The Potential of Milbemectin on the Formation of Galls and Egg Masses of Meloidogyne javanica on Eggplants
The Potential of Milbemectin on the Formation of Galls and Egg Masses of Meloidogyne javanica on Eggplants
Mohamed S. Khalil
Agricultural Research Center (ARC), Central Agricultural Pesticides Laboratory (CAPL), El-Sabaheya, Alexandria, Egypt.
Abstract | The root-knot nematode (Meloidogyne javanica) is one of the most destructive pests to vegetable crops worldwide. Non-fumigant and/or fumigant nematicides, as well as biological agents are the most utilized tools to manage the root-knot nematodes (RKNs). Environmental and health defects were notice with using synthetic nematicides. Thus, in this work an attempt was conducted to provide alternative approach could be listed in control measures of RKNs. Two pot experiments were conducted to assess the efficacy of the milbemectin on galls and egg masses formation of M. javanica in roots of eggplants. Milbemectin (Milbeknock 1% EC) was applied at 0.1% (1 ml/l) and 0.5% (5 ml/l) in compared with the non-fumigant nematicide fenamiphos (40% EC). Results revealed that all tested doses of milbemectin suppressed galls (ranged from 50.59 to 75.50%, of both experiment) and egg masses (ranged from 66.67 to 87.01%, of both experiment) significantly, while fenamiphos recorded moderate reductions. Also, the measured plant indices were augmented with all applied treatments. Therefore, from this study it can prove that milbemectin possess efficacy on galls and egg masses of root –knot nematode (M. javanica).
Received | October 23, 2021; Accepted | December 09, 2021; Published | December 17, 2021
*Correspondence | Mohamed S. Khalil, Agricultural Research Center (ARC), Central Agricultural Pesticides Laboratory (CAPL), El-Sabaheya, Alexandria, Egypt; Email: [email protected]
Citation | Khalil, M.S., 2021. The potential of milbemectin on the formation of galls and egg masses of Meloidogyne javanica on eggplants. Pakistan Journal of Nematology, 39(2): 106-110.
DOI | https://dx.doi.org/10.17582/journal.pjn/2021.39.2.106.110
Keywords | Meloidogyne javanica, Eggplants, Fenamiphos, RKN management, Milbemectin
Introduction
Root-knot nematodes (Meloidogyne spp.), are sedentary endoparasites that considered the most threats to vegetable crops production around the world (Ibrahim, 2011; Forghani and Hajihassani, 2020). The current management practices are not enough to manage Meloidogyne spp., when the most reciprocal strategy in Egypt or African countries is using synthetic nematicides (Khalil and Alqadasi, 2019). There are only six active ingredients of non-fumigant nematicides that authorized as for use in vegetable crops; oxamyl, ethoprophos, fenamiphos, fosthiazate, abamectin and fluopyram. The frequent use of these nematicides could exert high selective pressure on root-knot nematodes (RKNs) and soil microbiota. Thus, scientists are search for new effective nematicides with different modes of action (Talavera-Rubia et al., 2020).
Milbemectin belongs to milbemycin group which is an antibiotic isolated from the soil bacterium Streptomyces hygroscopicus subsp. Aureolacrimosus. Milbemectin was recorded acaricidal efficacy against spider mites, as well as nematicidal activity against Bursaphelenchus xylophilus and Hirschmanniella diversa (Yang et al., 2013; Bi et al., 2015; Takagi et al., 2020). Furthermore, milbemectin is a macrocyclic lactone with similar molecular structure to other members of the family used as biopesticides against RKN, as abamectin and emamectin benzoate (Rehman et al., 2009; Saad et al., 2017; Khalil and Darwesh, 2018; Radwan et al., 2019). Therefore, this investigation aimed to estimate the influence of milbemectin as soil application against galls and egg masses formation of M. javanica, on roots of eggplants.
Materials and Methods
Nematode inoculum
The population of root-knot nematode (Meloidogyne javanica) was obtained from EL-Bossaily area, Rashid region, Behera governorate, Egypt. The species of RKN was identified as M. javanica, by using female perineal patterns method according to Taylor and Netscher (1974). The eggs of root knot nematode (M. javanica) were extracted by the sodium hypochlorite method from infested roots (Hussey and Barker, 1973). Meanwhile, to count the egg masses they were stained by using aqueous solution of phloxine B (0.15 g/L water) for 15 minutes according to the method of Holbrook et al. (1983).
Pot experiments
Two pot experiments were carried out to evaluate the potential of milbemectin against M. javanica on eggplants. The used pots of 15 cm diameter were filled with 1 kg of sterilized sandy loam soil. Each pot was transplanted with one eggplant seedling (Solanum melongena L., cv. Black Beauty) of 40 days-old. Each pot was inoculated with 5000 nematode eggs after two days from transplanting time by pouring the nematode suspension into holes made 2-4 cm below the soil surface around the base of the plants.
The non-fumigant nematicide, fenamiphos (fenamiphos 40% EC) was applied to the soil at the recommended dose, while milbemectin (Milbeknock 1% EC) was applied at the suggested concentrations (0.1 and 0.5%) to the soil after a day of inoculation time. All pots were replicated four times and arranged in a complete randomized design on a bench in a greenhouse. The irrigation and fertilization were applied when appropriate.
After 65 days from inoculation time, plants were uprooted and the roots were washed free of adhering soil. Meanwhile, the plant height and fresh weights of shoot and root, as well as, the number of galls and egg masses /root system were estimated. The reductions (%) in root galls and egg masses were calculated as follow:

Furthermore, the increases (%) in plant indices were calculated by the next formula:
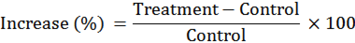
Statistical analysis
The obtained data were statistically analyzed according to CoStat Software (2005) Version: 6.303. Results of the present work were subjected to the analysis of variance test (ANOVA) as complete randomized design (CRD). Comparison among means was made via the least significant difference (LSD) test at the 5% level of probability.
Results and Discussion
Based on root galls, results indicated that milbemectin at 0.5% was the superior treatment which recorded 70.29% reduction, followed by fenamiphos and milbemectin at 0.1% with 61.76 and 50.59%, respectively, during the 1st experiment (Table 1). In the 2nd experiment, the same trend was noticed where milbemectin at 0.5% was the highest treatment followed by fenamiphos and milbemectin at 0.1% with suppression in root galls estimated by 75.50, 67.38 and 60.54%, consecutively.
On the other hand, the egg masses formation was affected by the applied treatments significantly. In the 1st experiment, results revealed that milbemectin at 0.5% recorded the highest potential by 75.00% reduction, while milbemectin at 0.1% and fenamiphos recorded 67.50 and 53.75%, respectively. Furthermore, during the 2nd experiment the application of milbemectin at 0.5% gave the highest decreasing (87.01%), followed by milbemectin at 0.1% (66.67%) and fenamiphos (62.34%). There weren’t significant differences between both milbemectin concentrations in galls and egg masses formation, except egg masses in the first experiment.
Only a single study was published recently showed that the efficacy of milbemectin against the second stage juveniles in soil (omit). Talavera-Rubia et al. (2020) found that using milbemectin at 1.5 and 2.5L/ ha reduced soil population by 52 and 59%, respectively. Milbeknock® found to minimized the egg masses of Meloidogyne spp., significantly by 99% in tomato roots compared to untreated nematode-infected plants (Talavera-Rubia et al., 2020). Also, in the same study, milbemectin decreased the egg hatching, soil population and tomato root infection. The mode of action of milbemectin against Meloidogyne species is mostly looks like other members of macrocyclic lactones (abamectin and emamectin benzoate). It was suggested that milbemectin is effect on the δ-aminobutyric acid (GABA) receptors and glutamate-gated chloride channels, which activate the influx of chloride ions causing hyperpolarizing in nerve and muscle cells, and disturbing the neuromuscular transmission leading to death (Martin et al., 2002).
Fenamiphos has efficacy to reduce gall index, egg-masses in carnation and population of J2 in the soil significantly (Kimenju et al., 2014). Moreover, Maggie et al. (2016) mentioned that fenamiphos reduced galls (90.64%) and egg masses (93.25%). In a study, it was recorded that application of fenamiphos and abamectin were minimized the formation of galls and egg masses by 91.73 and 90.80%, and 66.69 and 66.31%, respectively (Saad et al., 2017). The efficacy of fenamiphos against RKN is depending on the inhibition in acetylcholinesterase (AChE) at cholinergic synapses in the nematode nervous system (Opperman and Chang, 1990).
The effect of applied treatments on growth indices such as shoot and root height and weight of eggplants were recorded in the termination of two experiments (Table 2). During the 1st experiment the applied treatments increased shoot height at range of 12.99 to 29.87%, and shoot weight from 10.35 to 77.76%. Besides, the root length was augmented at range of 2.60 to 33.77%, while root weight was increased at range of 3.89 to 40.68%. Fenamiphos was the most effective treatment and there weren’t significant differences between milbemectin concentrations except in root system length.
Table 1: The influence of milbemectin against galls and egg masses formation of root-knot nematode (M. javanica) in roots of eggplants during two pot experiments.
|
Treatments |
1st Experiment |
2nd Experiment |
|||||||
|
Galls / root system |
Egg masses / root system |
Galls / root system |
Egg masses / root system |
||||||
|
Mean |
Decrease (%) |
Mean |
Decrease (%) |
Mean |
Decrease (%) |
Mean |
Decrease (%) |
||
|
Milbemectin 0.1% |
42.00b |
50.59 |
19.50bc |
67.50 |
69.25b |
60.54 |
38.50b |
66.67 |
|
|
Milbemectin 0.5% |
25.25c |
70.29 |
15.00c |
75.00 |
43.00c |
75.50 |
15.00c |
87.01 |
|
|
Fenamiphos |
32.50bc |
61.76 |
27.75b |
53.75 |
57.25bc |
67.38 |
43.50b |
62.34 |
|
|
Untreated check |
85.00a |
---- |
60.00a |
---- |
175.50a |
---- |
115.50a |
---- |
|
Within a column, numbers followed by different letter(s) are significantly different using LSD at p = 0.05, Means are the average of four replicates.
Table 2: The effect of milbemectin on the growth indices of egg plants infected with root-knot nematode (M. javanica).
|
Treatments |
Shoot system |
Root system |
|||||||
|
Height |
Weight |
length |
Weight |
||||||
|
Mean (cm) |
I% |
Mean (g) |
I% |
Mean (cm) |
I% |
Mean (g) |
I% |
||
|
1st Experiment |
|||||||||
|
Milbemectin 0.1% |
22.13ab |
14.94 |
4.29b |
10.35 |
19.75b |
2.60 |
2.39ab |
12.50 |
|
|
Milbemectin 0.5% |
21.75ab |
12.99 |
5.04b |
29.63 |
25.75a |
33.77 |
2.53ab |
19.10 |
|
|
Fenamiphos |
25.00a |
29.87 |
6.92a |
77.76 |
23.00ab |
19.48 |
2.98a |
40.68 |
|
|
Uninfected untreated check |
22.00ab |
14.29 |
4.29b |
10.35 |
20.25b |
5.19 |
2.20b |
3.89 |
|
|
Untreated check |
19.25b |
0.00 |
3.89b |
0.00 |
19.25b |
0.00 |
2.12b |
0.00 |
|
|
2nd Experiment |
|||||||||
|
Milbemectin 0.1% |
33.25ab |
15.65 |
5.40bc |
18.74 |
29.63b |
3.04 |
2.74bc |
25.24 |
|
|
Milbemectin 0.5% |
32.63ab |
13.48 |
7.09a |
55.77 |
34.50ab |
20.00 |
2.88ab |
31.58 |
|
|
Fenamiphos |
37.50a |
30.43 |
6.75ab |
48.35 |
36.38a |
26.52 |
3.43a |
56.62 |
|
|
Uninfected untreated check |
33.00ab |
14.78 |
5.20c |
14.18 |
29.50b |
2.61 |
2.53bc |
15.66 |
|
|
Untreated check |
28.75b |
0.00 |
4.55c |
0.00 |
28.75b |
0.00 |
2.19c |
0.00 |
|
Within a column, numbers followed by different letter(s) are significantly different using LSD at p = 0.05, Means are the average of four replicates.
In respect to the 2nd experiment, the augmentation in shoot height and weight were ranged from 13.48 to 30.43% and 14.18 to 55.77% over control, respectively. Meanwhile, root length was increased at range of 2.61 to 26.52%, while root weight recorded increment ranged from 15.66 to 56.62%. Fenamiphos was the superior treatment which increased all plant indices. Also, there weren’t any significant differences between milbemectin concentrations except in shoot system weight. The obtained results are in agreement with Maggie et al. (2016) who found that plant growth indices of tomato plants such as fresh weight, dry weight, root length and shoot height were enhanced. While, Al-Hazmi et al. (2017) indicated that fenamiphos as soil drench, seed dressing and seed dep increased the green beans fresh weight by 42.57, 59.40 and 48.52%, consecutively. Meanwhile, fenamiphos and abamectin increased the growth indices of tomato plants (Saad et al., 2017).
Conclusions and Recommendations
Plant parasitic nematode (Meloidogyne sp.) is very devastating disease to a lot of vegetable crops. As has been mentioned above, the present study revealed that milbemectin which is an acaricide was effective in reducing galls an egg masses of M. javanica in eggplants. Also, the impacts on plant growth improved relatively in compared with untreated check. More studies are needed in open field.
Novelty Statement
Globally, this investigation is the 2nd study for the nematicidal activity of milbemectin (acaricide) against the root-knot nematode (Meloidogyne javanica). Activity was proved and confirmed.
Conflict of interest
The authors have declared no conflict of interest.
Reference
Al-Hazmi, A.S., Dawabah, A.A.M., Al-Nadhari, S.N., and Al-Yahya, F.A., 2017. Comparative efficacy of different approaches to managing Meloidogyne incognita on green bean. Saudi J. Biol. Sci., 24(1): 149-154. https://doi.org/10.1016/j.sjbs.2016.05.013
Bi, Z., Gong, Y., Huang, X., Yu, H., Bai, L., and Hu, J., 2015. Efficacy of four nematicides against the reproduction and development of pinewood nematode, Bursaphelenchus xylophilus. J. Nematol., 47: 126–132.
Forghani, F., and Hajihassani, A., 2020. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci., 11: 1125. https://doi.org/10.3389/fpls.2020.01125
Holbrook, C.C., Knauft, D.A., and Dikson, D.W.A., 1983. Technique for screening peanut for resistance to Meloidogyne arenaria. Plant Dis., 57: 957-958. https://doi.org/10.1094/PD-67-957
Hussey, R.S., and Barker, K.R. 1973. A comparison of methods of collecting inocula on Meloidogyne spp., including a new technique. Plant Dis. Rep., 57:10251028.
Ibrahim, I.K.A., 2011. Nematode pests parasitic on agricultural field crops. Manshaat El. Maaref, Alexandria.
Khalil, M.S., and Alqadasi, B.A.A., 2019. Potential of non-fumigant nematicides at different formulations against southern root-knot nematode (Meloidogyne incognita) on tomato plants. Int. J. Phytopathol., 8(1): 23-28. https://doi.org/10.33687/phytopath.008.01.2899
Khalil, M.S., Darwesh, D.M., 2018. Some integrated practices to manage root-knot nematodes on tomatoes: A mini review. Innov. Tech. Agric., 3(2): 618-625.
Kimenju, J.W., Wachira, P.M., Lang’at, J.K., Otieno, W., and Mutua, G.K., 2014. Evaluation of selected methods in the control of plant parasitic nematodes infecting carnation. J. Agric. Sci., 6: 31-38. https://doi.org/10.5539/jas.v6n3p31
Maggie, E.M.H., Zawam, H.S., El-Nahas, S.E.M., and Desoukey, A.F., 2016. Comparison study between silver nanoparticles and two nematicides against Meloidogyne incognita on tomato seedlings. Plant Pathol. J., 15: 144-151. https://doi.org/10.3923/ppj.2016.144.151
Martin, R.J., Robertson, A.P., and Wolstenholme, A.J., 2002. Mode of action of the macrocyclic lactones. In macrocyclic lactones in antiparasitic therapy, (eds. J. Vercruysse and R.S. Rew) CABI: Wallingford, UK, pp. 125–140. https://doi.org/10.1079/9780851996172.0125
Opperman, C.H., and Chang, S., 1990. Plant parasitic nematode acetyl cholin-esterase inhibition by carbamate and organophosphate nematicides. J. Nematol., 22: 481-488.
Radwan, M.A., Saad, A.S.A., Mesbah, H.A., Ibrahim, H.S., and Khalil, M.S., 2019. Investigating the in vitro and in vivo nematicidal performance of structurally related macrolides against the root-knot nematode, Meloidogyne incognita. Hell. Plant Prot. J., 12(1): 24-37. https://doi.org/10.2478/hppj-2019-0005
Rehman, A.U., Javed, N., Ahmad, R., and Shahid, M., 2009. Protective and curative effect of bio-products against the invasion and development of root-knot nematodes in tomato. Pak. J. Phytopathol., 21: 37-40.
Saad, A.S.A., Radwan, M.A., Mesbah, H.A., Ibrahim, H.S., and Khalil, M.S., 217. Evaluation of some non-fumigant nematicides and the biocide avermectin for managing Meloidogyne incognita in tomatoes. Pak. J. Nematol., 35(1): 85-92. https://doi.org/10.18681/pjn.v35.i01.p85-92
Takagi, M., Goto, M., Wari, D., Saito, M., Perry, R.N., and Toyota, K., 2020. Screening of nematicides against the lotus root nematode, Hirschmanniella diversa Sher (Tylenchida: Pratylenchidae) and the efficacy of a selected nematicide under lotus micro-field conditions. Agronlogy, 10: 373. https://doi.org/10.3390/agronomy10030373
Talavera-Rubia, M., Vela-Delgado, M.D., and Verdejo-Lucas, S., 2020. Nematicidal efficacy of milbemectin against root-knot nematodes. Plants, 9: 1-10. https://doi.org/10.3390/plants9070839
Taylor, D.P., and Nelscher, C. 1974. An improved technique for preparing perineal patterns of Meloidogyne spp. Nematolo., 20: 268-269.
Yang, L.Y., Wang, J.D., Zhang, J., Xue, C.Y., Zhang, H., Wang, X.J., and Xiang, W.S., 2013. New nemadectin congeners with acaricidal and nematocidal activity from Streptomyces microflavus neau3 Y-3. Bioorg. Med. Chem. Lett., 23: 5710–5713. https://doi.org/10.1016/j.bmcl.2013.08.002
To share on other social networks, click on any share button. What are these?




