 |
|
Volume(cm3) |
12070.1± 7367.3 | 51845.7± 32786.5 | 306187.4± 226533.1 | 389042.3± 113693.0 |
21.193 |
<0.001 |
Data analysis
Statistical analysis was conducted following SPSS 19.0. The normality of data was tested using the Shapiro-Wilk test; if the data were not normally distributed, a logarithm transformation log (x+1) was used. One-way Analysis of Variance (ANOVA) and Tukey HSD test were used to test whether there were significant differences in the following features: i) nest length; ii) nest width; iii) nest height; iv) volume, among different habitats (lake, river and grassy swamp), nest types (natural island nest, grass mound nest, dirt mound nest and floating grass nest), and months (April, May, June). The Spearman correlation coefficient (r) was used to analyze the relationship between nest size (length, width, height and volume) and the date of nest-building. Significance level in all tests was set at P <0.05, highly significance level was set at P <0.01.
RESULTS
Nest types and nest-building period
Four types of nests were found during the survey, i.e., natural island nest, grass mound nest, dirt mound nest and floating grass nest, among which grass mound nests were the most abundant (n =64), while floating grass nests were the least abundant (n =4). All 83 nests were found in wetland habitat, including lakes (n =22), rivers (n =20) and grassy swamps (n =41). In the four types of nests, grass mound nests were found in all the three nesting habitats, natural island nests were found in rivers and lakes, while floating grass nests and dirt mound nests were found only in lakes.
The nest-building period varied between 0.5 and 40.0 days, with a mean value of 6.7±9.3 days (n =55). The longest mean nest-building period was spent on building a dirt mound nest (31.8±8.4 days, n =5), while the shortest time was needed for natural island nest (0.9±0.4 days, n =5). Other related parameters are given in Table I.
Nest size
The length of the nests varied between 32.0 and 135.0 cm, with a mean value of 84.8 ± 23.2 cm (n =83). The width of nests varied between 30.0 and 130.0 cm, with a mean value of 79.8 ± 20.4 cm (n =83). The height of nests varied between 1.5 and 52.6 cm, with a mean value of 11.9 ± 11.8 cm (n =83). The volume of nests varied between 1885.0 and 719,653.5 cm3, with a mean value of 82,169.3 ± 118,135.1 cm3 (n =83).
Comparison of nest size among different nest-types
Among the four types of nests, floating grass nests were the largest (mean volume: 389,042.3± 113,693.0 cm3 (n =4)); natural island nests were the smallest (mean volume: 12,070.1 ± 7367.3 cm3 (n =9)). The length, width, height and volume of the four types of nests were significantly different (P <0.01) and changed in the order of floating grass nests >dirt mound nests >grass mound nests and >natural island nests (Table II).
Comparison of nest size among different habitats
Among the three types of habitats, the lake held the four types of nests, and the river held two types of nests, i.e., natural island nest and grass mound nest, while the grassy swamp supported only the grass mound nests.
No significant difference was found in terms of length and width among habitats, but highly significant difference of nest volumes was found among habitats (P <0.0001). Both nest height and volume changed in the order of lake habitat >river habitat >grassy swamp habitat, while the nest length and width changed in the order: lake habitat >grassy swamp habitat >river habitat (Table III). There was a significant difference in volume between lake nests and nests in other habitats (P <0.01), but the difference was not significant between river habitat and grassy swamp habitat (P=0.858) (Table III).
Grass mound nest was found in lake, river and grassy swamp habitats, and was the most widely distributed and most abundant nest type. The height and volume of grass mound nest changed in the order: lake habitat >river habitat >grassy swamp habitat, and the length and width changed in the order: lake habitat >grassy swamp habitat >river habitat (Table III).
Comparison of nest size among different months
As stated before, the nesting period of black-necked crane at Ruoergai started from the early April and continued up to the end of June.
Table III.- Size of nests in three kinds of habitats of Black-necked Cranes in Ruoergai (Mean ± SD).
| Variables | Lake | River | Grassy swamp | F | P | |
| All nests | Length(cm) | 88.8±26.3(22) | 81.6±20.4(20) | 84.3±23.0(41) | 0.53 | 0.591 |
| Width(cm) | 82.4±23.5(22) | 77.5±17.6(20) | 79.5±20.3(41) | 0.311 | 0.734 | |
| Height(cm) | 21.6±19.4(22) | 9.1±4.0(20) | 8.1±3.5(41) | 12.904 | <0.001 | |
|
Volume(cm3) |
170175.7± 201367.4(22) | 53997.7± 38266.5(20) | 48688.6± 28640.8(41) | 10.184 | <0.001 | |
| Grass mound nests | Length(cm) | 85.3±31.5(4) | 83.5±19.0(19) | 84.3±23.0 (41) | 0.014 | 0.986 |
| Width(cm) | 80.3±31.1(4) | 79.2±16.2(19) | 79.5±20.3 (41) | 0.005 | 0.995 | |
| Height(cm) | 9.2±2.8(4) | 9.4±3.8(19) | 8.1±3.5 (41) | 0.917 | 0.405 | |
|
Volume(cm3) |
61757.7± 52852.1(4) | 56571.8± 37493.9(19) | 48688.6± 28640.8 (41) | 0.562 |
0.573 |
Table IV.- Size of nests of Black-necked Cranes in different months in Ruoergai (Mean ± SD).
| Variables | April | May | June |
T-testa |
Correlationb |
|||
|
F |
P |
r |
P |
|||||
| All nests | Length(cm) | 97.6± 19.5(38) | 78.9± 17.8(35) | 57.2± 22.0(10) |
20.58 |
<0.001 |
-0.543 |
<0.001 |
| Width(cm) | 89.8± 16.6(38) | 75.8± 16.6(35) | 55.5± 22.1(10) |
17.16 |
<0.001 |
-0.478 |
<0.001 |
|
| Height(cm) | 16.6± 15.3(38) | 8.4± 3.5(35) | 6.4± 9.7(10) |
6.265 |
0.003 |
-0.372 |
0.001 |
|
|
Volume(cm3) |
130790.8± 157457.6 (38) | 44053.7± 27432.8 (35) | 30812.2± 61825.8 (10) |
6.837 |
0.002 |
-0.484 |
<0.001 |
|
| Grass mound nests | Length(cm) | 97.8±15.7 (25) | 81.7± 16.9(30) | 53.8± 20.3(9) |
22.89 |
<0.001 |
-0.750 |
<0.001 |
| Width(cm) | 90.3±13.1 (25) | 78.6± 15.5(30) | 52.0± 20.3(9) |
20.59 |
<0.001 |
-0.720 |
<0.001 |
|
| Height(cm) | 9.4±3.1(25) | 9.4±2.8 (30) | 3.5±2.8 (9) |
15.91 |
<0.001 |
-0.568 |
<0.001 |
|
|
Volume(cm3) |
68321.3± 32435.1 (25) | 49993.4± 24961.5 (30) | 12254.9± 20640.5 (9) |
13.70 |
<0.001 |
-0.775 |
<0.001 |
|
a, Tukey HSD test; b, The Spearman correlation coefficient (r).
Nest parameters (length, width, height and volume) showed highly significant differences among different months (P <0.01). The nest length, width, height and volume were all changed in the order: April nests >May nests >June nests (Table IV), indicating that the size tended to decrease with time (Table IV). Spearman correlation analysis indicated that size of nests (length, width, height and volume) and date of nest-building were negatively correlated (P <0.01) (Table IV).
The analysis on 64 grass mound nests showed that their size decreased from April to June (P <0.0001) (Table IV). In addition, significant differences were found for all the nest parameters (length, width, height and volume) among different months (P <0.01). Correlation analysis indicated that size of nests (length, width, height and volume) and date of nest-building were negatively correlated (P <0.01).
DISCUSSION
Is nest size an indicator of nest-site quality in black-necked crane?
In the present study, we found that size of crane nests was influenced by different habitats. Similar results have been reported previously in the studies of Sandhill Crane (Grus canadensis) (Walkinshaw, 1973) and Penduline Tits (Remiz pendulinus) (Hoi et al., 1996). Nest size has been related to the strategy of birds to protect them from predation. For example, kestrels can make facultative adjustments to current reproductive investment in response to their perception of the risk of nest predation (Greenwood and Dawson, 2011). At the nest site with low predation risk, birds usually take the breeding strategy with maximizing the reproduction investment in first breeding (Slagsvold, 1984; Fontaine and Martin, 2006), such as building bigger nests (Martin, 1988), hence larger nests are usually associated with better nest-site habitats (e.g., low predation risk, low human disturbance etc.). The studies on the White Stork (Ciconia ciconia) (Vergara et al., 2010) and the Magpie (Pica pica) (Soler et al., 1995) have revealed that the nests located at better nesting sites had bigger sizes, therefore, nest size may be an indicator of nest-site habitat quality to some extent, i.e., a bigger nest means a better habitat. In three kinds of nesting habitats of Black-necked Crane, the predation risk in the lake was the lowest being larger and deeper water bodies (Li and Li, 2005; Wu et al., 2009).
Table V.- Nest parameters of Black-necked Cranes reported in different studies.
| Source |
Nest type |
habitat |
Sample size |
Nest length (cm) |
Nest width (cm) |
Nest height (cm) |
Nest depth (cm) |
| Lü et al. (1980) |
- |
- |
11 |
79.96 |
- |
10.61 |
4.49 |
| Wang et al. (1989) |
4 |
- |
9 |
112.23 |
93.83 |
7.13 |
- |
| Wu et al. (2009) |
- |
3 |
21 |
56.37 |
37.49 |
21.99 |
- |
| Dwyer et al. (1992) |
- |
- |
16 |
75.5 |
64.06 |
- |
- |
| Pfister (1998) |
- |
- |
5 |
74.6 |
66 |
- |
3.8 |
| Li and Li (2005) |
- |
- |
4 |
89 |
78 |
- |
5.75 |
| This study |
4 |
3 |
83 |
84.82 |
79.77 |
11.92 |
2.99 |
At lake habitats, because of the best nesting habitat quality with low predation risk and low human disturbance, Black-necked Cranes usually build bigger nests despite more energy and time investment on bigger nests.
Relationship between nest size and the dates of nest building
In the current study, nests built in April were the biggest and the smallest in June. In addition, parameters of nest size were significantly different among months, i.e., nest size decreasing with time. Correlation analysis indicated that size of nests (length, width, height and volume) and date of nest-building were negatively correlated. It has been proposed that the nest building behavior is the most important symbol that birds are entering the breeding cycle (Zheng, 2012), therefore we suggest that nest size was correlated with the time of Black-necked Cranes entering their breeding cycle.
Since the breeding season for birds is limited in high-cold and high-altitude areas (Lack, 1950, 1968; Gurney et al., 2011), birds can adjust their breeding cycle by adjusting size of nests (Geupel and DeSante, 1990). The studies on the Magpie Pica species (Soler et al., 1995) found larger nests associated with earlier breeding starting dates. Our results showed that size of nests was negatively correlated to starting dates of nest building, which is likely a strategy to shorten the breeding cycle.
Nest height has been suggested to be a strategy of conserving energy in the nest (Collias and Collias, 1984). Windsor et al. (2013) found that nest height was significantly related to nest cooling rates, with higher nests suitable for reducing heat loss; nest height has been found to be negatively related to atmospheric temperature. Among the same type of grass mound nests, height of nests built in April (lower temperature) was significantly higher compared to nests built in June, and nest height was negatively related to atmospheric temperature. Similar results have been reported previously in the studies of Eurasian tree sparrow (Passer montanus) (Pinowski et al., 2006).
Black-necked crane nest type and size
Black-necked Cranes could build four types of nests, the grass mound nest was the type with shorter building-nest period, more available nest-sites and most in number, but two nest types (floating grass nest and dirt mound nest) with longer building-nest period only were found in lake habitat. Floating grass nest was the nest type floating on water surface and needed larger nest volume and nest height to generate enough floatage to ensure that the nest and eggs were not flooded. Dirt mound nests were located in the lake water bodies and susceptible to water erosion and scour, hence only larger sized nests could effectively resist water erosion and scouring. Seen from the number of nest types of Black-necked Cranes, lake habitat >river habitat >swamp habitat, were sequentially secure habitats for nesting.
The current survey results on nest size of the Black-necked Crane (Table V) greatly differed with the studies already conducted. Compared with nests reported by Lü et al. (1980), Dwyer et al. (1992), Pfister (1998) and Wu et al. (2009), nests in this study were bigger; the results might have been different because of the total number of samples, measuring time, nest types etc.
CONCLUSION
In conclusion, our analyses indicate that the nest size of the Black-necked Crane was correlated with nesting habitat, nest type and nesting date. The birds build bigger nests and more types of nests in better nesting habitats. The study would help better understand the relationship of bird nesting and habitats.
ACKNOWLEDGEMENTS
Financial support for the study was provided by the International Crane Foundation. We are grateful to Ruoergai National Nature Reserve and Dr. Li Kui and other staff of flower lake scenic spot for assistance during field surveys. We thank the laboratory students for assistance in fieldwork.
Statement of conflict of interest
Authors have declared no conflict of interest.
REFERENCES
Bosque, C. and Bosque, M.I., 1995. Nest predation as a selective factor in the evolution of developmental rates in altricial birds. Am. Nat., 145: 234-260. https://doi.org/10.1086/285738
Britt, J. and Deeming, D.C., 2011. First-egg date and air temperature affect nest construction in blue tits Cyanistes caeruleus, but not in great tits Parus major. Bird Study, 58: 78-89. https://doi.org/10.1080/00063657.2010.524916
Broggi, J. and Senar, J.C., 2009. Brighter great tit parents build bigger nests. Ibis, 151: 588-591. https://doi.org/10.1111/j.1474-919X.2009.00946.x
Collias, N.E., 1997. On the origin and evolution of nest building by passerine birds. Condor, 99: 253-270. https://doi.org/10.2307/1369932
Coleman, R.M., Gross, M.R. and Sargent, R.C., 1985. Parental investment decision rules: a test in the bluegill sunfish. Behav. Ecol. Sociobiol., 18: 59-66.
De Neve, L. and Soler, J.J., 2002. Nest-building activity and laying date influence female reproductive investment in magpies: an experimental study. Anim. Behav., 63: 975-980. https://doi.org/10.1006/anbe.2001.1989
De Neve, L., Soler, J.J., Soler, M. and Pérez-Contreras, T., 2004. Nest size predicts the effect of food supplementation to magpie nestling on their immunocompetence: an experimental test of nest size indicating parental ability. Behav. Ecol., 15: 1031-1036. https://doi.org/10.1093/beheco/arh074
Dwyer, N.C., Bishop, M.A., Harkness, J.S. and Zhong, Z.Y., 1992. Black-necked cranes nesting in the Tibet autonomous region, P. R. china. N. Am. Crane Worksh., 6: 75-80.
Fontaine, J.J. and Martin, T.E., 2006. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol. Lett., 9: 428-434. https://doi.org/10.1111/j.1461-0248.2006.00892.x
Geupel, G.R. and DeSante, D.F., 1990. Incidence and determinants of double brooding in Wrentits. Condor, 92: 67-75. https://doi.org/10.2307/1368384
Greenwood, J.L. and Dawson, R.D., 2011. Risk of nest predation influences reproductive investment in American kestrels (Falco sparverius): an experimental test. J. Raptor Res., 45: 15-26. https://doi.org/10.3356/JRR-10-26.1
Gurney, K.E.B., Clark, R.G., Slattery, S.M., Smith-Downey, N.V., Walker, J., Armstrong, L.M., Stephens, S.E., Petrula, M., Corcoran, R.M., Martin, K.H., De Groot, K.A., Brook, R.W., Afton, A.D., Cutting, K., Warren, J.M., Fournier M. and Koons, D.N., 2011. Time constraints in temperate-breeding species: influence of growing season length on reproductive strategies. Ecography, 34: 628-636. https://doi.org/10.1111/j.1600-0587.2010.06622.x
Hansell, M. and Overhill, R., 2000. Bird nests and construction behaviour. Cambridge University Press, Cambridge, U.K. https://doi.org/10.1017/CBO9781139106788
Hoi, H., Schleicher, B. and Valera, F., 1996. Nest size variation and its importance for mate choice in penduline tits Remiz pendulinus. Anim. Behav., 51: 464-466. https://doi.org/10.1006/anbe.1996.0046
Jiao, S., Jian, Y.L., Zhang, L.S., Li, S., Wang, H.T. and Gao, W., 2010. Effects of parental investment during nest building on reproductive efficacy of Great Tit. J. Changchun Nor. Univ. (Nat Sci), 29: 76-79.
Julliard, R., McCleery, R.H., Clobert, J. and Perrins, C.M., 1997. Phenotypic adjustment of clutch size due to nest predation in the great tit. Ecology, 78: 394-404. https://doi.org/10.1890/0012-9658(1997)078[0394:PAOCSD]2.0.CO;2
Kern, M.D. and Van Riper, C., 1984. Altitudinal variations in nests of the Hawaiian honeycreeper Hemignathus virens virens. Condor, 86: 443-454. https://doi.org/10.2307/1366825
Lack, D., 1950. The breeding seasons of European birds. Ibis, 92: 288-316. https://doi.org/10.1111/j.1474-919X.1950.tb01753.x
Lack, D., 1968. Ecological adaptations for breeding in birds. Chapman and Hall, London.
Leito, A., Ojaste, I., Truu, J. and Palo, A., 2005. Nest site selection of the Eurasian crane Grus grus in Estonia: an analysis of nest record cards. Ornis Fenn., 82: 44-54.
Li, Z.M. and Li, F.S., 2005. Research on the black-necked crane. Shanghai Technological and Educational Press, Shanghai (In Chinese).
Lü, Z.B., Yao, J.C. and Liao, Y.F., 1980. Observation on breeding ecology of the black-necked crane. Chin. J. Zool., 1: 19-24 (In Chinese). http://caod.oriprobe.com/articles/32872870/hei_jing_he_fan_zhi_sheng_tai_de_guan_cha_.htm
Martin, T.E., 1988. Nest placement: implications for selected life-history traits with special references to clutch size. Am. Nat., 132: 900-910. http://www.jstor.org/stable/2462268
Martin, T.E., 1995. Avian life history evolution in relation to nest sites, nest predation and food. Ecol. Monogr., 65: 101-127. https://doi.org/10.2307/2937160
Martin, T.E., Scott, J. and Menge, C., 2000. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. B-Biol. Sci., 267: 2287-2293. https://doi.org/10.1098/rspb.2000.1281
Nager, R.G. and van Noordwijk, A.J., 1992. Energetic limitation in the egg laying period of great tits. Proc. R. Soc. B-Biol. Sci., 249: 259-263. https://doi.org/10.1098/rspb.1992.0112
Palomino, J.J., Martin-Vivaldi, M., Soler, M. and Soler, J.J., 1998. Functional significance of nest size variation in the Rufous Bush Robin Cercotrichas galactotes. Ardea, 86: 177-185.
Pfister, O., 1998. The breeding ecology and conservation of the Black-necked Crane (Grus nigricollis) in Ladakh, India. New Delhi, Univ. Hull, pp. 1-124.
Pinowski, J., Haman, A., Jerzak, L., Pinowska, B., Barkowska, M., Grodzki, A. and Haman K., 2006. The thermal properties of some nests of the Eurasian tree sparrow Passer montanus. J. Therm. Biol., 31: 573-581. https://doi.org/10.1016/j.jtherbio.2006.05.007
Ran, J.H., Liu, S.Y., Zeng, Z.Y., Shao, K.Q., Lin, Q. and Zhang, M., 1999. The population and distribution of Black-necked Cranes (Grus nigricollis) in Xiaman Nature Reserve in Sichuan. Chin. J. appl. environ. Biol., 5: 40-44.
Slagsvold, T., 1982. Clutch size, nest size, and hatching asynchrony in birds: experiments with the Fieldfare (Turdus pilaris). Ecology, 63: 1389-1399. https://doi.org/10.2307/1938866
Slagsvold, T., 1984. Clutch size variation of birds in relation to nest predation: on the cost of reproduction. J. Anim. Ecol., 53: 945-953. https://doi.org/10.2307/4669
Slagsvold, T., 1989. On the evolution of clutch size and nest size in passerine birds. Oecologia, 79: 300-305. https://doi.org/10.1007/BF00384308
Soler, J.J., Soler, M., Møller, A.P. and Martínez, J.G., 1995. Does the great spotted cuckoo choose magpie hosts according to their parenting ability? Behav. Ecol. Sociobiol., 36: 201-206. https://doi.org/10.1007/BF00177797
Soler, J.J., Møller, A.P. and Soler, M., 1998. Nest building, sexual selection and parental investment. Ecol. Evolut., 12: 427-441. https://doi.org/10.1023/A:1006520821219
Soler, J.J., De Neve, L., Martínez, J.G. and Soler, M., 2001. Nest size affects clutch size and the start of incubation in magpies: an experimental study. Behav. Ecol., 12: 301-307. https://doi.org/10.1093/beheco/12.3.301
Soler, J.J., Martin-Vivaldi, M., Haussy, C. and MØller, A.P., 2007. Intra- and interspecific relationships between nest size and immunity. Behav. Ecol., 18: 781-791. https://doi.org/10.1093/beheco/arm045
Tian, Y.B., 2005. The vegetation type and its distribution regularity under different habitats in Ruoergai Plateau. J. Yangtze Univ. (Nat Sci), 2: 1-6.
Tomás, G., Merino, S., Moreno, J., Sanz, J.J., Morales, J. and Garcia-Fraile, S., 2006. Nest weight and female health in the blue tit (Cyanistes caeruleus). Auk, 123: 1013-1021. https://doi.org/10.2307/25150216
Vergara, P., Gordo, O. and Aguirre, J.I., 2010. Nest size, nest building behavior and breeding success in a species with nest reuse: the white stork Ciconia ciconia. Annls. Zool. Fennici, 47: 184-194. https://doi.org/10.5735/086.047.0303
Walkinshaw, L.H., 1973. A history of Sandhill Crane on the Haehnle Sanctuary Michigan. Jack-Pine Warbler, 51: 54-74.
Wang, Y.H., Wu, Z.K., Li, Z.M., Li, D.H., Zhou, Z.J. and Mary, A.B., 1989. An Observation on the Nests, Eggs and Chickens of Black-necked Cranes. Guizhou Sci., 7: 50-56. (In Chinese)
Wang, Y., Zhang, Z.W., Zheng, G.M., Li, J.Q., Xu, Z.L., Ma, Z.J. and Biancucci, A.L., 2012. Ornithological research: past twenty years and future perspectives in China. Biodiv. Sci., 20: 119-137.
Windsor, R.L., Fegely, J.L. and Ardia, D.R., 2013. The effects of nest size and insulation on thermal properties of tree swallow nests. J. Avian Biol., 44: 305-310. https://doi.org/10.1111/j.1600-048X.2013.05768.x
Wu, H.Q., Zha, K., Zhang, M. and Yang, X.J., 2009. Nest site selection by Black-necked Crane Grus nigricollis in the Ruoergai Wetland, China. Bird Conserv. Int., 19: 277-286. https://doi.org/10.1017/S0959270909008168
Zheng, G.M., 2012. Ornithology (2nd ed.). Beijing Normal University Press, Beijing. (In Chinese)










