Advances in Animal and Veterinary Sciences
Research Article
The Study on Effect of Temperature Stress on Occurrence of Clinical Signs Caused by Aeromonas hydrophila in Capoeta damascina in In Vitro Condition
Laleh Yazdanpanah Goharrizi1, Mohammad Ebrahim Jalil Zorriehzahra2*, Milad Adel3
1Animal Sciences Dept., Kerman Agricultural and Natural Resources Research Center, Agricultural Research Education and Extension Organization (AREEO), Kerman; 2Aquatic Animal Health & Diseases Dept., Iranian Fisheries Sciences Research Institute (IFSRI); 3Aquatic Animal Health and Diseases Dept., Caspian Sea Ecology Research Center, Iranian Fisheries Science Research Institute (IFSRI), Agricultural Research Education and Extension Organization (AREEO),Tehran, I.R. Iran.
Abstract | Aeromonas hydrophila is one of the most important microbial pathogens affecting several fish species worldwide and economical loss of related mortality should be considered in world aquaculture industry. In this study, the effects of temperature stress on occurrence of clinical signs caused A. hydrophila on Capoeta damascina was carried out. For this purpose, 240 C. damascina were captured from Baft’s rivers in Kerman province in spring season of 2014 and transported to central laboratory. After adaptation period in first stage, 0.1 ml of 3× 108 cfu/ml of A. hydrophila was injected intraperitoneal to each fish. Then, fish were divided into 4 groups (0-10, 10 - 20, 20 - 30 and to over 30oC). After 2 weeks, clinical signs and mortality of each group were recorded. The results revealed that water temperatures can play an important role to pathogenicity of A.hydrophila on C. damascina. Most clinical signs and mortality were observed in 20-30oC and above groups, although, at 10-20oC and below 10oC only 40% and 1% of fish revealed typical clinical signs, respectively.
Keywords | Iran, Aeromonas hydrophila, Capoeta damascina, Temperature stress
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | April 28, 2015; Revised | June 09, 2015; Accepted | June 10, 2015; Published | June 19, 2015
*Correspondence | Mohammad Ebrahim Jalil Zorriehzahra, Aquatic Animal Health & Diseases Dept., Iranian Fisheries Sciences Research Institute (IFSRI), Iran; Email: [email protected]
Citation | Goharrizi LY, Zorriehzahra MEJ, Adel M (2015). The study on effect of temperature stress on occurrence of clinical signs caused by Aeromonas hydrophila in Capoeta damascina in in vitro condition. Adv. Anim. Vet. Sci. 3(7): 406-412.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.7.406.412
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Goharrizi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Aeromonas hydrophila is a ubiquitous gram-negative, motile and rod-shaped bacterium with wide distribution in aquatic habitats (Krovacek et al., 1994; Kerster et al., 1995; Maalej et al., 2004). A. hydrophila is an opportunist human pathogen which is widely distributed in aquatic environments (Rahman et al., 2001). This bacterium commonly isolated from fresh water ponds and is also a normal inhabitant of the gastrointestinal tract of fish. Aeromonas species in aquatic environments is hazardous, especially for fish and shellfish species as it was the causative agent of several infectious diseases in aquaculture (Santos et al., 1999). Some strains of Aeromonas are more virulent, which means that they possess special properties which enable them to cause more serious disease outbreaks (Bullock, 1968). If these more damaging strains become endemic in a population of fish (which means that they are there all of the time and the fish develop an immunity to them), it becomes difficult to introduce new fish into the water body without suffering major losses of newly-stocked fish (Colwell, 2000).
Motile Aeromonas Septicemia (MAS) is a common bacterial disease, caused by Aeromonas, which affects warm water fish, both in commercial production systems and in natural waters (Austin and Stobie, 1993). Frequently, MAS outbreaks are stress-related and elimination of the underlying stress factor may be sufficient to resolve the disease outbreak (Shelton, 2000).
The disease primarily affects freshwater fish such as catfish, bass, and many species of tropical or ornamental fish. Fish infected with A. hydrophila may have many different clinical signs (Chakrabaiti, 2006). These range from sudden death to in appetence, swimming abnormalities, pale gills, bloat and skin ulcerations. The skin ulcers may occur at any site on the fish and often they are surrounded by a bright red rim of tissue. Because of the variability of these clinical signs, the diagnosis of this disease based only upon the clinical presentation of the fish is highly unreliable and may be economically disastrous to the fish producer with intensive fish farming systems, whether these systems are outdoor ponds or indoor aquaria and tanks, predisposing factors are primarily responsible for the precipitation of this disease (Elbour et al., 2001).
Any time an Aeromonas infection persists as a chronic problem, it is important to make efforts to determine whether an underlying stress factor causes the fish to have insufficient immune protection from the bacteria (Chakrabaiti, 2006).
Several authors noted that the survival of A. hydrophila in aquatic environment was inhibited by entrance into a viable but non-cultivatable (VBNC) state, due to stress induced by aquatic conditions for the bacterial cell, which remained metabolically activity, but unable to undergo the sustained cellular division required for growth in different conditions (Maalej et al., 2004).
Stress is the one of the most important predisposing factor associated with this disease. Common sources of stress are water temperature, water poor quality, overcrowding, or rough handling. Temperature is one of the most common environmental stressors in aquaculture ecosystems. Temperature is a stressor when it ranges to near the fish’s high, long-term tolerance level and when it fluctuates rapidly by more than a few degrees (e.g. 3 to 5°C in less than one hour), especially if temperature was increased. A relationship between changes in water temperature and the incidence of Aeromonas spp. has been reported (Kersters et al., 1996).
The main objective of this study was to investigat the effects of temperature stress on occurrence of clinical signs of Aeromonas infections in C. damascina as native fish from rivers of Baft region in Kerman province, Iran.
Materials and Methods
Fish
About 240 C. damascina were captured from a native river of Baft region in spring season of 2014 in Kerman province and shifted to central laboratory immediately. In first stage, fish were acclimated to the condition of the laboratory for 7 days. In this time, fish were fed twice daily with commercial pellet (Mazan, Mazandaran). During the study, water temperature, pH and dissolved oxygen were daily measured as 18±2oC, 7-8 and 5.2- 5.8ppm, respectively.
Isolation of A. hydrophila
From Baft River, about 25 infected C. damascina were used for isolation of bacteria. Kidney sample were collected from infected fish and transferred to (tryptic soy agar) TSA medium and were then incubated for 48 h at 25oC. After 48 h, A. hydrophila were identified based on biochemical test (Mohajeri et al., 2011). Cell densities were photometrical obtained at 540 nm wavelength (Saulnier et al., 2000), based on the Mcfarland standard and 3×108 CFUmL-1 of bacteria was prepared.
Challenge Assay
About 20 fish from each subgroup were randomly selected for the challenge assay. The challenge was by intraperitoneal injection with 0.1 ml−1 suspensions of A. hydrophila in 0.9% (w/v) saline containing 3×108 CFUmL-1.
Experimental Group
The study was designed as three treatments, 0-10, 10 - 20, 20 - 30 and to over 30oC and control (30oC) in triplicates. Twelve glass aquariums were divided to the groups. Also, 20 C. damascina were kept to each aquarium for 2 weeks and clinical signs and mortality were recorded to each group. Aquarium heater was used to regular temperatures. The erosions of caudal and dorsal fins and kidney of moribund fish were selected as target tissues for isolation and confirmation of bacteria. Nutrient and Blood agar were used as primary media cultures. After separating and preparing the pure culture, some biochemical tests including: SIM, Nitrate, Indol, Motility, MR, VP, O/F, Oxidase, Urease, Maltase, Gelatinase, Hemolysin, Catalase, Esculine, Lysine and Arginine have been employed to characterization of A. hydrophila.
Statistical Analysis
All the obtained data were subjected to statistical analysis using the SPSS software, version 18. The statistical analysis was done by using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. P-value of <0.05 was considered significant.
Results
In this study, different temperature ranges were examined to survey its impacts on pathogenicity of A. hydrophila in C. damascina. The results of biochemical tests of A. hydrophila isolated from erosions of fins and internal organs of moribund fish were revealed in Table 1.
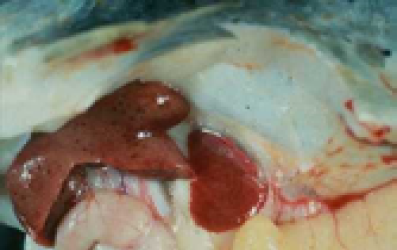
In this study, clinical signs including: exophthalmia, blackening of the skin, abdominal distension, hemorrhages in the eyes, base of the fins or on the skin and internal organs such as liver (Figure 1), kidney and spleen were observed. Internal signs were included fluid in the abdomen, swollen liver and spleen, fluid-filled and distended intestines. The results revealed that water temperatures play a major role to pathogenicity of A. hydrophila in C. damascina. Most clinical signs and mortality were observed in 20-30oC and above groups, although, at 10-20oC and below 10oC only 40% and 1% of fish showed clinical signs, respectively (Figure 2, Table 2 and 3).
Table 1: The result of biochemical test for bacterial isolation (Aeromonas hydrophila)
|
Gram |
- |
||
|
Nitrate |
NO2 |
Urease |
- |
|
SIM |
- or + |
Maltase |
+ |
|
H2S |
+ |
Gelatinase |
+ |
|
Indol |
+ |
TSI |
A/A |
|
Motility |
+ |
Hemolysin |
+ |
|
MR |
- |
Catalase |
+ |
|
VP |
+ |
Esculine |
+ |
|
O/F |
+ |
Lysine |
+SH2 |
|
Oxidase |
+ |
Arginine |
- |
Table 2: Variance analysis of infected fish with clinical signs of Aeromonas hydrophila in Capoeta damascina
|
Sources alteration |
Degree of freedom (df) |
Sum squares |
Mean Squares |
F |
|
Treatment |
3 |
329/67 |
109/89 |
0 |
|
Error |
8 |
5/33 |
0/667 |
|
|
Total |
11 |
335 |
Table 3: Mean Comparisons with Duncan’s multiple range test
|
Temperature |
T1 (0-10°C) |
T2 (10-20°C) |
T3 (20-30°C) |
T4 (above30°C) |
|
Average |
0.67 |
7 |
12 |
14.33 |
*Means in rows with different superscripts are significantly different at the level of (P<0.05).
Discussion
A. hydrophila and other motile aeromonads are among the most common bacteria in freshwater habitats throughout the world, and these bacteria frequently cause infectious diseases among cultured and feral fishes (Maalej et al., 2004). Speculated that septicemic infections in fish caused by motile Aeromonads that were common throughout Europe during the middle ages. Although the bacterial etiology of these early reports was inconclusive, the pathology was similar to that observed with red leg disease in frogs, in which A. hydrophila was identified as the causal organism. Because many bacteria isolated from fish with hemorrhagic septicemias in fish were often misidentified, it is now recognized that certain isolations of bacteria ascribed to the genera Pseudomonas, Proteus, Bacillus, Aerobacter, and Achromobacter actually belonged to the genus Aeromonas. The exact etiology of disease involving Aeromonads is complicated by the diverse genetic, biochemical, and antigenic heterogeneity that exists among members of this group (Mary et al., 2002). Consequently, motile Aeromonads are often referred to as a complex of disease organisms that are associated with bacterial hemorrhagic septicemias and other ulcerative conditions in fishes (Post, 1989). Although motile aeromonads appropriately receive much notoriety as pathogens of fish, it is important to note that these bacteria also compose part of the normal intestinal microflora of healthy fish (Trust et al., 1974). Therefore, the presence of these bacteria, by itself, is not indicative of disease and, consequently, stress is often considered to be a contributing factor in outbreaks of disease caused by these bacteria. Such stressors are most commonly associated with environmental and physiological parameters that adversely fish under intensive culture. Furthermore, Cipriano et al. (1984) have shown that the prevalence of motile Aeromonad septicemia in cultured and wild Nile tilapia (Oreochromis niloticus) was 10.0% and 2.5% respectively; it was 18.75% and 6.25% in cultured and wild Karmout catfish, respectively. Ventura and Grizzle (1987) produced systemic infections more readily among channel catfish (Ictalurus punctatus) by abrading their skin prior to exposing the fish to the bacterium. Under controlled laboratory conditions, Defigueredo and Plumb (2005) found that strains of motile aeromonads isolated from diseased fish were more virulent to channel catfish than were those isolated from pond water. Lallier et al. (1981) performed studies on rainbow trout (Oncorhyncus mykiss, formerly Salmo gairdneri to compare the relative virulence of A. hydrophila and A. sobria, as taxonomically described. Their results indicated that strains of A. hydrophila isolated from either healthy or diseased fish were more virulent than strains of A. sobria. Additionally, A. sobria was not isolated from fish with clinical signs of motile aeromonad septicemia (Bogosian et al., 2000; Paniagua et al., 1990), for example, collected aeromonad isolates along the River Porma, Leon Province (Spain) and found that their isolates grouped within three species; A hydrophila (n=74 strains), A. sobria (n =11 strains), and A. caviae (n = 12 strains). The authors additionally observed that 72.02% of A. hydrophila isolates and 63% of A. sobria isolates were virulent for fish by intramuscular challenge, but all of the strains of A. caviae were avirulent. Pathologic conditions attributed to members of the motile Aeromonad complex may include dermal ulceration, tail or fin rot, ocular ulcerations, erythrodermatitis, hemorrhagic septicemia, red sore disease, red rot disease, and scale protrusion disease (Desnues et al., 2003).
Table 4: Principal technology components and their relationship to stress management of fish in cage culture technology
|
Technology component |
Stress management component |
|
1. Select a high quality, relatively stable environment for culture |
1. Water quality variables fluctuate well within tolerance ranges of fish |
|
2. Construct rectangular or square cages of 1- to 4-m3 volume; enclose with net with > 13mm mesh |
2. Water exchanges maintain water quality inside cages within tolerance ranges of fish |
|
3. Place cages in open water, with individual cages spaced apart |
3. Water exchanges maintain water quality inside cages within tolerance ranges of fish |
|
4. Stock select, healthy fish |
4. Fish have natural resistance to stressors and pathogens |
|
5. Feed proper allowance of nutritionally complete feed of good physical quality in special feed enclosure |
5. Nutritional requirements are for growth and good health. Minimum pollution potential wastes are lost to the environment |
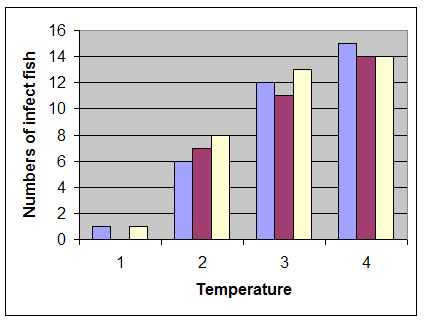
Figure 2: Number of infected fish to Aeromonas hydrophila in 3 different temperature ranges (1: 0-10˚C; 2: 10-20˚C; 3: 20- 30˚C; 4: Over 30˚C)
These researchers further revealed that A. hydrophila infected internal organs through the digestive tract or uninjured skin under conditions of crowding (13.1 g of fish/L) and high temperature (24oC). Such infections did not occurring when catfish were held at a lower density (5.2 g of fish/L) and temperature (18oC). In another study (Peters et al., 2000) subjected subordinate rainbow trout (Oncorhynchus mykiss) to social stresses of cohabitation with dominant cohorts and then exposed these fish to infection by A. hydrophila. By comparison to their dominant counterparts, the subordinate trout revealed physical evidence of stress based on elevated plasma glucose concentrations and increased leukocyte volumes. Following exposure to the pathogen, the bacterium was also recovered from more organs and with greater prevalence among the subordinate fish than from their dominant cohorts. In feral fish, other factors may be important stressors that trigger motile Aeromonad septicemias. For example, described the occurrence of such an epizootic associated with spawning stress among gizzard shad (Dorosoma cepedianum) in the Potomac River (Maryland, U.S.A.). It is important to submit fish suspected of being infected with Aeromonas to a diagnostic laboratory to confirm the disease, and to determine the antibiotic (Cipriano, 2001). Sensitivity of the strain of Aeromonas can cause some problems. In addition, because Aeromonas infections is stress-mediated disease, it is not unusual to find that infected fish are heavily parasitized or on currently infected with another systemic disease agents. When MAS, or any bacterial infection, is suspected in your fish, you should immediately submit a live, sick fish to the nearest diagnostic facility (Vila et al., 2002). If Aeromonas is diagnosed, you need to know what legal drug the isolate is sensitive to and whether or not other infectious agents are present. In addition, if the affected system is an indoor or closed system, good sanitation is essential to decrease the number of bacteria in the system. Techniques for preventing and minimizing stress are the principal techniques of any aquaculture technology system (Maalej et al., 2004). Principal technology components of high density cage fish culture and their purpose relative to stress management are presented in Table 4 By managing those specific chemical, biological and physical factors discussed earlier that may cause stress, fish health is almost assured but not guaranteed. Diseases do occur in fish that have not been stress mediated (Bogosian and Bourneu, 2001).
The behavior of A. hydrophila, like that of other bacteria, depends on the cells’ history and their ability to respond to environmental changes (Yesmin et al., 2004). The organism is ubiquitous in nature and is even found in the intestinal tract of the fish. In natural situations, infections of fish with A. hydrophila are probably a minor problem. However, with intensive fish-fanning systems, whether these systems are outdoor ponds or indoor aquaria or tanks, other factors must be considered. The common occurrence of this disease relates to stress conditions or factors of the fish (Holmes et al., 2005).
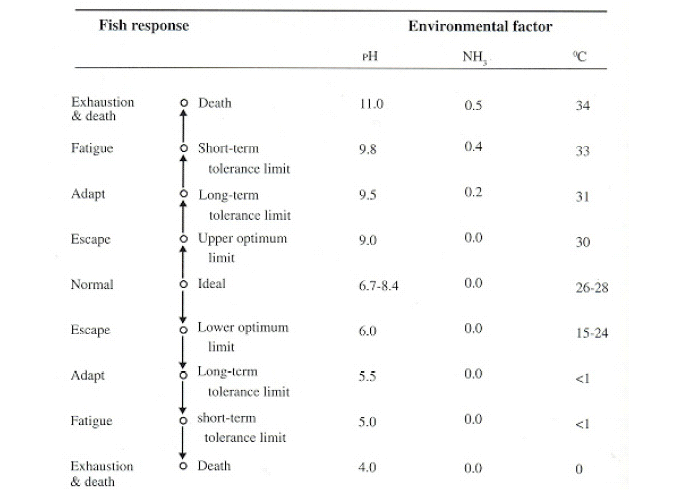
Figure 3: Generalized illustration of how warm water freshwater fish might respond to specific environmental factors under certain conditions
Fish experts agree that fish are easily stressed when mishandled, overcrowded, transported under poor conditions, are on a poor level of nutrition, have poor water quality (Shelton, 2000). Experimental demonstration shows that fish which are in poor environments, low water quality such as high nitrite levels, low levels of dissolved oxygen (DO) or high levels of carbon dioxide (CO2) are more susceptible to infection by Aeromonas hydrophila. Additionally, a seasonal incidence of a higher number of rep-ted fish deaths in the spring is associated with decreased water temperatures (El Mejri et al., 2008). Based on these observations, we suggest that low densities of A. hydrophila in internal organs, coupled with high water temperatures and stress induced by extreme trapping procedures and handling, are sufficient to create physiologic conditions conducive to rapid proliferation of the bacteria. Similar to our results, the results of the Rahman et al. (2001) indicated that temperature affects both the cell surface structure of A. hydrophila and the phagocytic activity of goldfish macrophages, resulting in different mortality rates of the fish at different temperatures.
In this study, it was clear that at 20-30 and above 30°C, the bacteria were more pathogenic than at 10-20 and lower than 20°C in C. damascina. This finding was similar to results that observed in goldfish at 17 and 25°C, the bacteria were more pathogenic than at 10°C (Rahman et al., 2001). Survey of O’Reilly and Day (1983) revealed that stimulation of the extracellular proteolytic activity of A. hydrophila was influenced by temperature. It is possible that A. hydrophila may have lost its virulence at 10°C and below. At 10°C, the metabolism and potential virulence mechanisms of A. hydrophila are decreased. On the other hands, Ishiguro et al. (1981) reported that A. salmonicida lost its virulence when cultured at high temperatures. Results of Sautour et al. (2003) and Koeypudsa and Jongjareanjai (2010) showed among the three factors tested including temperature, water activity and pH, temperature has the main influence on growth and survival of A. hydrophila in microcosm water. These results confirmed current findings.
It was suggest that the increase level of A. hydrophila in internal organs during increasing the water temperature was related to the production of sufficient quantities of lytic toxins that caused histopathology changing and subsequent mortality. So, A. hydrophila, in combination with stress factors, could increase mortality. It was recommended that during trapping, handling and manipulation of fish increasing of water temperatures should be eliminated.
Acknowledgement
We would like to thanks Mr. Mohamadreza Sabetpay for his kind collaboration in this study.
Author’s cONTRIBUTION
Authors declare that there is no conflict of interest.
Conflict of Interest
Laleh Yazdanpanah Goharrizi participated in the design and coordination of the study, Milad Adel and Mohammad Ebrahim Jalil Zorriehzahra participated in field sampling and conducted the laboratory work. Milad Adel analyzed data of this study and Mohammad Ebrahim Jalil Zorriehzahra reviewed the manuscript. All authors read and approved the final manuscript.
Reference