Advances in Animal and Veterinary Sciences
Research Article
Toxigenicity and Antimicrobial Resistance of Bacillus cereus Isolated from Raw and Pasteurized Milk
Ibrahim H. Amer, Esmat I. Awad, Salah F. Abd El-Aal, Rania M. Kamal, Reem M. Algendy*
Food Control Deptartment, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | Bacillus cereus (B. cereus) is an aerobic spore-forming bacterium that has a great public health hazards due to its high distribution in the environment, especially in dairy products in addition to its production to toxins. A total of 25 each of raw and pasteurized milk samples were examined for the presence of B. cereus. Microscopic examination, biochemical tests followed by detection of virulence genes and the antimicrobial susceptibility patterns of recovered B. cereus isolates were performed. From 37 samples that yielded bacillus like growth, there were 15 positives for B. cereus group. All Gram-positive B. cereus isolates were positive for glucose utilization test but were negative for indole test. The distribution of emetic cereulide gene (ces) among the examined isolates was 66.67% (10 15), while the enterotoxigenic non haemolytic gene (nhe) was not detected in any of the tested isolates. B. cereus isolates showed an absolute resistance to penicillin (10 U) (100%), followed by ampicillin (10 mg) (93.33%), cefoxitin (30 mg) and amoxicillin (20 mg) (80% each). The isolates were susceptible to enrofloxacin (5 mg) and erythromycin (15 mg) (100 % each), vancomycin (30 mg) (93.33%) and doxycycline (30 mg) (80%). In conclusion, the presence of toxigenic and resistant B. cereus in raw milk and pasteurized milk samples indicates a potential risk to food safety and, therefore, monitoring of the dairy items is suggested.
Keywords | Bacillus cereus, Virulence genes, Enterotoxigenic gene, Cereulide gene, Antibiotic susceptibility
Received | September 12, 2019; Accepted | October 26, 2019; Published | December 19, 2019
*Correspondence | Reem M. Algendy, Food Control Deptartment, Faculty of Veterinary Medicine, Zagazig University, Egypt; Email:
Citation | Amer IH, Awad EI, El-Aal, Kamal RM, Algendy RM (2019). Toxigenicity and antimicrobial resistance of bacillus cereus isolated from raw and pasteurized milk. Adv. Anim. Vet. Sci. 7(s2): 123-128.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.123.128
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Amer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bacillus cereus (B. cereus) is an aerobic and facultative anaerobic, motile, spore formers, Gram positive bacilli, beta hemolytic on blood agar, catalase positive and non-mannitol fermenter (Ceuppens et al., 2013). B. cereus is found commonly in the soil as a natural inhabitant opportunistic pathogen and it also does exist in various types of foods such as milk and dairy products (Larsen and Jorgensen, 1997).
The high distribution of B. cereus in the environment is attributed to its capability to withstand different conditions as it can live in a wide range of temperature (10-48 oC) and pH (4.9- 9.3) (OSPBH, 2005). This bacterium contaminates the milk and the temperature of pasteurization can’t destroy their spores completely (Andersson et al., 1995). Some of them can live at a temperature of 140-145 °C for 2-5 seconds (Scheldeman et al., 2006). Moreover, B. cereus is capable for growing at a low temperature (4-7 °C), which is the temperature of refrigerator and this mainly determines the shelf life of pasteurized milk and derived milk products (Granum, 2005). Bacillus cereus acquired its virulence from the toxins they produce. They comprise toxins responsible for emesis (Agata et al., 1995) and other diarrheal toxins, which include haemolysin BL or hbl (Beecher and Wong, 1997), non-haemolytic enterotoxin or nhe (Lindback et al., 2004) and cytotoxin K or cyt K (Lund et al., 2000). The most important diarrheal factors for B. cereus are hbl, nhe and cytK proteins (Lund et al., 2000). Recently, a high prevalence of contamination by B. cereus in dairy products especially in pasteurized milk was recorded (Yobouet et al., 2014; Dréan et al., 2015; Chaves et al., 2017; Lan et al., 2018). Moreover, B. cereus that harbored virulence genes responsible for toxicity was found with high percentage rates in various dairy products in Egypt (EL-Zamkan and Mubarak, 2017). Therefore, this work was conducted to reveal the presence of B. cereus in raw and pasteurized cow’s milk samples collected from different markets at Zagazig city, Sharkia Governorate, Egypt. Also, we determined the antimicrobial susceptibility patterns and the virulence genes of the recovered B. cereus isolates.
MATERIALS AND METHODS
Sampling
Fifty samples, 25 each of raw and pasteurized milk samples were randomly collected from different markets at Zagazig city, Sharkia Governorate during the period from March 2018 to June 2019 for isolation of B. cereus. All the samples were collected from clinically healthy cows and about 250-500 ml of each sample was collected aseptically in sterile screw cap tubes and transported within six hours in an ice box to the food safety laboratory at Faculty of Veterinary Medicine, Zagazig University (APHA, 2004).
Isolation and identification of B. ceures group isolates
Raw and pasteurized milk samples were heated to 85 oC for ten minutes to inactivate all vegetative bacteria except heat stable ones. The prepared samples were cultivated on polymyxin egg yolk mannitol bromothymol blue agar (PEMBA) supplemented with polymyxin B sulphate antibiotic (Oxoid, Basingstok, UK) and then the plates were incubated at 30 oC for 24-48 hours in an aerobic condition (EN ISO, 2004), trimethoprim was added to media to increase its selectivity to B. cereus against staphylococcus and other microorganisms which grow on this media (Kang et al., 2017).
Suspected B. cereus group isolates exhibited colonies with a turquoise to peacock blue appearance (typically mannitol negative) and precipitation zone around these colonies (lecithinase positive) (Mossel et al., 1967; Fricker et al., 2008). The suspected isolates were confirmed by microscopic examination of Gram stained film and motility followed by biochemical tests that included catalase, oxidation fermentation (glucose and mannitol), indole, voges proskauer, nitrate reduction, starch hydrolysis and gelatin liquefaction tests as per method described previously (Kim et al., 2010). Moreover, B. cereus group isolates were examined for their hemolytic activities through streaking a loopful of nutrient broth inoculated with suspected isolates onto 5% sheep blood agar plates. After incubation of the plates at 37 oC for 24 h, they were observed for the pattern of hemolysis around the colonies (Nour et al., 2002). These basic characteristics are shared by members of the B. cereus group. Therefore, differentiation of B. cereus strains from other members of the group was made while performing rhizoid test and protein toxin crystal formation test (FDA, 2012).
PCR detection of B. cereus group identification and virulence genes
After culturing the recovered B. cereus group isolates in brain heart infusion broth (BHI) at 37 oC for 24 h, DNA template was extracted using QLA amp DNA mini Kit, Qiagen, CA, USA) according to manufacturer’s instructions.
PCR amplification was carried out in a total reaction volume of 25 µL containing 12.5 μL of Emerald Amp GT PCR master mix (Takara, France), 0.1 μL of 100 pmol of each primer (Sigma Aldrich, Co., St. Louis, USA), 2 μL of extracted DNA and the volume of the reaction mixture was completed to 25 μL using DNase/RNase-free water. Three sets of primers targeting gro EL, nhe and ces virulence genes. The sequences of the used primers in addition to the cycling conditions and the amplicons` sizes for the analyzed genes are listed in Table 1. The amplified PCR products (533, 766 and 1271 bp) for gro EL, nhe and ces genes, respectively were analyzed using 1.5 % agarose gel stained with ethidium bromide. DNA bands were photographed under an ultraviolet transilluminator (Spectroline, USA) with a 100 bp DNA ladder (Fermentas, USA), which was used as a molecular size marker. Both positive (B. cereus ATCC11778) and negative control (distilled water) were used.
Antimicrobial susceptibility testing of B. cereus isolates
B. cereus isolates were subjected to antibiotic susceptibility tests following the standard agar disc diffusion method according to clinical and laboratory standard institute (CLSI) (CLSI, 2012). A total of 10 commercial antibiotic discs included amoxicillin (AML, 20 mg), ampicillin (AMP, 10 mg), cefoxitin (FOX, 30 mg), erythromycin (E, 15 mg), kanamycin (K, 30 mg), tetracycline (TE, 30 mg), vancomycin (VA, 30 mg), doxycycline (D, 30 mg), enrofloxacin (ENR, 5 mg) and penicillin (P, 10 U). The results were classified as susceptible, intermediate and resistant according to the standardized criteria listed in Table 2 (Quinn et al., 1999; CLSI, 2012). The procedure was performed in triplicates for each of the isolate. The multi-drug resistance (MDR) pattern of the isolates was calculated. MDR strains are those strains showing resistance to three or more antibiotics of different antimicrobial classes. Moreover, MAR index was calculated using the formula a/b, in which, a is the total number of antibiotics to which the organism was resistant and b is the total number of antibiotics to which the organism was tested.
Table 1: Oligonucleotide primers sequences targeting B. cereus group identification and B. cereus virulence genes with their respective cycling conditions and amplicons length.
| Target gene |
Primer sequence (5'-3') |
Amplicon size (bp) | Cycling conditions | Reference |
| groEL | F: TGC AAC TGT ATT AGC ACA AGC T | 533 |
94 oC / 5 min; 35 cycles (94 oC/30 sec; 55 oC /40 sec; 72 oC /45 sec), final extention of 10 min at 72 oC |
|
| R: TAC CAC GAA GTT TGT TCA CTA CT | ||||
| nhe | F: AAG CIG CTC TTC GIA TTC | 766 |
94 oC / 5 min; 35 cycles (94 oC/30 sec; 49 oC /40 sec; 72 oC /45 sec), final extention of 10 min at 72 oC |
|
| R: ITI GTT GAA ATA AGC TGT GG | ||||
| ces | F: GGT GAC ACA TTA TCA TAT AAG GTG | 1271 |
94 oC / 5 min; 35 cycles (94 oC/30 sec; 49 oC /40 sec; 72 oC /1 min), final extention of 12 min at 72 oC |
|
| R: GTA AGC GAA CCT GTC TGT AAC AAC A |
Table 2: Guidelines for interpretation of B. cereus antimicrobial susceptibility test.
| Antimicrobial agent | Symbol | Disc potency (μg) |
Inhibition zone diameter (mm) |
||
| Resistance (≤) | Moderate | Susceptible (≥) | |||
| Amoxicillin | AML | 20 | 13 | 14–17 | 18 |
| Ampicillin | AMP | 10 | 28 | – | 29 |
| Cefoxitin | FOX | 30 | 21 | – | 22 |
| Erythromycin | E | 15 | 13 | 14-22 | 23 |
| Kanamycin | K | 30 | 13 | 14–17 | 18 |
| Tetracycline | TE | 30 | 14 | 15–18 | 19 |
| Vancomycin | VA | 30 | 9 | 10–11 | 12 |
| Doxycycline | D | 30 | 12 | 13–15 | 16 |
| Enrofloxacin | ENR | 5 | 15 | 16–20 | 21 |
| Penicillin | P | 10 unit | 28 | - | 29 |
RESULTS
Occurrence and phenotypic characterization of B. cereus group isolates
From 37 samples who yielded bacillus like growth (20 from raw milk and 17 from pasteurized one), there were 15 positive for B. cereus group isolates (10 from raw milk and 5 from pasteurized one) with a total recovery rate of 30% of the examined samples. The total isolation rates of B. cereus group isolates from the examined raw cow’s and pasteurized milk samples were 40% and 20%, respectively. All the isolates showed common phenotypic and biochemical characters that were consistent with the identification of B. cereus group isolates. The isolates were shaped bacilli, showed positive reactions with catalase, lecithinase, voges proskauer, nitrate reduction, starch hydrolysis tests and utilized glucose. All the isolates were negative for indole, gelatin liquefaction tests and none of them fermented mannitol. According to the criteria used to differentiate B. cereus strains from other members of the group, B. cereus strains were accurately identified as they were motile unlike B. anthracis, strongly hemolytic and did not produce rhizoid colonies like B. mycoides or protein toxin crystals as B. thuringiensis.
PCR identification of B. cereus isolates and distribution of virulence genes
Notably, gro EL gene was found in 100% (15/15) of the analyzed isolates (Figure 1). The ces gene was found in 66.67% (10 15) of B. cereus group isolates; 7 from raw milk samples (28%) and 3 from pasteurized milk samples (12%) (Figure 2). Furthermore, nhe gene was not detected in any of the examined isolates.
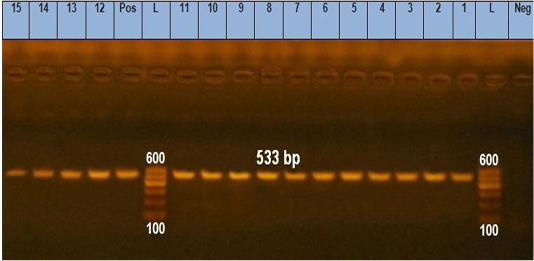
Figure 1: Agarose gel electrophoresis showing pattern of gro EL gene of B. cereus isolates identification. Lane L: 100 bp DNA ladder, lane Neg: the negative control, lane Pos: the positive control (B. cereus ATCC11778), lanes 1-10: PCR amplicons of B. cereus strains from raw milk, lanes 11-15: PCR amplicons of B. cereus strains from pasteurized milk.
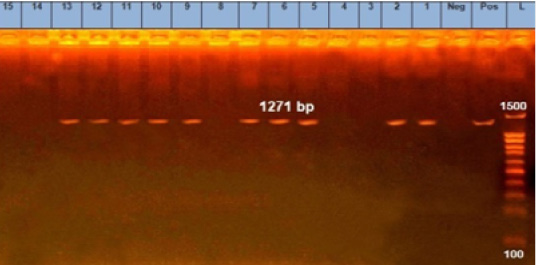
Figure 2: Agarose gel electrophoresis showing pattern of emetic cereulids gene (ces) of B. cereus strains isolates. Lane L: 100 bp DNA ladder, lane Neg: the negative control, lane Pos: the positive control (B. cereus ATCC11778), lanes 1, 2, 5, 6, 7, 9 and 10: PCR amplicons of B. cereus strains from raw milk, lanes 11, 12 and 13: PCR amplicons of B. cereus strains from pasteurized milk.
Results of antimicrobial susceptibility tests of B. cereus isolates
As shown in Table 3, B. cereus isolates had a higher resistance to penicillin (100%), ampicillin (93.33%), cefoxitin and amoxicillin (80% each), but they were highly susceptible to erythromycin and enrofloxacin (100% each), vancomycin (93.33%) and doxycycline (80%). The MDR pattern was present in 53.33% (8/15) of the tested isolates. Out of the examined isolates, 20 % (3/15) showed MAR index of ≤ 0.2, 26.67% (4/15) showed MAR index of 0.4 and 53.33% (8/15) showed MAR index of 0.6. The MAR index of higher than 0.2 was seen in 80% (12/15) of the isolates and this gives an indication about risk of contamination.
Table 3: Antibiotic susceptibility results of B. cereus group isolates (n= 15).
| Antibiotic agent (Abbreviation) |
Number of isolates showing susceptibility pattern (%) |
||
| Resistance | Intermediate | Susceptible | |
| Amoxicillin (AML) | 12 (80) | 0 (0) | 3 (20) |
| Ampicillin (AMP) | 14 (93.33) | 0 (0) | 1 (6.67) |
| Cefoxitin (FOX) | 12 (80) | 0 (0) | 3 (20) |
| Erythromycin (E) | 0 (0) | 0 (0) | 15 (100) |
| Kanamycin (K) | 7 (46.67) | 0 (0) | 8 (53.33) |
| Tetracycline (TE) | 8 (53.33) | 1 (6.67) | 6 (40) |
| Vancomycin (VA) | 0 (0) | 1 (6.67) | 14 (93.33) |
| Doxycycline (D) | 1 (6.67) | 2 (13.33) | 12 (80) |
| Enrofloxacin (ENR) | 0 (0) | 0 (0) | 15 (100) |
| Penicillin (P) | 15 (100) | 0 (0) | 0 (0) |
DISCUSSION
Bacillus cereus has a great interest in food safety. It also has major public health hazards owing to its ability to spoil food and production of toxins (Organji et al., 2015). The occurrence rate of B. cereus isolates in raw cow’s milk in Zagazig city was 40%. A similar isolation percentage of B. cereus (38.80%) from raw milk samples was reported in a previous research conducted in Haramaya district in Ethiopia (Abraha et al., 2017). On the other hand, a higher occurrence rate of B. cereus isolates (66.60%) from raw milk samples was recorded in a previous study carried out in Egypt (Organji et al., 2015). Improper sanitation during collection, transferring and handling of milk is the main cause of this high prevalence rate of B. cereus in marketed raw milk samples (Abraha et al., 2017). With regard to pasteurized milk, our study detected B. cereus isolates in 20 % of the collected 25 pasteurized milk samples. This result was quite similar to the results obtained by several authors in Abidjan (27%) (Yobouet et al., 2014) and Brazil (27.37%) (Reis et al., 2013). However, the study isolation rate was lower than those reported in previous researches conducted in India (37%) (Rather et al., 2011) and Ghana (47%) (Owusu-Kwarteng et al., 2017). The high prevalence of B. cereus in pasteurized milk is attributed to the heat stable B. cereus spores in raw milk and the post-pasteurization contamination along the milk processing lines (Saleh-Lakha et al., 2017).
Studies on virulence genes of B. cereus documented that these genes or enterotoxins are associated with emesis and diarrhea (Jeßberger et al., 2015). In this study, ces (emetic) gene was detected with a percentage of 66.67% of B. cereus isolates, while nhe (diarrheal) gene was not detected. These results contradict those obtained by several authors where nhe gene was detected in 96 and 100% of B. cereus isolates in France (Glasset et al., 2016) and China (Cui et al., 2016), respectively. Regarding results of emetic gene, our prevalence rate is higher than those obtained previously in Holland (10.20%) (Biesta-Peters et al., 2016) and Ghana (9%) (Owusu-Kwarteng et al., 2017).
As infections and diseases caused by B. cereus can lead to death (Lund et al., 2000; Dierick et al., 2005), so effective antibiotic therapy must be chosen to treat B. cereus infections. The resistance to penicillin and ampicillin as well as to cephalosporin among Bacillus spp. has most frequently been observed (Murray et al., 2005). This gives an indication about the potential risk for B. cereus infections. Our results revealed that B. cereus isolates had higher resistance rates against penicillin (100%), ampicillin (93.33%), cefoxitin (86.67%) and amoxicillin (80%), but they were highly susceptible to erythromycin and enrofloxacin (100% each), vancomycin (93.33%) and doxycycline (80%) (Table 3). These results are comparable with those discussed previously by several others (Luna et al., 2007) where 95% of B. cereus isolates were resistant to each of penicillin and ampicillin. Moreover, recent researches proved that B. cereus isolates were resistant to beta-lactam antibiotics (da Silva Fernandes et al., 2014; Kim et al., 2015; Yibar et al., 2017). In another research carried out in Egypt, tested B. cereus isolates showed 100% resistance to penicillin and 100% susceptibility to each of erythromycin and vancomycin (Organji et al., 2015). As proven in our results, the most effective antibiotic used for treatment of B. cereus infection was vancomycin (Godič Torkar et al., 2016; Tatara et al., 2013). The MDR pattern was present in 53.33% of the tested isolates which highlight the fact that choice of therapy is limited and difficult.
CONCLUSION
Existnce of virulent strains of B. cereus is very common in raw milk and dairy products and they are resistant to many of commonly known or applied antibiotics. Necessary food safety programmes and public awarness on dairy food consumption should be implemented for their effective control and, if infection occure, managemnet and treatment of infection using appropriate antibiotic.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the food safety laboratory at the Food Control Department, Faculty of Veterinary Medicine, Zagazig University, for providing the tools required for isolation and identification of B. cereus isolates.
Authors Contribution
Ibrahim H. Amer, Esmat I. Awad, Salah F. Abd El-Aal, Rania M. Kamal and Reem M. Algendy designed the article idea and experiments. Reem M. Algendy carried out sample collection and laboratory work and performed the experiments (researcher). Reem M. Algendy and Rania M. Kamal analyzed the data and wrote the article. All authors revised, read and approved the final manuscript.
CONFLICT OF INTEREST
No conflict of interest is declared.
REFERENCES






