Advances in Animal and Veterinary Sciences
Research Article
A New Approach to the Treatment of Lumpy Skin Disease Infection in Cattle by using Propolis Encapsulated within ALG NPS
Tarek Korany Farag1*, Asmaa S. El-Houssiny2, Eman Hussein Abdel-Rahman1, Ahmed G. Hegazi3
1Department of Parasitology and Animal Diseases, National Research Centre, Giza, Egypt; 2Department of Microwave and Dielectric, National Research Centre, Dokki, Giza, Egypt; 3Department of Zoonotic Diseases., National Research Centre, Dokki, Giza, Egypt.
Abstract | Background: Lumpy skin disease (LSD) is a contagious disease caused by the Lumpy skin disease virus (LSDV) which belongs to the genus Capripoxvirus of the family Poxviridae. Cattle are the only animal species affected, with high morbidity and mortality rate in the young. LSD causes economic losses, abortions in females, and sterility in males. Nanoparticles are one of the novel strategies that adopted in the treatment of diseases in the last few years owing to their pioneering and functional properties. Aim: This study aimed to treat the clinically infected cattle with LSDV using Propolis-Alginate nanoparticles (Propolis-ALg NPs) through different routes such as eye drop, oral route and topical spray. Materials and methods: The Propolis-ALg NPs were characterized through Transmission Electron Microscope (TEM), Differential Scanning Calorimetry (DSC), in-vitro cytotoxicity, and hemolysis assays. The animal study was carried out during the outbreak of LSD in July 2018 at Beni-suef Governorate, Egypt. 35 infected cows with different ages were used in the present study. The animals were divided into two groups; group A (20 animals) and group B(15 animals). Animals in group A were treated with Propolis-ALg NPs and animals in group B were treated with tetracycline antibiotics. Confirmation of the isolated LSDV was done by polymerase chain reaction (PCR) using universal primers. Results: The Propolis-ALg NPs showed spherical morphology with small particle sizes. The thermal analysis revealed the successful encapsulation of the propolis within the ALg NPs. Also, the in-vitro cytotoxicity study confirmed the safety of the Propolis-ALg NPs. In addition to that, the prepared nanoparticles were found to be non-toxic when they come in contact with blood. On the other hand, the clinically infected cattle with LSDV which treated with the Propolis-ALg NPs revealed a 100 % recovery. While, the animals treated with the tetracycline showed only 13.3% recovery. Conclusion: Therefore, the Propolis-ALg NPs were shown to be a potential candidate in the therapy against LSDV infections.
Keywords | Propolis, Alginate, Nanoparticles, Lumpy Skin Disease infection, Cattle.
Received | September 07, 2020; Accepted | September 15, 2020; Published | November 15, 2020
*Correspondence | Tarek Korany Farag, Department of Parasitology and Animal Diseases, National Research Centre, Giza, Egypt; Email: [email protected]
Citation | Farag TK, El-Houssiny AS, Abdel-Rahman EH, Hegazi AG (2020). A new approach to the treatment of lumpy skin disease infection in cattle by using propolis encapsulated within alg nps. Adv. Anim. Vet. Sci. 8(12): 1346-1355.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1346.1355
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Farag et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lumpy skin disease (LSD) is a contagious, fatal skin disease affecting cattle and caused by LSDV which belongs to the family Poxviridae. LSDV is a double-stranded DNA virus (Onyejekwe et al., 2019). It is a member of the genus Capripoxvirus of Poxviridae with the size of its genome 151-kbp (Bhanuprakash et al., 2006). LSDV is a transboundary high-impact cattle disease characterized by fever, nodular formation, a rapid eruption of skin nodules, enlarged superficial lymph node, generalized lymphadenitis, and edema with great economic losses (Abera et al., 2015, Şevik et al., 2016; Rouby et al., 2019). It is usually more prevalent during the wet summer and autumn months, especially in the low-lying areas of water. However, outbreaks can also occur during the dry season (Tuppurainen et al., 2012).
Mechanical transmission of LSDV occurs by numerous blood-feeding insects such as mosquitoes and flies (Tuppurainen et al., 2013). The virus can be transmitted through blood, nasal discharge, lacrimal secretions, semen and saliva. It can also be transmitted through infected milk to suckling calves. In experimentally the infected cattle, LSDV was persisting for 11 days in saliva, 22 days in semen and 33 days in skin nodules after the development of fever (Babiuk et al., 2008). LSDV can remain viable in infected tissue for more than 120 days and quarantine restrictions are of limited use (Gumbe, 2018).
DNA bands of LSDV isolates were visualized using an ultraviolet trans-illuminator after the agarose gel was stained with Red Safe. The amplification products were identified on 1.5% agarose gel electrophoresis at the expected positions for the fusion gene at 472 bp and for the P32 at 587 bp (Allam et al., 2020).
Vaccination with attenuated virus offered the most promising method of control. Despite annual mass vaccination with sheep pox vaccine (Veterinary Serum and Vaccine Research Institute, Egypt) by the Egyptian authorities, LSDV still circulates almost every summer. For instance, during the summer of 2006, an extensive LSD outbreak hits Egypt, affecting 16 provinces (Awadin et al., 2011), and it reemerged in 2011, 2014 and 2016 (Amin et al., 2015; El-Tholoth & El-Kenawy et al., 2016; Abdallah et al., 2018).
Nanotechnology is an emerging field of applied science and cutting-edge technology that utilizes the physicochemical properties of nanomaterials to control their size, surface area, and shape to generate promising characteristics (Prasad, 2008). Different materials, proteins, lipids, inorganic materials can be used for the creation of nanoparticles. However, the polymeric nanoparticles such as alginate NPs seem the most interesting and promising ones (Girija 2019). Alginate NPs (a biodegradable and biocompatible co-polymer of guluronic acid and mannuronic acid) have already received permission from the United States Food Drug Administration (US FDA) for human use and it is commonly administered orally (Ahmad et al., 2006). Since their NPs size is very small, they maximize tissue compatibility, reduce cytotoxic effects, and enhance bioavailability.
The use of the polymeric nanoparticles can be extended to the development of antiviral nanoparticles that act by interfering with the viral replication, during attachment or entry (Loutfy et al., 2020). Some studies have emerged showing that the alginate NPs can be used as an effective carrier for the antiviral agents against most of the viruses such as human immunodeficiency virus (HIV) (Joshy et al., 2018). The nanoparticles were found to be biocompatible and the in-vitro cellular internalization studies indicated significantly higher internalization efficiency (Albarqi, 2019).
Propolis is a natural product the extensively used in the food and beverages to improve the health status and prevent diseases and show immunomodulatory properties (Silva-Carvalho et al., 2015). It can play an important role as an antiviral agent (Abd El-Hady et al., 2002; 2007; Hegazi et al., 2004; Hegazi and Abd El-Hady, 2008).
This work aimed to focus on assessing the usage of Propolis encapsulated into ALg NPs in the treatment of LSD infection in cattle reared in Beni-Suef Governorate, Egypt.
Materials and Methods
Propolis Source and Extraction
Propolis sample were collected from the apiary farm near El-Mansoura City, Dakahlia Governorate, Egypt. The resinous materials were kept in a dark bag in the refrigerator until being extracted with ethanol. Sodium Alginate was supplied by ROTH, Germany. Calcium chloride was supplied by Qualikems, India. The materials used are with analytical grade.
Fifty grams of propolis sample was cut into small pieces and extracted at room temperature with 500 ml of 70% ethanol (twice after 72 hours). The alcoholic extract was evaporated under vacuum at 50oC until dryness. The percentage of the extracted sample is 5.1 g/dry weight (Hegazi et al., 2019).
Preparation of Alginate-Propolis NPs
Alginate-Propolis NPs were prepared by the controlled gellification method reported by (Rajaonarivony, et al., 1993) based on the ionotropic gelation of polyanion with CaCl2. Alginate with concentration (0.1% w/v) was dissolved in distilled water at room temperature. Then, 2 ml of propolis with a concentration of 5mg/ml ethanol was mixed with the alginate solution for 24 hours. After a mechanical stirring, 5 ml of CaCl2 solution (36 mM) was added dropwise under constant stirring to 95 ml of the Alginate-Propolis solution to induce gellification. The nanoparticle suspensions were further stirred for three hours at room temperature. Finally, the nanoparticles were freeze-dried for storage (El-Houssiny et al., 2017).
Characterization of Nanoparticles
Transmission Electron Microscopy (TEM): The morphological characteristics of the prepared Propolis-ALg NPs were examined by transmission electron microscope (TEM) (JEM- HR- 2100, Japan). One drop of the particle suspension was spread onto a carbon-coated copper grid and stained using phosphotungstic acid. The sample was left for drying at room temperature. Then, the sample was placed for TEM imaging at 120 kV accelerating voltage (El-Houssiny et al., 2016).
Differential Scanning Calorimetry (DSC): Differential scanning calorimetry (SETARAM Inc., France) was used to characterize the thermal behavior of propolis, ALg NPs, and Propolis-ALg NPs. The instrument was calibrated using the standards (Indium, Tin, Lead, Zinc, and Aluminium). Nitrogen and Helium were used as the purging gases. The lyophilized samples were weighted in standard aluminum pans and heated from 25 °C to 300 °C at a heating rate of 5 °C/min (Baygar et al., 2020).
In-vitro Cytocompatibility of the ALg-Propolis NPs (MTT Assay): Cell viability of the ALg-Propolis NPs was assessed by the mitochondrial-dependent reduction of yellow MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) to purple-colored formazan crystals (Raheem et al., 2019). For cell culture experiments, RPE1 cells (normal retinal pigment epithelial cells) were suspended in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM-F12) under 5% CO2 at 37ºC. The RPE1 cell line was provided by the Bioassay-Cell Culture Laboratory, National Research Centre, Egypt. The medium was supplemented with 1% antibiotic-antimycotic mixture (10,000 U/ml Potassium Penicillin, 10,000 µg/ml Streptomycin Sulphate and 25µg/ml Amphotericin B), 1% L-glutamine, and 10% fetal bovine serum. Then, the cells were detached from the plate with Trypsin and resuspended in the growth medium for further experiments.
Cells were cultured for 10 days and then seeded at a concentration of 104 cells/well in 96-well plates under 5% CO2 at 37ºC for 24 hours. After that, the media were aspirated, fresh medium was added and the cells were incubated either alone (negative control) or with different concentrations of ALg-Propolis NPs sample to give a final concentration of (100 -50 -25 -12.5 μg /ml). After 48 hours of incubation, the medium was aspirated, 40 µl MTT (2.5μg/ml) was added to each well and incubated for a further 4 hours. To stop the reaction and dissolving the formed crystals, 200 µL of 10% sodium dodecyl sulfate (SDS) was added to each well and incubated overnight at 37 ºC. The absorbance of the solution was measured at wavelength 595 nm using a microplate multi-well Elisa reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA). The samples were analyzed three times for each concentration of the nanoparticles. A statistical significance was tested between the sample and negative control using an independent t-test by SPSS 11 program.
Hemolysis Assay: The in-vitro hemolytic property of ALg-Propolis NPs was studied by quantifying the free hemoglobin (Hb) released into plasma after lysis of red blood cells (RBC) upon interaction with nanoparticles (Sasidharan et al., 2016). Fresh blood was taken from the jugular vein of cattle into heparin containing tubes as an anticoagulant. The ALg-Propolis NPs were added to 1 ml of blood and incubated for 1 h at 37 ºC. Normal saline-treated blood was used as a negative control (0% lyses) and distilled water-treated blood as a positive control (100% lyses). The treated blood was then centrifuged for 10 minutes at 4500 rpm. The absorbance of the plasma was measured using a spectrophotometer at 415, 380, and 450 nm. The amount of plasma hemoglobin was calculated from the equation as follows:
Where, A380, A415, and A450 are the absorbance values at 380, 415, and 450 nm, respectively. The A380 and A450 are correction factors applied for absorption by uroporphyrin which falls in the same wavelength spectra, like that of hemoglobin and A415 is the sorbent band absorption of oxyhemoglobin. A correction factor, 1.655 is applied owing to interference from turbidity of the plasma sample and E is 79.46, the value of molar absorptivity of oxyhemoglobin at 415 nm. The percentage of hemolysis was expressed as:
Animals Study
Ethical Approval: All experimental procedures were performed under the institutional guidelines of the Animal Research Committee, National Research Centre, Egypt, under protocol number 16/219.
Study Area: Samples were collected from local cattle reared in Beni Suef Governorate, (29.076°N 31.097°E) located in Upper Egypt about 120 km south of Cairo, the capital of Egypt.
Animals
Treatment Protocol (Babiuk et al., 2008): A total of 35 clinically infected animals were collected from Beni-Suef Governorate, Egypt during the outbreak of lumpy skin disease virus (LSDV) circulating in the upper Egypt during the summer of 2018. They were divided into two groups (A & B) as described in Table (1).
Group (A): 20 infected animals were treated three successive days with ALg-Propolis NPs in a dose of 300 μl/animal through the oral route. Eye drops and the spray of ALg-Propolis NPs were applied in some cases.
Group (B): 15 infected animals treated three successive days with tetracycline (10%) in a dose of 1CC/10 kg per animal body weight through intramuscular injection. Tetracycline ointment was locally applied in some cases.
Animal Clinical Examination: From May to July 2018, infected cattle reared in Bani-Suef Governorate, Egypt were examined clinically. These cattle suffered from biphasic fever (40°C-41.5°C), depression, inappetence, salivation and ocular-nasal discharges. Superficial lymph nodes were markedly enlarged, especially the prescapular and precrural lymph nodes.
Animals Samples Preparation: Skin biopsies comprising of epidermis, dermis and subcutis of the nodular skin lesions were collected from infected local cattle reared in Beni-suef Governorate, Egypt. Samples were used for isolation and molecular studies as our previous study by (Allam et al, 2020).
Table 1: Treatment protocol of animals with ALg-Propolis NPs
| Treatment | Number of animals | Total | ||
| Eye lesions | Skin lesions | Wound lesions | ||
| Group (A): ALg-Propolis NPs | 1 | 16 | 3 | 20 |
| Group (B): Tetracycline (10%) | 0 | 12 | 3 | 15 |
Results
Transmission Electron Microscopy (TEM)
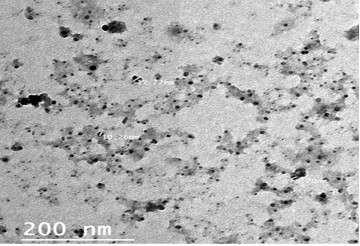
The particle size and morphology of the ALg-Propolis NPs were examined by TEM as illustrated in Figure 1. The prepared NPs size was on average of 10 nm. Moreover, the TEM view confirmed homogenous, no aggregation, and spherical shape nanoparticles with a smooth surface.
Differential Scanning Calorimetry (DSC)
The DSC thermogram of the propolis (Fig. 2-A) showed a sharp endothermic peak at 74°C followed by a weak endothermic peak at 206°C. The DSC thermogram of the ALg NPs (Fig. 2-B) showed a sharp exothermic peak at 238°C, while the DSC thermogram of the Propolis-ALg NPs (Fig. 2-C) showed absence of the endothermic peaks related to propolis. Moreover, it wasn’t observed the sharp exothermic peak at 238°C in Propolis-ALg NPs which become depressed and broad.
In-vitro Cytocompatibility of the ALg-Propolis NPs (MTT Assay)
The in-vitro cytotoxicity of the ALg-Propolis NPs was evaluated using normal retinal pigment epithelial cell line (RPE1) and the results were explained in (Figure 3), it is clear that there is no significant effect on the cell viability of the cells treated with different concentrations of NPs samples. The results obtained indicated that the cell viability decreased only by 13.5% for the highest concentration of the NPs sample.
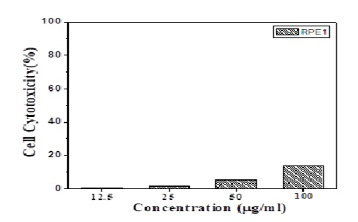
Figure 3: In-vitro cytotoxicity of the Propolis- ALg NPs in normal retinal pigment epithelial cells (RPE1).
Hemolysis Assay
Quantitative evaluation by spectrophotometer revealed that the ALg-Propolis NPs have no hemolytic effect on the red blood cells with clear plasma supernatant similar to the negative control (saline). While, the RBCs which exposed to water (positive control) showed a leakage of hemoglobin into plasma (supernatant).
Clinical Signs
Clinical symptoms of LSDV infected animals started with fever, salivation, ocular and nasal discharges, enlarged superficial lymph nodes and nodular formation. The nodules are round, raised over the skin and 1-5 CC2 in diameter. Udder, teats, eyes, genital tracts and the mucosa of the upper respiratory tract also showed nodular formation. Edema was developed on legs and brisket. Skin rupture was observed in some cases.
Gene Bank Accession
In this study, we reported the genetic characterization of the isolated gene fusion protein and protein P32 in the specimen from cattle reared at Beni-Suef Governorate, Egypt. The sequenced PCR products of both genes of isolates were analyzed, forwarded to the Gene Bank, and registered with accession numbers. The accession numbers for the nucleotide sequences of isolates from Beni-Suef Governorate are MN694826 for the fusion-coding gene and MN694827for the P32 coding gene (Allam et al., 2020).
Treatment of Clinically Infected Animals
Treatment of clinically infected animals revealed that 2 years old cow showed a nodular formation specific to LSDV in the shoulder with 2 cm in diameter (Fig.4-A). After treatment of the animal with Propolis-ALg NPs, the shoulder nodules disappeared and the animal’s skin returned healthy (Fig.4-B).
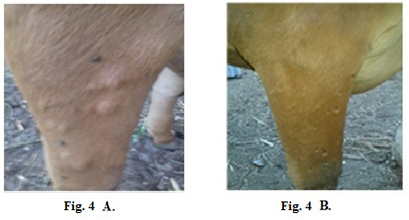
Figure 4: Lumpy skin disease viral in the shoulder of a cow 2 years old- 2 cm in diameter- a photo: (A) before treatment and (B) after treatment with Alginate-Propolis NPs.
Also, 5 years old cow exhibited LSDV in the submaxillary space with 2 cm in diameter (Fig.5-A). It showed the disappearance of the skin nodules after treatment with Propolis-ALg NPs spray (Fig.5-B). While in (Fig.6-A), a cow of 4 years old showed a nodular formation in the eye with 1 cm in diameter. After treatment of the animal with Propolis-ALg NPs eye drops, the eye nodules disappeared, and the eye returned healthy (Fig.6-B).
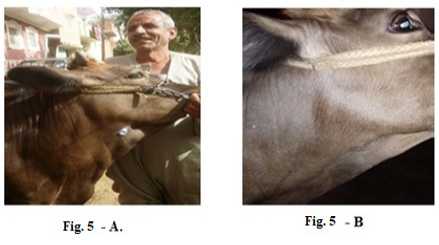
Figure 5: Lumpy skin disease viral in the submaxillary space of a cow 5 years old -2 cm in diameter- a photo: (A) before treatment and (B) after treatment with Alginate-Propolis NPs spray.
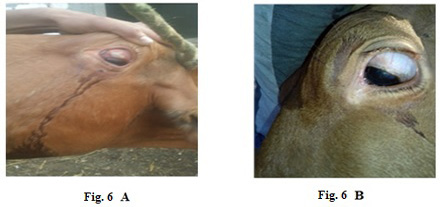
Figure 6: Lumpy skin disease viral in the eye of a cow 4 years old- 1 cm in diameter- a photo: (A) before treatment and (B) after treatment with challenged ALg-Propolis NPs eye drops.
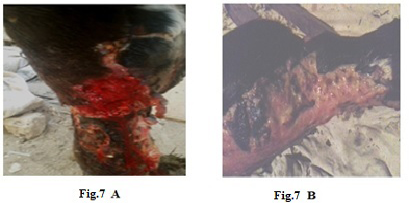
Figure 7: Lumpy skin disease viral in the leg of a cow 6 years old -1cm in diameter - a photo: (A) before treatment and (B) after treatment with challenged ALg-Propolis NPs spray.
Also (Fig.7-A) showed skin rupture in the leg of a cow 6 years old, which is specific to LSDV infection. After the
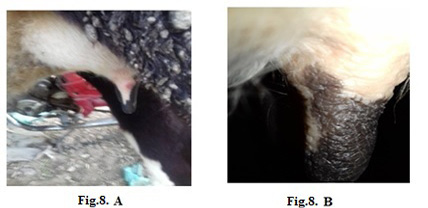
Figure 8: Lumpy skin disease viral in the teat of a cow 5 years old-1 cm in diameter- a photo: (A) before treatment and (B) after treatment with challenged ALg-Propolis NPs spray.
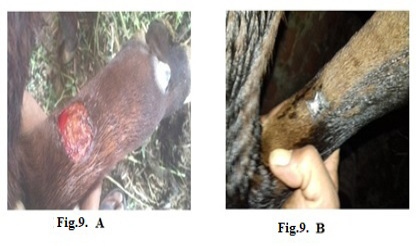
Figure 9: Lumpy skin disease viral in the forelimb of a young cow 1-year-old- 4 cm in diameter- a photo: (A) before treatment and (B) after treatment with challenged ALg-Propolis NPs spray.
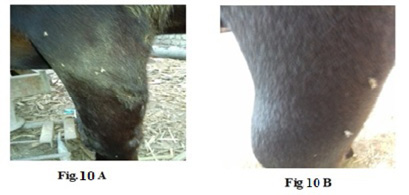
Figure 10: Lumpy skin disease viral Oedema in the forelimb of a cow 4 years old - a photo: (A) before treatment and (B) after treatment with challenged ALg-Propolis NPs.
topical treatment of the animal with Propolis-ALg NPs spray, the wound became healthy (Fig.7-B). Furthermore, 5 years old cow showed nodular formation on the teat with 1cm in diameter (Fig.8-A). After the topical spraying of the animal with Propolis-ALg NPs, the teat nodule disappeared, and the animal appeared healthy (Fig.8-B). Moreover, a cow of 1 year old showed LSDV wounds in the forelimb with 4 cm diameter (Fig.9-A). The wounds disappeared and the animals appeared healthy after the treatment with Propolis-ALg NPs spray (Fig.9-B). Also, after treatment of a cow 4 years old infected with LSDV edema in the forelimb by Propolis-ALg NPs (Fig.10-A), the edema disappeared and the animal appeared healthy (Fig.10-B).
The PCR was run after different treatments with Propolis-ALg NPs or antibiotics to check the presence of LSDV. The results were illustrated in Table 2.
Table 2: Distribution of samples collected and processed from Beni-Suef Governorate after treatment.
| Treatment | Number of examined Samples | Number of Negative by PCR | Recovery percentage % |
| Treated with Propolis-ALg NPs | 20 | 20 | 100.00 |
| Treated with Antibiotics | 15 | 2 | 13.33 |
After treatment the infected animals with Propolis-ALg NPs, the virus was undetectable by PCR with a recovery percentage of 100%. Otherwise, the isolated samples from the infected animals treated with the antibiotic were positive and the virus was still present with a recovery percentage of 13.38%.
After treatment with Propolis-ALg NPs, the temperature decreased to 39oC, the nodules disappeared over the skin within 3-7 days, the superficial lymph nodes became normal and the animal had a good appetite. The percentage of recovery was 100 % in the clinically infected animals group (A) treated with Propolis-ALg NPs as illustrated in Table 3.
The result of the clinically infected animals treated with antibiotic tetracycline 30% (group B) revealed recovery 13.33% after a considerably longer time, 6 weeks more than that taken by the Propolis-ALg NPs. In group B, two animals died after the treatment with antibiotic tetracycline. The post mortem examination showed nodules over the skin and edema on the legs. Also, the gastrointestinal tract showed the presence of nodules. Healing of lesions and complete disappearance of clinical signs was quite rapid among the cattle of group A than group B by five weeks.
Discussion
Alginate is a generally accepted polymer with low cytotoxicity, good biocompatibility, and biodegradability. It already has been used because its biodegradation products are non-toxic and safe (Cheaburu-Yilmaz et al., 2019).
Table 3: Percentage of recovered animals after treatment
| Animals | Number of animals treated with ALg-Propolis NPs Group A | % of animals treated with ALg-Propolis NPs | Number of animals treated with antibiotics Group B | % of animals treated with antibiotics |
| Recovered animals | 20/20 | 100 | 2/15 | 13.33 |
| Total number | 20 | 15 |
MTT assay was used as a quantitative, sensitive, and reliable calorimetric technique that measures the viability of cells. It is known that the reduction of cell viability by more than 30% is considered to have a cytotoxic effect (ISO 10993-5). The different concentrations of prepared NPs showed no significant effect on the cell viability especially, for the highest concentration where the cell viability decreased only by 13.5%. Hence, these results confirmed the cytocompatibility of the ALg-Propolis NPs.
Hemolysis is the rupturing of erythrocytes or red blood cells and leakage of hemoglobin into the plasma. ALg-Propolis NPs would come in contact with blood upon the systemic administration of nanoparticles as drug delivery carriers. This necessitates the hemocompatibility analysis of the ALg-Propolis NPs. Quantitative evaluation of the samples by spectrophotometer revealed that the ALg-Propolis NPs have no hemolytic effect on the red blood cells with clear plasma supernatant similar to the negative control (saline). In contrast, the red blood cells exposed to water (positive control) showed leakage of hemoglobin into plasma (supernatant) displaying apparent hemolysis behavior. This confirmed the excellent hemocompatibility of the ALg-Propolis NPs (Spadari et al., 2019).
Particle size is one of the most important physicochemical characteristics of nanoparticles. Small nanoparticle size can enhance the cellular uptake, efficacy, and bioavailability of the active drug compounds in a formulation. The prepared NPs have a small particle size (Fig. 1) in the nanometer range with an average particle size of 10 nm which in agreement with Barbosa et al. (2019). Therefore, the TEM results suggested that the ALg-Propolis NPs can permeate through cell membranes more effectively due to their small particle sizes. Hence, this can be reflected in the treatment of the LSDV infection in cattle and their rapid wound healing.
The DSC was performed to characterize the physical state of the active ingredient inside the nanoparticles. Moreover, it can provide information about whether the drug is successfully encapsulated within the NPs or not. A perfect inclusion of the drug inside the NPs may cause the disappearance of any Exo and endothermic peaks. The DSC thermogram of the propolis (Fig. 2-A) was in accordance with the results stated by by do Nascimento et al. (2019). The sharp endothermic peak corresponded to water volatilization and starting of melting processes of low molecular weight compounds such as flavonoids and other phenolic compounds present in the propolis. The weak endothermic peak was attributed to the end of the melting transition (Raheem et al., 2019). The DSC thermogram of the ALg NPs (Fig. 2-B) showed a sharp exothermic peak as mentioned by El-Houssiny et al. (2016). This exothermic peak resulted from the degradation of the polymer due to the dehydration and depolymerization reactions; most probably due to the partial decarboxylation of the protonated carboxylic groups and oxidation reactions of the polymer. While the DSC thermogram of the Propolis-ALg NPs (Fig. 2-C) showed the absence of the endothermic peaks related to propolis, indicated that the crystalline structure of the propolis did not form and the Propolis-ALg NPs were in an amorphous state. Moreover, it wasn’t observed the sharp exothermic peak at 238 °C in the Propolis-ALg NPs which become depressed and broad due to the overlap between the propolis and ALg NPs. These results confirmed the successful encapsulation of the propolis in the ALg NPs matrix and indicated that there was a thermal compatibility between them.
The Propolis-ALg NPs play an important role in the treatment of clinically infected animals with LSDV. The results revealed that the ALg-Propolis NPs were an accurate specialist treatment for LSDV which was characterized by withdrawing the symptoms. Also, it was an aid in lowering the feverish cases and improved the general health condition of clinically infected animals. Moreover, the nodular formations were disappeared, and the wounds were rapidly restored. The main constituents of propolis are caffeic acid, p-coumaric acid, benzoic acid, galangin, pinocembrin and chrysin (Hegazi et al., 2019). The propolis is well known to accelerate the wound healing and shorten the time of reepithelialization (Al-Waili et al., 2018). This also observed in our results in which the ALg-Propolis NPs accelerated the wound healing of ruptured wounds in the infected animal more than the group treated with antibiotics. Also, the propolis reduced the wound contamination in our study as the propolis possesses high antimicrobial activity (Hegazi et al., 2019). Moreover, the ALg-Propolis NPs treatment disappeared the nodules over the skin within 3-7 days. On the other hand, propolis was studied previously as an antiviral agent against herpes simplex virus type 1 (HSV-1) (Schnitzler et al., 2010), infectious Bursal Disease Virus and Reo-Virus (Hegazi, et al., 2004; Abdel-Hady et al., 2007), and HSV-1 and HSV-2 which caused a significant decrease in the number of viral copies (Yildirim et al., 2016). Other studies reported that the propolis showed antiviral activity due to interfering with the virion envelope structures, prevention of virus adsorption to host cells (Amoros et al. ,1992; Serkedjieva et al., 1992) or entry into the host cells (Kwon et al., 2020) or by inhibiting the virus RNA replication (Yildirim et al., 2016).
Alginate NPs were frequently used in nanomedicine due to its biodegradability by natural pathways, biocompatibility and its capacity to deliver lipophilic, amphiphilic or hydrophilic drugs. Alginate NPs were used as an antiviral therapeutics carriers against human immunodeficiency virus (HIV) (Joshy et al., 2017). The results indicated that the synthesized ALg NPs represented a potential carrier for zidovudine and increased its efficiency as an anti-HIV drug. Also, the chitosan/alginate NPs encapsulated Bee Venom (CH/AL-BV) were used as a nasal delivery system against porcine reproductive and respiratory syndrome virus (PRRSV) (Lee et al., 2018). The results demonstrated that the CH/AL-BV could be a promising strategy for overcoming the disadvantages of classical vaccination and can be applied as a preventive agent against PRRSV.
The mode of action of nanoparticles was their adsorption on the virus surface which leads to local transformations of the surface, such as agglutination of glycoproteins, thus preventing the virus penetration into the cells (Elechiguerra et al., 2005; Zhong et al., 2010). Therefore, integrating the benefits of propolis as a natural antiviral agent with the innovative profits of nanotechnology generate a novel delivery system that aids in the treatment of clinically infected animals with LSDV.
Conclusion, This study highlighted the intrinsic worth of the developed Propolis-ALg NPs as a promising treatment against LSDV infections. The obtained nanoparticles showed a small particle size in the nanometer scale. Also, the thermal analysis indicated the probable interaction between the propolis extract and the ALg NPs. In addition to that, the cytocompatibility results revealed that Propolis-ALg NPs did not display any significant adverse effect on the cell viability of normal retinal pigment epithelial cells (RPE1). Moreover, the incubation of the red blood cells with Propolis-ALg NPs did not show hemolysis, suggesting that the nanocarrier is safe.
On the other hand, the clinically infected animals with LSDV revealed a complete recovery after giving the Propolis-ALg NPs as the nodular formation was disappeared and the wounds were rapidly restored. Therefore, the Propolis-ALg NPs was a very excellent treatment for lumpy skin disease viral infections (LSDV).
Acknowledgments
This study was supported by the National Research Centre, Egypt (Grant No. 11020201) entitled: “Evaluation of the antimicrobial effect of propolis & Moringaon Fasciola gigantica and Clostridium oedematous (Clostridium novyi) type B”.
Conflict of interest
There are no conflicts of interest to disclose.
Authors contribution
Tarek Korany Farag: Collect and grouping of animals, Observation of the infected animals, Treatment of the infected animals, Help in righting of the article and wrote the discussion related to study in the original manuscript draft. Asmaa S. El-Houssiny: Preparing the the ALg nanopaticles, encapsulation of propolis within ALg NPs, Characterization of the NPs, Studies of cytotoxicity, Discussion of the results and Writing the paper. Eman Hussien Abdel-Rahman: Designer of the experiment, Coordinatization of the team, plan of the study Discussion of the results and wrote and revising the original manuscript draft. Ahmed G. Hegazi: prepared the original idea, Designer of the experiment, Coordinatization of the team, Preparation and extraction of the propolis, conceptualized the aim, design, Did the statistical analysis, plan of the study, Discussion of the results and wrote and revising the original manuscript draft. All authors contributed to revising the final draft of the manuscript. All authors read and approved the final manuscript.
References