Advances in Animal and Veterinary Sciences
Research Article
Increased Profile Fertilization and the Process of Cleavage of Kacang Goat In Vitro Fertilization Using Intracytoplasmic Sperm Injection
Widjiati1*, Zakiyatul Faizah2, Ninik Darsini2, Viski Fitri Hendrawan3, Epy Muhammad Luqman4, Sutiman B. Sumitro5
1Post Graduate School of Universitas Airlangga Surabaya, Indonesia; 2Departement of Biomedical Science Faculty of Medicine Universitas Airlangga Surabaya, Indonesia; 3Departement of Reproduction Faculty of Veterinary Medicine Universitas Brawijaya Malang, Indonesia; 4Departement of Veterinary Anatomy Faculty of Veterinary Medicine Universitas Airlangga Surabaya, Indonesia; 5Departement of Biology Faculty of Science Universitas Brawijaya, Malang, Campus B Universitas Airlangga, Jalan Airlangga 4-6 Surabaya.
Abstract | The weakness of in vitro fertilization (IVF) was not able to observe whether in vitro fertilized oocytes were all mature. Intra Citoplasmic Sperm Injection (ICSI) was able to solve IVF failure as spermatozoa was directly injected to oocyte. The research was divided into 2 groups, T1 group: oocyte was fertilized conventionally (maturated oocyte was supplamanted with spermatozoa) and T2 group: fertilization using ICSI method. Next, oocyte was mixed with spermatozoa. After ICSI was done, it was put into incubator CO2 5 %, with the temperature of 38.5oC. Then, fertilization rate and embryo growth at cleavage stage were observed. The data presented as means Standard Error of the Mean (S.E.M) and were compared using the Student’s t-test. Research result showed that fertilization rate of ICSI group was higher significant than conventional IVF group (p<0.05). Also, cleavage stage (2 cells, 4 cells, 8 cells) of ICSI group was higher significant than conventional IVF group (p<0.05). The research concluded that fertilization rate and embryo cleavage of IVF using ICSI technique were higher than conventional IVF.
Keywords | Sperm injection, Oocytes, Cell block, Fertilization rate, Embryo growth
Received | May 06, 2020; Accepted | July 07, 2020; Published | July 20, 2020
*Correspondence | Widjiati, Post Graduate School of Universitas Airlangga Campus B Universitas Airlangga, Jalan Airlangga 4-6 Surabaya, Indonesia; Email: [email protected]
Citation | Widjiati, Faizah Z, Darsini N, Hendrawan VF, Luqman EP, Sumitro SB (2020). Increased profile fertilization and the process of cleavage of kacang goat in vitro fertilization using intracytoplasmic sperm injection. Adv. Anim. Vet. Sci. 8(8): 868-872.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.8.868.872
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Widjiati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Kacang goat was an Indonesian local goat that has good indurance as it adapts with the conditions in Indonesia. Increase of local goat population was not as good as that of other types of goat. Many factors were influenced it, including the small Kacang goat posture in terms of economical cheap price. Kacang goat starts being marginalized due to rapid breeding of big-sized goats. Therefore, technology to help accelerate Kacang goat production is required (Restitrisnani et al., 2013).
Breakthrough technology to create kacang goat embryo was able to be conducted using in vitro fertilization (IVF) method either conventionally done by supplementing mature oocyte with spermatozoa outside body or by intra citoplasmic sperm injection (ICSI) method. Both of these conventional IVF and ICSI methods had their advantages and disadvantages, and depend on the quality of oocytes and spermatozoa as sources of gamete cells (Merchant et al., 2011).
Oocyte quality as gamete source highly influences success of IVF. Several researches explain that conventional IVF was not able to measure maturity level of fertilized oocyte as in vitro maturated oocyte was directly mixed with spermatozoa with certain concentration. Therefore, it was not sure that all in vitro maturated oocytes reach metaphase II stage. It was different from ICSI technology, before spermatozoa was injected to ooplasm of oocyte, oocyte was first denudated to know oocyte maturity level after in vitro maturation. ICSI was done only to mature oocyte which is shown by existence of polar body (Uehara and Yanagimachi, 1997).
Low fertilization rates in conventional fertilization need to be overcome with technology that can overcome the process of fertilization through a pass cell block. ICSI was able to solve IVF failure as spermatozoa was directly injected to oocyte. The aim of this study was to compare the rate of fertilization and embryonic development (2, 4 and 8 cells) of the IVF method with ICSI.
MATERIALS AND METHODS
The research was carried out to observe Fertilization rate and embryo growth. Techniques used to yield fertilization rate and embryo growth were in vitro fertilization method and ICSI. The research was conducted at Bio-medic Laboratory Medical Faculty Universitas Airlangga. The research used Completely Randomized Design (CRD), with the assumption that all treatments were treated equally from sample taking to execution as well as laboratory condition. In vitro experiments were carried out at least in duplicate, oocytes recovered from five Kacang goat (1.5- 2 years old) were randomly assigned to the IVF method and ICSI group. Stages of the research were as follows: oocyte collection, oocyte maturation, conventional in vitro fertilization, spermatozoa preparation, fertilization using intra citoplasmic sperm injection method, in vitro embryo culture and examination of embryo quality at cleavage stage.
Methods
Oocyte collection
Oocytes of kacang goats that were cleaned from hooks and fat tissue were washed with physiological NaCl 0.9% which was supplemented with gentamycin sulfate 50 µl and put in a sterile tube and warmed in waterbath at the temperature of 30-35oC. Oocytes were collected using aspiration of follicles with diameter surface of 8-12 mm using disposable syringe 10 ml with 18 G needle which was previously filled with MEM medium. Collected oocytes were cleanly washed with MEM medium.
Oocyte maturation
Collected oocytes were washed 3 times using maturation medium MEM (Minimun Essential Medium) which was supplemented with newborn calf serum (Sigma, USA) 10% (v/v) which was inactivated, follicle stimulating hormone (Denka, Japan) 0.01 µg/ml dan gentamycin sulphate 50 µg/ml.Oocytes were cultured in 100 µl MEM and covered with mineral oil or parafin liquid (Sigma, USA). Maturation was done in incubator CO2 5% with the temperature of 38ºC for 20-22 hours until cumulus cell expansion existed.
Conventional in vitro fertilization method
Collected oocytes were then respectively washed three times in PBS and MEM medium. Washed oocytes were next moved to Fertilization medium while waiting for spermatozoa that was prepared for IVF. After spermatozoa was prepared, it was immersed in fertilization medium. Oocytes that were mixed with spermatozoa then were incubated in incubator CO2 5% with the temperature of 38.5° C for 20 hours, after that granulosa cell was shed to observe 2 pn.
Spermatozoa preparation
Collected spermatozoa was supplemented with silk select medium and centrifuged with the speed of 2500 rpm for 10 minutes, next it was put in a drop of well shape. Spermatozoa that was motile in small well was used for ICSI.
Fertilization using intra citoplasmic sperm injection method
Oocytes were denudated to shed cumulus cell so that polar body position was seen. A motil spermatozoa was taken, then it was immobilized by cutting its tail using injector, after that the immobilized spermatozoa was put into injector and injected into oocyte that had polar body.
In vitro embryo culture
After 2 pn existed, zygote was moved to culture medium and incubated in incubator C02 5% at the temperature of 38.5oC. Culture medium was changed once in two days until embryo reached cleavage stage.
Examination of embryo quality at cleavage stage
Examination of embryo quality at cleavage stage was conducted until embryo reached 8 cell stage three days after IVF or ICSI was done.
Statistical analysis
The data presented as means Standard Error of the Mean (S.E.M) and were compared using the Student’s t-test. They were considered to be significantly different if P < 0.05.
RESULT AND DISCUSSION
Examination of fertilization rate
Data of fertilization rate examination showed that fertilization rate of kacang goat using ICSI method was higher than that of using conventional method. It can be seen at Table 1.
Table 1: In vitro fertilization rate of kacang goat using conventional method and intra cytoplasmic sperm injection.
| Group | Mean ± SD |
| T1 |
10.25 ± 5.32a |
| T2 |
2.50 ± 1.00b |
Notes: Different supercrips in the same coloum have significant difference <0.05; T1: In vitro fertilization using intra cytoplasmic sperm injection method; T2: In vitro fertilization using conventional method.
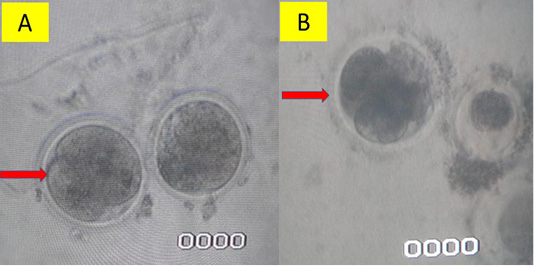
Figure 1: In vitro fertilization using intra cytoplasmic sperm injection method (A) and conventional method (B)/a>
Embryo growth examination
Zygote formed from in vitro fertilization using either conventional In Vitro Fertilization method or ICSI was next cultured further until zygote split into stage of 2 cells, 4 cells and 8 cells. Data of embryo growth can be seen at Table 2.
Table 2: Kacang goat embryo growth resulted from in vitro fertilization using conventional method and intra cytoplasmic sperm injection.
|
Zygote (mean±SD) |
2 cells (mean±SD) |
4 cells (mean±SD) |
8 cells (mean±SD) |
|
| ICSI |
8.50 ± 0.65a |
8.50 ± 0.65 a |
5.75 ± 0.45 a |
5.75 ± 0.45 a |
| Conventional |
4.25 ± 0.33b |
2.75 ±.0.96 b |
1.75 ± 0.13 b |
1.75 ± 0.13 b |
Note: Different supercrips in the same coloum have significant difference (p< 0.05); T1: In vitro Fertilization using intra cytoplasmic sperm injection method; T2: In vitro Fertilization using conventional method.
The research result at Table 1 above showed that IVF using ICSI technique had higher fertilization rate than that of conventional in vitro fertilization technique. In conventional technique, sperm was supplemented to drop of oocyte culture with certain concentration, then incubated. Conventional IVF technology was by mixing oocyte and spermatozoa, spermatozoa would go to and penetrate oocyte motility of spermatozoa highly determined movement to be able to penetrate and fertilize oocyte. However, IVF technology was not able to observe whether all oocytes fertilized were mature.
The new fertilization technology to yield fertilization was ICSI. ICSI technique was by injecting a sperm which was the most motile one with normal morphology into mature oocyte shown by existence of polar body or oocyte at Metaphase II condition. Tail of spermatozoa was immobilized so spermatozoa did not move, then it was put into oocyte by injecting it to ooplasm of mature oocyte using injector needle (Bhattacharya et al., 2001).
Nowadays ICSI technology at IVF scope has developed and has been applied to animals and humans. ICSI became a technological breakthrough to increase fertilization rate and production of in vitro embryo. ICSI on livestock was able to be valuable research device to learn fundamental aspects of oocyte and sperm interaction during fertilization, particularly related to oocyte activation and formation of male pronucleus at early growth of embryo (García‐Roselló et al., 2009; Bhattacharya et al., 2001; Parmar et al., 2013).
ICSI was current technology at in vitro fertilization scope to solve fertilization failure. ICSI helped several important processes during fertilization. ICSI used was aimed to help spermatozoa penetrate oocyte and increase fertilization rate. Fertilization using ICSI technology as a way to increase gestation rate.
Research result at Table 2 showed that there was a significant difference of embryo growth resulted from ICSI technique compared to that resulted from conventional in vitro Fertilization. Cleavage process of embryo at ICSI group was higher than that of conventional in vitro fertilization group. ICSI gave rate change of the success of in vitro embryo production significantly. It was due to ICSI was able to prevent fertilization failure. Rate of live born embryo of ICSI was higher than that of conventional IVF, so ICSI was still better than conventional IVF (Speyer et al., 2019). Fertilization rate of cattle ranged between 50-80%, higher than fertilization rate of pigs. Embryo growth at blastocyst stage ranged from 6.1% to 40.1%. Whereas, blastocyst rate of small ruminants ranged from 11.7% to 35%. Rate of blastocyst formation on goat was caused by failure of pre-puberty oocyte to maturate and fertilize (García‐Roselló et al., 2009).
Cell membrane of oocyte consisted of mix of protein and lipid membrane. Lipid level in membrane covered 20% up to 80%, depended on cell type and cell function. Lipid had a role in membrane flexibility, while protein membrane regulated chemical level in cell and organized chemical transport in membrane. Phospholipid was a main component in cell membrane. Lipid bilayer was semi-permeable. Only several molecules could diffuse through membrane. Cholesterol was the biggest lipid component of cell and was distributed equally so it could prevent membrane from being hard due to tighly bound phospholipid (Li et al., 2017).
Structural protein of oocyte was composed of protein that had important role to keep shape and size of cell. Receptor in protein cell membrane had important role in cell communication with external environment by hormone use, neuro-transmitter and other molecular signals. Membrane was able to fix themselves post ICSI by means of molecular lipid in membrane that regulated their position to reduce exposure of their hydrophopic area to water (Anzalone et al., 2016).
Membrane improvement process highly relied on physical condition of the membrane itself. When membrane had swelling or was hydrated and exposed with water post ICSI, membrane got thin, released pressure externally so oocyte got ruptured. On the other hand, if embryo was on compression phase/embryo was dehydrated, lipid molecule tended to migrate to the surface of hole formed by micro-injector to release compression, and consequently membrane regeneration got faster and better. Therefore, embryo dehydration post ICSI would increase regeneration of cell membrane and accelerate embryo to continue with cell cleavage (Burruel et al., 2014).
ICSI was a more invasive option than conventional IVF, which was able to succeed despite bad semen quality. Report which showed that higher fertilization rate post ICSI showed that this technique might be better than conventional IVF method to be used by a couple who wanted to have IVF. ICSI increased fertilization rate, it was possible that embryo from ICSI had better quality and had bigger potential of implantation and better gestation rate (Jimenez-Macedo et al., 2005).
Embryo of kacang goat which was created in vitro had important role to increase livestock productivity. By creating in vitro embryo, genetic selection was able to be done, so it was possible to maintain excellent genetics. This technology was also able to be used to improve infertility which was not able to yield gestation on livestock. In vitro Fertilization technology was able to be used as a method to handle infertility caused by failure of ovulation, fertilization and other causes (Zhu et al., 2012; Larbuisson et al., 2017).
Success of in vitro fertilization technology pushed development to micro fertilization, which was aimed to solve factors that induced fertilization failure if using in vitro fertilization technology. Weakness of in vitro fertilization technology was existence of polyspermy as oocyte was fertilized by more than one sperm so it made bad embryo growth, decreased success of fertilization rate and made embryo undevelop. Development of micro Fertilization technology either in terms of reseach or application was getting better, kept up with technological development. Development of micro fertilization technology was aimed to perfect in vitro Fertilization technology to cope with failure of in vitro fertilization and existence of polyspermy. ICSI was a way to increase fertilization success as it was ensured that 1 sperm was penetrated to cytoplasm of oocyte using a device (Neri et al., 2014; Larbuisson et al., 2017).
Conclusion
The research concluded that fertilization rate resulted from IVF using ICSI technique was higher than that resulted from conventional In vitro Fertilization. Also, embryo cleavage of Kacang goat had higher rate when it used ICSI technique than conventional IVF. An increase in the rate of fertilization and embryo development in ICSI reached 100-400% So that the ICSI implementation in Kacang goats was very promising to be developed in the future.
Acknowledgements
We would like to thank Universitas Airlangga through Post-graduate Program and Research Bureau and Inovasi that have funded the research from mandatory research scheme year 2019.
Authors Contribution
All authors contributed equally to the manuscript.
Conflict of Interest
The Authors have declared no conflict of interest.
REFERENCES