Advances in Animal and Veterinary Sciences
Research Article
Artificial Insemination vs Natural Mating and Genetic PRL/PstI Locus Polymorphism and Their Effect on Different Productive and Reproductive Aspects in Duck
Hanaa Mohamed Ghanem1*, Ahmed Ibrahim Ateya1, Rasha Mohamed Saleh2, Mamdouh Said Hussein3
1Department of Animal Husbandry and Wealth Development, Faculty of Veterinary Medicine, Mansoura University, 35516, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Mansoura University, Egypt; 3Department of Theriogenology, Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Mansoura University, Egypt.
Abstract | The aim of this study was to investigate the impact of artificial insemination (AI) versus natural mating and genetic PRL/PstI locus polymorphism on different duck productive and reproductive aspects. The birds were divided into two groups; 5000 female for each group and the data (body weight of birds, hatchability%, fertility %, mortality %, hatching egg % and birth weight of day old chick) were analyzed using PROC MIXED procedure of SAS V9. Concerning PRL/PstI locus polymorphism, blood samples were collected from the experimental birds for DNA extraction and PCR-PstI digestion for a fragment of exon 5 (400-bp) of PRL gene was carried out. AI breeding group had significantly higher productive and reproductive parameters (average egg number, birth weight of day-old chick, fertility and hatchability %) compared to the natural mating group except for mortality %. PCR-PstI of 400-bp of PRL gene revealed two fragments (254 and 146-bp) for genotype TT, three fragments (400, 254 and 146-bp) for genotype TC and undigested fragment (400-bp) for genotype CC. The χ2-test showed that the genotype distributions in the duck population were in Hardy–Weinberg equilibrium (P<0.05). Statistical analysis revealed a significant association between PRL genotypes and the studied traits; where CC genotype was higher than both TC and TT genotypes in these traits. This research highlights the artificial insemination, natural mating, and PRL/PstI locus as candidates for different duck productive and reproductive traits.
Keywords | Duck, Artificial insemination, Natural mating, PRL gene, Productive, Reproductive traits
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 25, 2017; Accepted | April 17, 2017; Published | April 21, 2017
*Correspondence | Hanaa Mohamed Ghanem, Department of Animal Husbandry and Wealth Development, Faculty of Veterinary Medicine, Mansoura University, 35516, Egypt; Email: [email protected]
Citation | Ghanem HM, Ateya AI, Saleh RM, Hussein MS (2017). Artificial insemination vs natural mating and genetic prl/psti locus polymorphism and their effect on different productive and reproductive aspects in duck. Adv. Anim. Vet. Sci. 5(4): 179-184.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.4.179.184
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Ghanem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Muscovy duck (Cairina Moschata), Pekin duck (Anas platy rhynchos domestic) and their cross-bred (Mulards) (Baeza, 2006) represent the main species for duck meat production. The higher performance of modern domestic White Pekin duck than the modern broiler chicken in terms of growth traits was attributed to genetic improvement (Adzitey and Adzitey, 2011).
Artificial Insemination (AI) becomes a very common practice in the current animal husbandry, livestock and poultry breeding programs (Foote, 2002; Gee et al., 2004; Blanco et al., 2009; Dhama et al., 2007; Bakst and Long, 2010) since it enables the breeder to overcome different breeding problems of the livestock production and preserves the genetic material of many species threatened by the damage of their natural habitat, allows the addition of extra males or females in the breeding population (Watson, 2009). The sign of genetically judged cases within a population to improve the reproductive efficiency of poultry is also increased.
Semen collection and the assessment of sperm deliver valued data about male fertility. AI becomes of major concern since it increases mating ratio, utilizes benefits of older males from outstanding performers and effective cross breeding. However, under natural conditions, cross breeding is very successful but occasionally there is thoughtful of color discrimination; some birds will not mate with a male of a different color unless they have been reared together (Dhama et al., 2014).
Prolactin (PRL) hormone is a polypeptide in nature belongs to the growth hormone family and is synthesized in the anteriorpituitary gland of all vertebrates (Wang et al., 2011). In mammals, it is the main regulator for miscellaneous biological activitiesduring pregnancy, lactation and subsequent embryo growth (Byrnes and Bridges, 2005; Bonomo et al., 2007; Lu et al., 2010). Regarding avian species, PRL initiates, up keeps of incubation behavior and rules the follicular development (Sharp et al., 1988; Reddy et al., 2002). The duck PRL gene size is 10kb, has 5 exons and 4 introns, codes for 229 amino acids. Duck PRL gene has sequence identity 92.0%, 91.7% and 91.4% at the cDNA level with PRL of other fowl including chicken, turkey, and quail, respectively (Kansaku et al., 2005). Identification of new polymorphic sites in PRL gene has been focused recently. However, PRL/PstI polymorphism and its association with productive and reproductive traits in duck is scare. Therefore, in addition to reveal the effect of both artificial insemination and natural mating on duck productive and reproductive traits, we aimed to investigate the PRL/PstI variant effect on such traits.
MATERIALS AND METHODS
Experimental Birds And Management
A total number of 10000 Pekin duck and 1000 Muscovy drakewere used as experimental birds. The birds were brooded together in the brooding unit (deep litter system) under the same environmental conditions as one grouptill the age of the first egg (165 days) with average 2.500 to 3.250 kg BW. Concerning microenvironment conditions, 100W electric bulb, 30◦C brooding temperature and 14h of light per dayincreased gradually to 16 h during the laying period. Supplementary lighting was provided as required to maintain the Production (Randall and Bolla, 2008). Good ventilation and fresh air were provided to reduce ammonia concentration in the housewith available water all time. Vitamin E and AD3E with prophylactic antibiotics and anti-coccidial drugs were added to water to control infection and cover any deficiencies. The birds were handled according to the animal care principles during the experiment.
The diet was formulated to meet the nutrient requirements of birds following NRC (1994). The birds were divided into two groups of 5000 female each (5000 female for natural mating (5 female: 1male ratio) and 5000 birds for the artificial group) at the age of the first egg (165 days). The following performance parameters were measured; fertility% (F), hatchability% (H), mortality%, hatching egg%, the number of egg production and body weight of day old chick (DOC). This study protocol was approved by the committee on animal welfare and ethics, faculty of veterinary medicine, Mansoura University.
Semen Collection
A collection of semen was carried out using abdominal massage method. Collection and insemination process occurred twice in a week to produce excellent fertility. Collected semen was assessed for motility, viability, and concentration. The semen of good quality has been diluted to maintain the viability of the sperm and maximize the number of birds that can be inseminated by increasing the volume (Donoghue and Wishart, 2000). The semen diluent was prepared in our laboratory and composed of Sodium Chloride (NaCl) =68 gm, Potassium Chloride (KCl) =17.33 gm, Calcium Chloride (CaCl2) =6.42 gm, Magnesium Chloride (MgCl2) =2.50 gm, Sodium bicarbonate=24.50 gm, Distilled water =10000 ml. normally the ratio of added diluents is (semen: diluents) = (1:1). All chemicals were purchased from Sigma-Aldrich.
Insemination
A 1-mL tuberculinsyringe was used for inseminationsdirectly into the oviduct. In order to evert the oviduct, pressure was applied to the left side of the abdomen around the vent to avert the cloaca and to protrudethe oviduct. Each duck receives a 0.02 mL of diluted semen (containing1-2 × 106spermatozoa). To be able to push out the whole volume of diluted semen 0.1 mL of air was first drawn into the syringe. As the semen expelled by the inseminator, pressure around the vent was released, this assisted the bird in relating sperm in the vagina or the oviduct.
Egg Collection And Incubation
Individual egg production data was recorded once/day and cumulated up to 48 weeks of age. The eggs laid were individually judged as proper or improper for incubation (because too small, too big or with a broken shell). The collection of eggs for incubation was carried out from the second day of the first artificial insemination. Artificial insemination (AI) day was the day 0 of fertilization. After a single insemination, eggs were collected 5 times/ day from the 2nd-day post insemination with grading. After grading, fumigation and storing of the eggs was completed with egg store room temperature between 16 -18°C. Discarding was done for dirty and cracked eggs that were unsuitable for incubation. On the 18th day of incubation, candling was done for identification of the fertile eggs transferred from setter to hatchers in the hatchery for the rest period with recording the fertility, number of hatched and dead chicks.
Data Measurements
The following data was measured and recorded; egg production and the age at first egg laid, birth weight of newly hatched chicks, the egg fertilization rate (F%) (Calculated for each female as the ratio of fertile eggs to eggs incubated, Ozcelik et al. (2006), hatchability rate (H%) and daily mortality rate of each group.
Experimental Samples And DNA Extraction
Whole blood was collected from native female duck population (n=10000) and subsequent F2 generation (n=500) of each breed on disodium EDTA anticoagulant and stored at -20°C for DNA extraction. The Genomic DNA extraction from the collected samples was carried out using DNA extraction kit (Thermo scientific, Lithuania).
PCR-RFLP For PRL Gene
The PCR was done for amplification of a fragment of exon 5 (400-bp) of PRL gene using one pair of primersdesigned according to the published duck DNA sequence with Gen Bank Accession no: AB158611
Forward: 5‘--TGCAAACCATAAAAGAAAAGA -3‘
Reverse: 5‘-CAATGAAAAGTGGCAAAGCAA -3‘
The reaction components of PCR were done in a total volume 25 µL contained: 3 µL DNA, 8.5 µL H2O, 12.5 µL PCR master mix (Jena Bioscience, Germany), 0.5 µL of each primer. The PCR temperature protocol was started by an initial denaturation at 940C for 4 min followed by 34 cycles of 940C for 30s for denaturation, primer hybridization temperature at 520C for 30s, primer extension at 720C for 30s and the final elongation at 720C lasts for 10 min. PCR products of PRL gene subjected to the action of PstI restriction end nuclease (NEW ENGLAND BIOLABS, USA) at 370C for 1 hr. The RFLP reaction volume was done in 20 µL consistedof 5 µL PCR product of PRL gene, 11.5 µL H2O (d.d water), 2.5 µL bufferand 1 µL restriction enzyme. The variable fragments obtained from RFLP were noticed by a garose gel electrophoresis and envisioned under UV by means of gel documentation system.
Statistical Analysis
Allele and genotype frequencies were calculated by direct counting. Hardy–Weinberg equilibrium (HWE) assessed by the Chi-square (χ2) test using POPGENE software (Version 1.31; Yeh et al., 1999).
Associations between PRL genotypes and productive and reproductive traits were analyzed using PROC MIXED procedure of SAS V9 (SAS Inst. Inc., Cary, NC, USA) implementing the following mixed model:
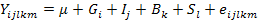
Where:
Yijklm: value of growth traits; Fertility (%), Hatchability (%), Annual egg production, Age at the first egg, Body weight at the first egg, Egg weight on mth chicken; µ: overall mean for each trait; Gi: fixed effect of ith PRL genotypes with 3 levels (i = CC, TC and TT); Ij: fixed effect of insemination technique (j= natural, artificial); Bk: fixed effect of breed with 2 levels (k= Pekin and Muscovy); Sl: random sire effect; eijk: random residual effect.
The Bonferroni correction in LSMEANS statement was used to counteract the problem of multiple comparison adjustments of the P-values for pair-wise comparisons of means. Data are reported as least squares means± standard error of the mean (SE). Also, the resultants P-values were also expressed P value of < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Artificial Insemination Versus Natural Service And Their Effect On Productive And Reproductive Performance Of Duck
The present study showed that natural mating group had the lowest average egg number, body weight, Fand H% compared to artificial insemination group.This may attributed to AI aidedin matingof the males and females having no mating increasing hatchability percentage. However, AI group had the highest mortality % comparing to the natural mating group (Table 1).
Table 1: Comparison of means in A.I and natural mating
|
Traits |
Natural mating |
Artificial insemination |
P-value |
|
Average egg number |
765b±0.18 |
801a±0.57 |
0.024* |
|
Average body size (g) |
60b±0.10 |
100a±0.20 |
0.04* |
|
Mortality % |
14b± 0.01 |
41a± 0. 03 |
0.001** |
|
Fertility% |
60b± 0.39 |
90a± 0.41 |
0.034* |
|
Hatchability % |
49b ±0.05 |
80a±0.33 |
0.001** |
*: indicates significant differences at P <0.05; **: indicates highly significant differences at P <0.01
The outcomes of the present study indicated that AI group achieved higher values for average egg number and average body weight compared to natural mating one. This may be
Table 2: Frequency of genotypes and alleles in the PRL locus
|
Number of genotypes (frequencies) |
Allelic frequencies |
P-value |
||||
|
CC |
TC |
TT |
C |
T |
||
|
Native population (n=10000) |
3480 (34.8) |
4838 (48.38) |
1682 (16.82) |
0.590 |
0.410 |
0.994 |
|
F2 (n=500) |
173 (34.6%) |
242 (48.4%) |
85 (17.0%) |
0.588 |
0.412 |
0.981 |
Deviation from H-W equilibrium was assessed using Pearson’s goodness-of-fit chi-square with (d.f=1)
Table 3: Associations of PRL genotypes with productive and reproductive traits
|
Traits |
CC |
TC |
TT |
P value |
|
Fertility (%) |
89a± 0.63 |
81b± 0.72 |
77c± 0. 59 |
0.03* |
|
Hatchability (%) |
80a± 0. 69 |
71b± 0. 49 |
63c± 0. 61 |
0.001** |
|
Annual egg production (n) |
375.53a± 0. 69 |
300.22b± 0.78 |
275.50c± 0.88 |
0.02* |
|
Age at the first egg (d) |
165.53 ± 0.71 |
167.23 ± 0.76 |
168.76 ±0.89 |
0.55 |
|
Body weight at the first egg (kg) |
3.250± 0.35 |
3.200 ± 0.37 |
3.195 ± 0.40 |
0.63 |
|
Egg weight (g) |
81.51a± 0.45 |
78.23b± 0.79 |
75.43c± 0.80 |
0.01* |
a,b: Means within the same row carrying different superscripts are sig. different at P < 0.05 based on Tukey’s Honestly Significant Difference (Tukey’s HSD) test; *: indicates significant differences at P <0.05; **: indicates highly significant differences at P <0.01
attributed to large size of the Muscovy male compared to that of the Pekin female in the natural mating group. The large size may cause reproductive problems via its effect on egg production, size of the egg and consequently average body weight of the newly hatched chicks. Moreover, natural mating recorded lower F and H%. This may relate toterritorial fighting and homosexual activity behavior between Muscovy male with lower paid attention to the Pekin females, preferential mating (monogamous mating and color discrimination), male large size and its role in transmission of different diseases. Concerning mortality, this study showed that AI group had the highest (41%) (P<0.001) mortality % compared to the natural mating group (14%). The latter may be attributed to the practical applied permanent stress on the flock, no wastage of the fertilized zygotes and the high percentage of the inseminated fertilized eggs. Also, during the technique of AI; uterine prolapse or even syringe penetration may be produced. These stressful factors can be minimized during the processes before and after the technique of insemination and the personnel collecting the semen should have adequate knowledge regarding proper collection methods that may help in excluding various defects or mortality %. However, the natural mating exhibits slow stages without any stress. According to the results, AI practice will develop and propagate economically and profitable viable poultry flocks; hence it will be a substitute for natural mating. However the breeder should be conscious to overcome the mortality problems.
These results are in accordance with those obtained by Brun and Larzul (2003), Gee et al. (2004) and Dhama et al. (2007) who recorded that AI exhibits superior fertility than natural mating. The authors supposed that these results enable rapid genetic improvement rate via increasing selection differential; where thousands of females were mated with one highly selected superior sire. On contrary, Koohpar (2010) indicated that the fertility and hatchability traits were not significantly differ; the F% in the AI flock was higher than the natural mating but without the reasonable level. It was suggested that both more accurate insemination and expected fertility were the principle cause.
Effect of PRL/PstI gene Polymorphism
PCR for PRL gene yielded a fragment length of 400-bp. PCR-PstI of 400-bp of PRL gene revealed two fragments (254 and 146-bp) for genotype TT, three fragments (400, 254 and 146-bp) for genotype TC and undigested fragment (400-bp) for genotype CC. The frequency of PRL genotypes and alleles in the native duck population and the subsequent generation are shown in Table 2. The χ2-test showed that the genotype distributions in the duck population were in Hardy–Weinberg equilibrium (P<0.05) (Table 3). Statistical analysis revealed significant association of PRL genotypes with productive and reproductive traits. Where, CC genotype was higher in the all studied traits than both TC and TT genotypes (Table 3).
In this study, PCR amplification of a fragment of exon 5 of PRL gene generated PCR product length of 400-bp. The restriction fragments obtained by action of PstI restriction end nuclease were; TT genotype with digested (254 and 146-bp) fragments, TC genotype with three fragments (400, 254 and 146-bp) and CC genotype with undigested fragment (400-bp). The χ2-test showed the genotypes distribution in the duck population in Hardy–Weinberg equilibrium (P<0.05). This balance attributed to the higher number of the heterozygous genotype (TC) than those homozygous genotypes (CC and TT) which keeps the balanced allelic frequencies in the population (Abdel- Kafy et al., 2015). PRL/PstI locus was significantly associated with productive and reproductive traits; where CC genotype was higher than both TC and TT genotypes. Association between PRL/PstI and productive and reproductive traits in ducks is scare. However, (Wang et al., 2011) reported PRL/PstI genetic diversity in native Chinese ducks and its association with egg production traits. PCR-PRL-Pst1 digestion of 400-bp resulted in three genotypes similar to the attained genotypes in our study. Moreover, Chi-square fitness test revealed the duck population was in Hardy-Weinberg equilibrium. Regarding the association analysis, results revealed significant association of PRL/PstI polymorphism with egg production and egg weight: where, the ducks with the genotype CC have higher egg production and egg weight than those with the CT and TT genotypes.
The limitation of the present study should be acknowledged. First, further studies need to be done on wide range of duck breeds to establish the association of artificial insemination Vs natural Mating with productive and reproductive traits to determine the best type for poultry farms. Second, limited number of candidate gene markers may influence the conclusion. Accordingly, such shortcoming should be considered in further investigations.
SUMMARY
It can be inferred that in near future, a strategic artificial insemination practice in birds will developand propagate economically viable poultry flocks. Moreover, this study highlights PRL/PstI locus as candidate for duck productive and reproductive traits.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Mohamed Afifi, assistant lecturer of statistic, faculty of veterinary medicine, Zagazig University for his kind cooperation. Also, many cardinal thanks for all members of Biotechnology lab, Faculty of Veterinary Medicine, Kafrelsheikh University for their valuable advices.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHORS’ CONTRIBUTION
HM Ghanem conceived and designed the experiments; HM Ghanem and AI Ateya performed the productive and genetic part of experiments and wrote the manuscript; MSHusseincontributed the semen collection process and the technique of artificial insemination and RM Saleh analyzed the data.
REFERENCES






