Reproductive Biology of Mahseer (Tor tambroides) from Atu Suasah and Lawe Melang Rivers in Aceh Province to Support Sustainable Fisheries Management
Reproductive Biology of Mahseer (Tor tambroides) from Atu Suasah and Lawe Melang Rivers in Aceh Province to Support Sustainable Fisheries Management
Teuku Fadlon Haser1*, Muh. Saleh Nurdin2, Eddy Supriyono3, Deni Radona4, Fauziah Azmi1 , Kukuh Nirmala3, Widanarni3, Tri Heru Prihadi4, Tatag Budiardi3 and Riris Yuli Valentine5
1Faculty of Agriculture, Samudra University, Aceh 24354, Indonesia
2Faculty of Animal Husbandry and Fishery, Tadulako University, Palu 94148, Indonesia
3Faculty of Fisheries and Marine Sciences, IPB University, Bogor 16151, Indonesia
4Research Institute for Freshwater Aquaculture Research and Fisheries Extension, Bogor 16129, Indonesia
5Polytechnic of Marine and Fisheries, Kupang 85351, Indonesia
ABSTRACT
Information on reproductive biology is crucial in formulating policy to manage and conserve mahseer in Atu Suasah and Lawe Melang Rivers, Aceh, Indonesia. This research aimed to analyse and compare of mahseer biology including sex ratio, length and weight relationship, Fulton and relative condition factor and fecundity to estimate the reproductive potential of mahseer in these two rivers. The study was conducted from July to December 2019. Mahseer samples were obtained from fishermen who captured the fish using gillnet with mesh size 1.5-2.0 inch. The specimens collected were placed in a coolbox and transported to the Aquatic Resources Management Department of IPB University in Bogor for further analysis. Regression, chi-square, Mann Whitney test and t-test were applied to analyze reproductive biology parameters. There was no significant between-site difference in the reproductive parameters of mahseer from Atu Suasah and Lawe Melang Rivers. The sex ratio was balanced. The growth pattern was allometric negative and the Fulton condition remained stable over the six month study period. Mahseer in the 610-710 mm length class made the largest contribution to total egg production. Management and conservation of this fish should include the designation of a reservation area to mantain larval production as the source of recruits.
Article Information
Received 31 August 2020
Revised 29 September 2020
Accepted 29 October 2020
Available online 15 Februay 2021
(early access)
Published 04 January 2022
Authors’ Contribution
TFH, MSN and FA presented the concept. TFH, ES, DR planned the methodology. TFH, ES, KN, THP, RYV and TB did sample collection and laboratorium analysis. TFH, MSN, ES and DR performed data analysis. FA, KN, W, THP, TB, RYV wrote the manuscript.
Key words
Mahseer, Sex ratio, Length and weight relationship, Fecundity, Aceh.
DOI: https://dx.doi.org/10.17582/journal.pjz/20200831050805
* Corresponding author: teukufadlon@unsam.ac.id
0030-9923/2022/0002-0561 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
INTRODUCTION
Mahseer (Tor tambroides) is the fish commonly found in freshwater system in the south, east and south east of Asia Continent. Their habitat is characterised by fast or slowing moving water with high water clarity, high dissolved oxygen content with a preference for sand and gravel as substrates (Khare et al., 2014; Ambili et al., 2014; Pinder et al., 2019). The fish are abundant in intact water, especially in tropical regions of Indonesia, Malaysia, Thailand and India (Yadav et al., 2012; Hoang et al., 2015). In particular, Mahseer are found in freshwater riverine systems in Aceh, where they are one of the fishes exploited by local residents.
Four mahseer species are found in Aceh, Tor soro, Tor tambra, Tor douronensis and Tor tambroides. Based on body size, Tor tambroides is the biggest mahseer of Indonesia (Haryono et al., 2009). Among the four species Tor tambra population is the most abundant (Maghfiriadi et al., 2019). Local people usually refer to this fish as kereuling or jurong. This fish is highly appreciated by locals for the delicacy of its meat (Muchlisin et al., 2015; Wibowo and Dwirastina, 2016). Mahseer also have cultural value and are used in many cultural ceremonies (Kristanto et al., 2007; Haryono, 2017).
Mahseer are rare in Java Island, whilst in Kalimantan and Sumatera the populations of these fish are also reported to be declining due to overexploitation and ecosystem degradation, especially in places where the fish spawn (Haryono, 2006; Gupta et al., 2014; Sarkar et al., 2015; Roesma et al., 2017). Hence, management and conservation efforts are needed to sustain mahseer populations.
Information on reproductive biology is required to devise policies on management and conservation of mahseer but is still scarce. Previous studies have reported progress with respect to biological information on mahseer including taxonomy (Zulfahmi et al., 2018), growth performance (Muchlisin et al., 2016), morphology (Akmal et al., 2018) and genetics (Arifin et al., 2015). However, there is a lack of information on aspects such as sex ratio, condition factor, fecundity and reproductive potential. Therefore, this study aimed to assess this gap through analysing and comparing these aspects of mahseer reproductive biology in Atu Suasah and Lawe Melang Rivers, in Aceh Province, Indonesia.
MATERIALS AND METHODS
The study was conducted from July to December 2019 in the Atu Suasah and Lawe Melang Rivers, Aceh Province, Indonesia (Fig. 1).
Mahseer samples were obtained from fishermen who captured the fish using gillnet with mesh size 1.5-2.0 inch. On one trip, the fishermen usually operated gill nets 10-15 m in length. The nets were set up at upstream and at the downhill in each of the two rivers. One trip is equaled to 16 h of fishing activity counting when the operation of gillnet at 16.00 in the afternoon and the lift-up of gillnet at 08.00 am. In total 146 mahseer specimens were collected, consisting of 74 fish from Atu Suasah and 72 from Lawe Melang River. The specimens collected were placed in a coolbox and transported to the Aquatic Resources Management Department of IPB University in Bogor for further analysis. The total length (mm) and weight (g) of each specimen were measured, and the sex was determined. Weight was obtained from digital scale with 0.1 g accuracy whilst total length was measured using ruler with 1 mm accuracy. Three parts of each eggs from anterior, middle, and posterior parts were subsampled to counted fecundity. The eggs subsamples were immersed in Gilson solution for 24 h to dissolved the outer membrane therefore makes it easier to count. Fecundity was estimated using a gravimetric method (Bagenal, 1968) by counting the eggs in the subsamples under a microscope.
Sex ratio was determined by dividing the number of male with the number of females. Chi-square (Zar, 2010) was used to estimate sex ratio difference between the two populations following formula:
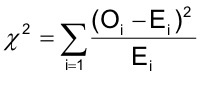
where Oi is the observed value and Ei is the expected value of the ith variable.
The length-weight relationship was calculated using the equation in Le Cren (1951):
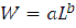
where W is the weight (g) and L is the total length (mm); a is the intercept and b is the slope of the log-transformed regression of weight against length.
The relative condition factor (Wr) and Fulton condition factor (K) were used to evaluate condition factor of each individual. Relative condition factor was computed refer to Rypel and Richter (2008):
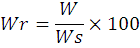
where Wr is the relative condition factor, W is weight of the specimen and Ws is the predicted standard weight for that same fish as calculated from a composite of length–weight regressions throughout the range of the species.
A value of the relative condition factor < 100 indicates low food availability while Wr > 100 suggests abundant or surplus food availability (Anderson and Neumann, 1996).
The Fulton condition factor was calculated using the following equation (Froese, 2006).
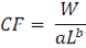
CF is the Fulton condition factor; W is the weight (g of the specimen; L is the total length (mm) of the specimen; a and b are the constants from the length-weight regression model.
The average difference between male and female condition factor was obtained using the Mann-Whitney test (Wilks, 1995):
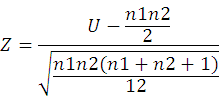
Z is the statistics, U is the smallest value of U1 and U2; n1 and n2 are sample size.
Fecundity was estimated following gravimetric protocol with formula:
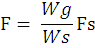
where F is fecundity, Fs is the number of eggs counted in the gonad sub- sample, Wg is total gonad weight, and Ws is the weight of the gonad sub-sample.
The between-population difference in mahseer total fecundity was analysed using the t-test (Zar, 2010), where t was calculated using the equation:
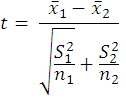
where x̅1 is the mean fecundity of specimens sampled from population 1, x̅2 is the mean fecundity of specimens sampled from population 2, S12 is the sample variance from population 1, S22 is the sample variance from population 2, n1 and n2 are the sample sizes of the two populations.
Reproductive potential was calculated using the following formula (Tuwo, 1993):

RESULTS
The mahseer (Tor tambroides) specimens from the Atu Suasah River consisted of 51.35% male and 48.65% female, with a male to female sex ratio of 1.61:1. While the mahseer specimens from Lawe Melang River consisted of 56.94% male and 43.06% female (1.31:1). Chi-square analysis results (χ2=0.054; df=1; P>0.05 in Atu Suasah River and χ2=2; df=1; P>0.05 in Lawe Melang River) suggest that the sex ratio is not significantly different from 1 in either river.
The length and weight relationship regression analysis showed that the four fish groups have a negative allometric (b<3) growth pattern (Table I). This indicates that these fish generally grow faster in length than in weight. The coefficient of determination shows that length explains a higher proportion of weight gain in female mahseer compared to males in both Atu Suasah River and Lawe Melang River.
The mean values and range of the Fulton condition factor were similar for male and female mahseer sampled from the Atu Suasah River (1.03±0.23, 1.04±0.30) and Lawe Melang River (1.02±0.30 and 1.06±0.27s) with mean values slightly greater than 1 (Fig. 2). The relative condition factor was greater than 100 for all groups. The Mann Whitney-test showed no significant difference (P>0.05) in either condition factor between male and female fish or between the two study sites. The results also suggest that male and female fish condition remained similar throughout the six month sampling period.
Table I. Length and weight relationship of mahseer in Atu Suasah and Lawe Melang River.
|
Parameter |
Atu Suasah River |
Lawe Melang River |
||
|
Male |
Female |
Male |
Female |
|
|
Growth model |
W=1.72L1.56 |
W=0.41L1.12 |
W=1.15L1.76 |
W=0.34L1.10 |
|
R2 |
0.65 |
0.82 |
0.56 |
0.74 |
|
Type of growth |
Negative allometry |
|||
Mahseer fecundity ranged from 3,600 to 5,466 eggs (average 4,476±911 eggs) in the Atu Suasah River and 2,433 to 4,203 eggs (average 3,212±631 eggs) in Lawe Melang River. The student t-test showed no significant between-site difference in fecundity (P>0.05). Mean reproductive potential was estimated at 10,444±4,404 in the Atu Suasah River and 4,818±3,870 in the Lawe Melang River (Table II).
DISCUSSION
Theoretically, a balanced sex ratio (1:1) is ideal to maintain a population (Ball and Rao, 1984). Studies on mahseer from several localities, report a female-biased sex ratio; e.g. a study on Tor putitora in India (Joshi et al., 2018), and a study in Kalimantan (Haryono, 2006) where the male to female sex ratio was 1:2. Bhatt et al. (2004) and Wibowo and Kaban (2014) found that male mahseer are mostly young fish while females are predominately adult fish. The proportion of young fish in the samples collected from the Atu Suasah and Lawe Melang Rivers was 57.14% from and 47.22%, respectively. Therefore, the balanced sex ratio of fish in this study related to the balance between young and adult fish in the samples collected such what had been found in Manna River and Tarusan River (Wibowo and Kaban, 2014).
Table II. Average fecundity and reproductive potential of mahseer in Atu SuasahRiver and Lawe Melang River.
|
Size class (mm) |
Quantity (fish) |
Mean fecundity (eggs) |
Reproductive potential |
|
Atu Suasah River |
|||
|
410-500 |
2 |
3,362 |
6,723 |
|
510-600 |
2 |
4,652 |
9,303 |
|
610-700 |
3 |
5,102 |
15,306 |
|
Lawe Melang River |
|||
|
410-500 |
1 |
2,433 |
2,433 |
|
510-600 |
1 |
2,943 |
2,943 |
|
610-700 |
3 |
3,533 |
10,599 |
|
710-800 |
1 |
3,297 |
3,297 |
Mahseer are pelagic fish which swim actively due to their habitat in fast flowing foothills streams with high-water quality (Dinesh et al., 2010; Ambili et al., 2014; Raghavan et al., 2017). Actively swimming fish which live in fast flowing water usually have a value of b < 3 because more energy is allocated for swimming than for growth in terms of weight gain (Shukor et al., 2008; Muchlisin et al., 2010; 2015). The same phenomenon was observed in the mahseer populations at both study sites.
However, growth patterns of mahseer reported in previous studies are varied (Tabel III). Muchlisin et al. (2015) also found that Tor tambra in Nagan and Sikundo Rivers had an allometric negative growth pattern. Tor putitora found in their spawning ground (Himalayan foothills) in Pakistan also had an allometric negative growth pattern (Naeem et al., 2011). On the other hand, Ali et al. (2014) who also studied Tor putitora in four different locations in the Indian Himalayan report an isometric growth pattern. The variability in fish growth patterns is usually closely related to the environment, including the biological and geographical condition of the habitat (Froese, 2006; Pervais et al., 2012).
Aside from food availability, biotic and abiotic factors, age, and length (size class) can also affect the fish condition factor (Le Cren, 1951; Blackwell et al., 2000). The Fulton condition factor of male and female mahseer was relatively stable over the sampling period. The value of relative weight exceeded 100 which indicates good food availability for the fish in both rivers. Mahseer in Atu Suasah and Lawe Melang are considered as omnivores with a tendency towards being predominantly herbivorous. While the main food sources of these fish are aquatic plants and algae, they also occasionally prey on insects (Pervais et al., 2012; Nautiyal, 2014; Mohapatra et al., 2017). Sulastri et al. (1985) suggested that, apart from relying on videoreceptor capability in searching for food, mahseer also use their barbels as mechanoreceptors.
Fecundity is considered the most reliable indicator to estimate fish reproductive potential (Bhatt and Pandit, 2015; Achmad et al., 2020). The estimates of mahseer fecundity in this research fall within the range of mahseer fecundity reported from other studies (Table IV). The highest fecundity was found in the Nayar River population (India) (Nautiyal and Lal, 1985). The lowest fecundity was found in Manna River population, West Sumatera Indonesia (Wibowo and Kaban, 2014). The fecundity found in this study is lower than that of mahseer found in Barito (Haryono, 2006), but higher compared to that reported from the Manna River. These variabilities may happens due to variabilities of fish size among populations.
Table III. Comparison of mahseer growth pattern from several locations.
|
No |
Species |
Location |
b Value |
Reference |
|
1 |
Tor tambroides |
Atu Suasah River, Aceh, Indonesia |
1.12 |
This study |
|
2 |
Tor tambroides |
Lawe Melang River, Aceh, Indonesia |
1.10 |
|
|
3 |
Tor tambra |
Nagan River, Aceh Indonesia |
2.84 |
|
|
4 |
Tor tambra |
Sikundo River, Aceh Indonesia |
2.63 |
|
|
5 |
Tor putitora |
Himalayan foothills, Pakistan |
2.85 |
|
|
6 |
Tor putitora |
Indian Himalayan |
3.10 |
Table IV. Comparison of mahseer fecundity from several locations.
|
No |
Species |
Location |
Fecundity (eggs) |
Reference |
|
1 |
Tor tambroides |
Atu Suasah River, Aceh, Indonesia |
3,600-5,466 |
This study |
|
2 |
Tor tambroides |
Lawe Melang River, Aceh, Indonesia |
2,433-4,203 |
|
|
3 |
Tor putitora |
Lesser Himalayan, India |
4,217-8,365 |
|
|
4 |
Tor tambroides |
Barito River, Indonesia |
3,125-8,201 |
|
|
5 |
Tor tambroides |
West Sumatera, Indonesia |
2,508 |
|
|
6 |
Tor tambroides |
Nayar River, India |
26,997-98,583 |
|
|
7 |
Tor putitora |
Pong Dam, India |
15,504 |
|
|
8 |
Tor putitora |
Bhimtal, India |
7,076-18,525 |
Table IV shows that the fecundity of mahseer populations can be different even they come from the same or similar species within the genus Tor. Bagenal (1971) argued that length and weight of fish tend to increase linearly. Additionally, variability in fish mahseer fecundity can be affected by species, spawning pattern, and estimating techniques (gravimetry and volumetry) (Pathani, 1981; Johal and Negi, 2003).
Mahseer from the 610-700 mm length class contributed the most to overall egg production. This size class was also the most abundant (42.86% and 50% of the total number of females within the two river population samples). Female mahseer with a length more than 710-800 mm had the lowest fecundity and only comprised 16.67% of the females sampled from the populations. Therefore, this group appears to make a relatively low contribution to total egg production.
Mahseer exploitation in the Atu Suasah and Lawe Melang Rivers is highly intensive due to excessive demand for the fish. To protect the fish populations, spawning grounds of the fish must be determined and protected. Establishing a reservation area in each of the two rivers could improve larval production through protection for the source of new recruits. This approach could also affect fisher incomes therefore its implementation must be accompanied by education for the local fishermen.
CONCLUSION
There was no significant between-site difference in sex ratio, length and weight relationship, condition factor or fecundity between mahseer captured in the Atu Suasah and Lawe Melang Rivers. The sex ratio was balanced and the growth pattern was negative allometric. Fish condition factor remained stable throughout the sampling period. Mahseer from the length class 610 – 700 mm made the largest contribution to total egg production. To improve new recruitment, priority should be given to the protection of mahseer spawning grounds by establishing a reservation (protected area) in order to maintain egg production.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Achmad, D.S., Sudirman., Jompa, J. and Nurdin, M.S., 2020. Estimating the catchable size of orange-spotted grouper (Epinephelus coioides) in Kwandang Bay, Gorontalo Utara District, Indonesia. IOP Conf. Ser. Earth environ. Sci., 473: 1-8. https://doi.org/10.1088/1755-1315/473/1/012133
Akmal, Y., Zulfahmi, I. and Rahardjo, M.F., 2018. Morphology of appendicular skeleton of the Thai mahseer’s Tor tambroides (Bleeker, 1854). Indonesian J. Ichthyol., 18: 261-274. https://doi.org/10.32491/jii.v18i3.443
Ali, S., Barat, A., Kumar, P., Sati, J., Kumar, R. and Haldar, R.S., 2014. Study of length-weight relationship and condition factor for the golden mahseer, Tor puttiora from Himalayan rivers from India. J. environ. Biol., 35: 225-228.
Ambili, T.R., Manimekalan, A. and Verma, M.S. 2014. Genetic diversity of genus Tor in river Chaliyar, Southern Western Ghats, Kerala, through DNA barcoding. J. Sci., 4: 206-214.
Anderson, R.O. and Neumann, R.M., 1996. Length, weight, and associated structural indices. Am. Fish. Soc., pp. 447–482.
Arifin, O.Z., Subagja, J. and Hadie, W., 2015. Biometric characterization three population of semah mahseer Tor douronensis (Valenciennes, 1842) in support to conservation of genetic resources. Indonesian J. Ichthyol., 15: 143-154. http://dx.doi.org/10.32491/jii.v15i2.68.
Asih, S., Nugroho, E., Kristanto, A.H. and Mulyasari, M., 2016. Determination of genetic variation of Tor soro from North Sumatra and West Java using Random Amplified Polymorphism DNA (RAPD) methods. Jurnal Riset Akuakultur, 3: 91-97.
Bagenal, T.B., 1968. The ecologycal and geographical aspects of the fecundity of the plaice. J. mar. Biol. Assoc., 46: 161–186. https://doi.org/10.1017/S0025315400017628
Bagenal, T.B, 1971. The interrelation of the size of fish eggs, the date of spawning and the production cycle. J. Fish Biol., 3: 207-219. https://doi.org/10.1111/j.1095-8649.1971.tb03665.x
Ball, D.V. and Rao, K.V., 1984. Marine fisheries. Tata McGraw-Hill publishing company. New Delhi.
Bhatt, J.P. and Pandit, M.K., 2015. Endangered Golden mahseer Tor putitora Hamilton: A review of natural history. Rev. Fish Biol. Fish., 26: 25-38. https://doi.org/10.1007/s11160-015-9409-7
Bhatt, J.P., Nautiyal, P. and Singh, H.R., 2004. Status (1993-1994) of the endangered fish Himalayan Mahseer Tor putitora (Hamilton) (Cyprinidae) in the mountain reaches of the river Ganga. Asian Fish. Sci., 17: 341-355.
Blackwell, B.G., Brown, M.L. and Willis, D.W., 2000. Relative weight (Wr) status and current use in fisheries assessment and management. Rev. Fish. Sci., 8: 1-44. https://doi.org/10.1080/10641260091129161
Dinesh, K., Nandeesha, M.C., Nautiyal, P. and Aiyappa, P., 2010. Mahseers in India: A review with focus on conservation and management. Indian J. Anim. Sci., 80: 26–38.
Froese, R., 2006. Cube law, condition factor and weight length relationships: history, meta-analysis and recommendations. J. appl. Ichthyol., 22: 241-253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
Gupta, N., Sivakumar, K., Mathur, V.B. and Chadwick, M.A., 2014. The ‘tiger of Indian rivers’: stakeholders’ perspectives on the golden mahseer as a flagship fish species. Area, 46: 389-397. https://doi.org/10.1111/area.12124
Haryono, S.J., 2008. The population and habitat of Tambra fish, Tor tambroides (Bleeker, 1854) in Muller Mountain waters Central Kalimantan. Biodiversitas, 9: 306-309. https://doi.org/10.13057/biodiv/d090414
Haryono, 2006. Biological aspects of tambra fish (Tor tambroides Blkr.) that exotic and rare for its domestication. Biodiversitas, 7: 195-198. https://doi.org/10.13057/biodiv/d070222
Haryono, T.A.H., and Wahyudewantoro G., 2009. The introduction of tambra fish that have high commercial value and are vulnerable to extinction to support their domestication. In: Domestication process and reproduction of tambra fish that rare to cultured (eds. Haryono and M.f. Rahardjo). LIPI Press, Jakarta. pp. 1-16.
Haryono, 2017. Some sacred fishes in Indonesia. Warta Iktiologi, 1: 7-13.
Hoang, H.D., Pham, H.M., Durand, J.D., Tran, N.T. and Phan, P.D., 2015. Mahseers genera Tor and Neolissochilus (Teleostei: Cyprinidae) from southern Vietnam. Zootaxa, 4006: 551-568. https://doi.org/10.11646/zootaxa.4006.3.8
Johal, M.S. and Negi, R.K., 2003. Maturity, fecundity and sex ratio of an endangered fish golden mahseer Tor putitora (Ham.) from Pongdam reservoir. Panjab Univ. Res. J., 53: 57-65.
Joshi, K.D., Das, S.C.S., Pathak, R.K., Khan, A., Sarkar, U.K. and Roy, K., 2018. Pattern of reproductive biology of the endangered golden mahseer Tor putitora (Hamilton, 1822) with special reference to regional climate change implications on breeding phenology from lesser Himalayan region, India. J. appl. Anim. Res., 46: 1289-1295. https://doi.org/10.1080/09712119.2018.1497493
Khare, P., Mohindra, V., Barman, A.S., Singh, R.K. and Lal, K.K., 2014. Molecular evidence to reconcile taxonomic instability in mahseer species (Pisces: Cyprinidae) of India. Org. Divers. Evol., 14: 307-326. https://doi.org/10.1007/s13127-014-0172-8
Kristanto, A.H., Asih, S. and Winarlin, W., 2016. Reproductive and morphometric characterization of Tor soro from two locations (North Sumatra and West Java). Jurnal Riset Akuakultur, 2: 59-65. https://doi.org/10.15578/jra.2.1.2007.59-65
Le Cren, E.D., 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol., 20: 201-219. https://doi.org/10.2307/1540
Maghfiriadi, F., Zulfahmi, I., Paujiah, E. and Sarong, M.A., 2019. Ichthyofauna of Alas River, around Soraya Research Station, Leuser Ecosystem Area, Subulussalam, Aceh. Indonesia. J. appl. Ichthyol., 19: https://doi.org/10.32491/jii.v19i3.502
Mohapatra, B.C., Sahoo, S.K., Gupta, S.D. and Gupta, S.D., 2017. Biology of mahanadi mahseer, Tor mosal mahanadicus (David) reared in freshwater pond culture system. Curr. agric. Res. J., 5: 244-251. https://doi.org/10.12944/CARJ.5.2.13
Muchlisin, Z.A., Arisa, A.A., Muhammadar, A.A., Fadli, N., Arisa, I.I. and Siti-Azizah, M.N., 2016. Growth performance and feed utilization of keureling (Tor tambra) fingerlings fed a formulated diet with different doses of vitamin E (alpha-tocopherol). Fish. aquat. Life, 24: 47-52. https://doi.org/10.1515/aopf-2016-0005
Muchlisin, Z.A., Batubara, A.S., Azizah, M.N.S., Adlim, M., Hendri, A., Fadli, N., Muhammadar, A.A. and Sugianto., 2015. Feeding habit and length weight relationship of keureling fish, Tor tambra Valenciennes, 1842 (Cyprinidae) from the western region of Aceh Province, Indonesia. Biodiversitas, 16: 89-94. https://doi.org/10.13057/biodiv/d160112
Muchlisin, Z.A., Musman, M. and Siti Azizah, M.N., 2010. Length-weight relationships and condition factors of two threatened fishes, Rasbora tawarensis and Poropuntius tawarensis, endemic to Lake Laut Tawar, Aceh Province, Indonesia. J. appl. Ichthyol., 26: 949-953. https://doi.org/10.1111/j.1439-0426.2010.01524.x
Naeem, M., Salam, A. and Ishtiaq, A., 2011. Length–weight relationships of wild and farmed Tor putitora from Pakistan. J. appl. Ichthyol., 27: 1133-1134. https://doi.org/10.1111/j.1439-0426.2010.01613.x
Nautiyal, P. and Lal, M.S., 1985. Food and feeding habits of Garhwal Himalayan Mahseer in relation to certain abiotic factors. Matsya, 11: 31-35.
Nautiyal, P., 2014. Review of the art and science of Indian mahseer (game fish) from nineteenth to twentieth century: road to extinction or conservation? Proc. natl. Acad. Sci. India B Biol. Sci., 84: 215-236. https://doi.org/10.1007/s40011-013-0233-3
Pathani, S.S., 1981. Fecundity of mahseer Tor putitora (Ham.). Proc. Anim. Sci., 90: 253-260. https://doi.org/10.1007/BF03185999
Pervaiz, K., Iqbal, Z., Mirza, M.R., Javed, M.N., Naeem, M. and Ishtiaq, A., 2012. Length weight, length length relationships and feeding habits of wild Indus Mahseer, Tor macrolepis, from Attock, Pakistan. J. appl. Ichthyol., 28: 673-676. https://doi.org/10.1111/j.1439-0426.2012.01953.x
Pinder, A.C., Britton, J.R., Harrison, A.J., Nautiyal, P., Bower, S.D., Cooke, S.J., Lockett, S., Everard, M., Katwate, U., Ranjeet, K. and Walton, S., 2019. Mahseer (Tor spp.) fishes of the world: status, challenges and opportunities for conservation. Rev. Fish Biol. Fish., 29: 417-452. https://doi.org/10.1007/s11160-019-09566-y
Raghavan, R., Dahanukar, N. and Britz, R., 2017. The type locality of Tor mosal (Hamilton, 1822) (Teleostei: Cyprinidae). Zootaxa, 4317: 593-596. https://doi.org/10.11646/zootaxa.4317.3.12
Roesma, D.I., Tjong, D.H., Munir, W., Agesi, A.V. and Chornelia, A., 2017. Genetic diversity of Tor douronensis (Pisces: Cyprinidae) in West Sumatra, Indonesia. Biodiversitas, 18: 1018-1025. https://doi.org/10.13057/biodiv/d180320
Rypel, A.L. and Richter, T.J., 2008. Empirical percentile standard weight equation for the blacktail redhorse. N. Am. J. Fish. Manage., 28: 1843-1846. https://doi.org/10.1577/M07-193.1
Sarkar, U.K., Mahapatra, B.K., Saxena, S.R. and Singh, A.K., 2015. Mahseer in India: An overview on research status and future priorities. J. Ecophysiol. Occup. Hlth., 15: 45-52.
Shukor, M.N., Samat, A., Ahmad, A.K. and Ruziaton, J., 2008. Comparative analysis of length-weight relationship of Rasbora sumatrana in relation to the physicochemical characteristics in different geographical areas in Peninsular Malaysia. Malays. appl. Biol., 37: 21-29.
Sulastri, S., Rachmatika, I. and Hartoto, D.I., 1985. Feeding habit and reproduction of Tor spp. as basic information on its aquaculture. Berita Biologi, 3: 84-90.
Tuwo, A., 1993. Biologie et ecologie de trois especes d’holothuries: Holoturia forskall, Aslia lefevrei et Pawsonia saxicola, en Bretagne Occidentale. These du Doctorat Nouveau Regime, Université de Bretagne Occidentale. Brest, France.
Wibowo, A. and Dwirastina, M., 2016. Growth comparison of mahseer (Tor tambroides) from Manna and Tarusan River in Western Sumatera River. Indones. Fish. Res. J., 22: 1-8. https://doi.org/10.15578/ifrj.22.1.2016.1-8
Wibowo, A. and Kaban, S., 2014. Reproductive characteristics of indonesia mahseer (Tor tambroides, Bleeker, 1854), in two different rivers in Western Sumatera. Indones. Fish. Res. J., 20: 49-57. https://doi.org/10.15578/ifrj.20.2.2014.49-57
Wilks, D.S., 1995. Statistical methods in the atmospheric sciences an introduction. Academic Press, New York.
Yadav, K., Lakra, W.S., Sharma, J., Goswami, M. and Singh, A., 2012. Development and characterization of a cell line TTCF from endangered mahseer Tor tor (Ham.). Fish Physiol. Biochem., 38: 1035-1045. https://doi.org/10.1007/s10695-011-9588-7
Zar, J.H., 2010. Biostatistical analysis. 5th edn. Prentice Hall. Upper Saddle River, New Jersey.
Zulfahmi, I., Akmal, Y. and Batubara, A.S., 2018. The morphology of Thai mahseer’s Tor tambroides (Bleeker, 1854) axial skeleton (ossa vertebrae). Indones. J. Ichthyol., 18: 139-149. https://doi.org/10.32491/jii.v18i2.329
To share on other social networks, click on any share button. What are these?










