Advances in Animal and Veterinary Sciences
Research Article
Supplementation with the Methanolic Extract of Lycium shawii or Rhanterium epapposum Enhances Immune Responses of Broilers
Saleh M Albarrak
Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia.
Abstract | The effects of Lycium shawii and Rhanterium epapposum supplementation on humoral and cellular immune responses of chickens have not been investigated. Chickens supplemented with either R. epapposum or Lycium shawii extracts and challenged with sheep erythrocytes (SRBC) exhibited a significant increase in body weight gain as compared to the control group (P<0.05). The data showed a significant increase in the serum activities of catalase (CAT) and superoxide dismutase (SOD) in the chickens pretreated with the R. epapposum extract (P<0.05). In contrast, only the activities of the SOD and TAC enzymes were significantly elevated in the Lycium shawii recipient group (P<0.05). Significant increases (P<0.05) in the in-vitro phagocytic index of chickens supplemented with either extract was noted, with the phagocytic index remaining significantly elevated (P< 0.01) throughout the experiment only in the R. epapposum group. In response to mitogenic stimulation, lymphocytes obtained from the R. epapposum and Lycium shawii groups consumed significantly higher amounts of glucose relative to the control group (P< 0.01 and P< 0.05, respectively). Chickens pretreated with Lycium shawii had significantly higher levels of serum IgM (P<0.05), with the IgG levels being significantly higher in the chickens pretreated with either extract, as well as in the SRBC group (P<0.01). The IgG levels remained significantly elevated (P<0.01) in the Lycium shawii recipient chickens until the experiment’s termination. It can be concluded that the plant extracts used in the present study had stimulatory effects on the chicken’s immune systems and broilers’ performance in general.
Keywords | Lycium shawii, Rhanterium epapposum, Immune responses, Ross chicken, Antioxidants
Received | May 20, 2021; Accepted | July 14, 2021; Published | September 25, 2021
*Correspondence | Saleh M Albarrak, Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia; Email: [email protected]; [email protected]
Citation | Albarrak SM (2021). Supplementation with the methanolic extract of Lycium shawii or Rhanterium epapposum enhances immune responses of broilers. Adv. Anim. Vet. Sci. 9(11): 1816-1828.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1816.1828
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Albarrak et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Because of our expanding knowledge regarding the role of antioxidants and free radicals in health and sickness (Wang et al., 2019), there has been an increasing interest in many traditional herbs as sources of antioxidants. The enrichment of plants with antioxidants as well as immunomodulatory agents is well established, with pharmacological activities largely depending on the presence of bioactive compounds such as alkaloids, flavonoids, and phenols in the plant extracts (Xu et al., 2017; Roleira et al., 2015).
The desert of Saudi Arabia harbors many plants utilized in traditional medicine. Among these plants are Lycium shawii and Rhanterium epapposum (Aldoweriej et al., 2016). The Lycium shawii, locally known as “Awsaj,” is a species of thorny shrub that belongs to a genus of flowers producing plants in the nightshade family, Solanaceae. The genus of Lycium shawii (Solanaceae) includes more than 90 species of thorny shrubs located in tropical areas. Plants of the Solanaceae family display a broad variety of secondary metabolites, with diverse antimicrobial, antioxidant, anti-inflammatory, and anti-cancer properties. Owing to such properties, they play significant roles in nutritional and pharmaceutical applications (Lee et al., 2012; Usha et al., 2016; Almoulah et al., 2017). Recent studies have confirmed the abundance of many biologically active components, such as flavonoids, terpenoids, and alkaloids, in Lycium species (Phondani et al., 2016). Traditional healers have been using a dry powder made from the aerial shoots and flower parts of the L. shawii as an anti-diabetic and hypotensive drug (Jia et al., 2003; Tahraoui et al., 2007). Another study has demonstrated that the L. shawii extract exhibits hypoglycemic activity in vivo (Sher and Alyemeni, 2011). Additionally, extracts made from L. shawii have been shown to possess antiplasmodial, antitrypanosomal, antimicrobial, and antioxidant actions (Fuchs and Steller, 2011; Ali et al., 2020a).
Rhanterium epapposum is another medicinal plant found in Saudi Arabia. It is a little bush with an exceedingly branched-out stalk that reaches heights of up to 70 cm. It has moderately limited leaves and yellow blooms (Demirci et al., 2017). R. epapposum is locally known as “Al-Arfaj,” and has been utilized in conventional medicine as a medication for skin illnesses and gastrointestinal issues (Younis and Adam, 2008; Phondani et al., 2016). The essential oil extracted from the leaves, flowers, and stems of R. epapposum growing in the northern part of Saudi Arabia was shown to be rich in monoterpenes that possess biological activities (Awad and Abdelwahab, 2016). The major component was linalol, representing 55.6% of the oil. Non-terpenoid aliphatic and fragrant structures were also detected by the study (Awad and Abdelwahab, 2016). Recent studies have demonstrated the anti-microbial activity of R. epapposum oil (Demirci et al., 2017; Mohammed et al., 2019). Adam et al. (2011) examined different R. epapposum extracts in relation to a few pathogenic microbes, including S. aureus, B. cereus, and P. vulgaris. Their study reported that the methanol extracts’ effects were modest (Adam et al., 2011). Boussoussa et al. (2016) have also done some screening studies and demonstrated the antimicrobial effects of R. epapposum (Boussoussa et al. 2016). Larvicidal effects have been also reported for the ethanolic extract of R. epapposum (Eltahir and Dahab, 2019). Phytochemical analysis of the R. epapposum ethanolic extract revealed the presence of flavonoids, alkaloids, triterpenes, and tannins, with alkaloids and flavonoids being most abundant (Eltahir and Dahab, 2019).
The effects of Lycium shawii and R. epapposum supplementation on poultry have not been investigated. To highlight the potential use of medicinal plants for therapeutic and preventive purposes, the present study attempted to examine the effects of methanol extracts of Lycium shawii and R. epapposum on chickens’ humoral and cell-mediated immune responses. The Ross breed of broilers was chosen to determine the effects of the extracts given in drinking water on antibody titers, body weight, daily weight gain, daily feed consumption, and feed conversion ratio. In order to complete the assessment, a leukogram, phagocytic index (in vitro), glucose consumption test, and histopathology of the spleen and bursa were also performed. The serum activity of antioxidative enzymes, such as superoxide dismutase, and catalase, as well as the total antioxidant capacity, were quantified. The indicator parameters were evaluated from 1 day up to 42 days of age in a controlled experiment carried out in the chicken housing facilities available in the Agricultural and Veterinary Research Station at Qassim University.
MATERIALS AND METHODS
Birds treatment and study design
The current investigation utilized 60 unsexed one-day-old broilers (Ross breed) compassionately provided by the Alwatanya company for poultry. The birds were randomly gathered into four groups of 15 birds each. Birds were placed in one squared meter of the pen using an allocating wire network. They were given 24 hours of light: sunlight during the day and artificial light during the evening. The chicks were served with starter and finisher feed till the end of the investigation (week 6). All chicks were raised under indistinguishable environmental, dietary, and biosecurity conditions. The immunization program was accurately applied to all birds involved in the experiment. Feed and water were given to all chicks as needed. The chicks were handled and treated as per the guidelines of the animal care panel at Qassim University. The compositions of their starting and finishing diets are listed in Table 1.
Chicks in the first group were given the starter and finisher diets and considered the control group (C). Chicks in the second group were fed on the starter and finisher and inoculated intramuscularly with sheep red blood cells (SRBC). They were considered the challenged control group (SRBC group). Chicks in the third and fourth groups were treated similarly to the SRBC group, but also received one of the extracts in water for the first two weeks of life and were considered the treatment groups (R. epapposum or Lycium shawii group). The suggested dose was 18 mg/bird, on the understanding of day-by-day water utilization. To the best of our knowledge, studies on plant extracts supplementation to broilers in drinking water are lacking. The extract dose was chosen based on a previous study (Alharbi et al., 2017) where each rat (average body weight 150 g) received approximately 37.5 mg of the plant extract orally. The average body weight for a 1- to 2-week-old broiler chicken is approximately 72 g. The dose was calculated as follows:
Table 1: Calculated composition of the diets fed for different treatments throughout the experiment.
| Calculated composition (%) | Diet type | |
| Starter (1day-21 day) | Grower (22-42 day) | |
| Moisture | 8.45 | 11.66 |
| Crude Protein | 21.16 | 20.11 |
| Crude Fat | 4.04 | 5.98 |
| Crude Fiber | 2.77 | 2.90 |
| Starch | 39.11 | 37.76 |
| Ash | 4.99 | 5.65 |
| Calcium | 1.22 | 0.82 |
| Phosphorus | 1.01 | 1.03 |
| Chloride | 0.52 | 0.47 |
| Manganese | 1.22 | 1.32 |
| Potassium | 1.38 | 1.02 |
| Premix (Vitamins+Mineral mix) | 1.77 | 1.98 |
SRBC was used as a foreign antigen for the induction of specific antibody responses. Thirty ml of whole blood from a healthy ewe was gathered into sterile vacutainer tubes containing an equivalent volume of Alsever׳s buffer 0.1, molar pH 6.1. The cells were separated by centrifugation at 900 g for 10 minutes. The cells were then washed multiple times with PBS (pH 8) and suspended in PBS to a 1% concentration to be utilized for the birds’ inoculation (Trombetta et al., 2018).
Growth performance observations and feed conversion ratio
The birds’ body weight was read weekly until the completion of the study, using a second decimal scale. To determine the feed efficiency, body weight, feed consumption, weight gain, and feed conversion were assessed weekly and then calculated on a three-week-interval basis. Feed conversion (kilograms feed intake per kilograms BW gain) was calculated for each group.
Relative weights of lymphoid organs
Three birds from each group were picked randomly, weighed separately, and slaughtered on the 14th, 28th, and 42nd days of the experiment. Lymphoid organs (bursa Fabrica and spleen) were dissected, weighed, and calculated as a percentage of live body weight.
Plant extracts preparations
Plants were recovered from their habitat during the flowering period. The airy parts of the plants were washed under pouring tap water, air-dried, ground, and then extracted. 200 g of the powdered plant were resuspended with 2L of methanol (99.9%), stirred, and left for 3 days (73 hours), followed by filtration. The filtrate was collected in a clean flask. This process was repeated 4 times until total exhaustion, and clear filtrate were obtained. The methanol extracts were collected under reduced pressure using a Rotary Evaporator (BUTCHI, Switzerland) at a temperature not passing 50 °C, and the yield percentages were noted. The concentrated extract was saved at – 4°C.
Extracts preparations for gas chromatography-mass spectral analysis (GC-MS analysis)
Twenty gm of the plant powder were combined with 100 ml of methanol (99.9%) and left for 24 hours, succeeded by filtration utilizing Whatman No.1 filter paper. The debris was excluded, and then the powder was dehydrated with sodium sulfate to discard moisture traces (Soumya et al., 2014).
GC/MS analysis
Analysis of the methanol extracts using GC/MS was done parallel to a method described by Soumya et al. (2014) utilizing Agilent GC (Model 6890N coupled to 5973 Mass Selective Detector, USA) (Soumya et al., 2014). To confirm the presence of phytochemicals, the obtained results were compared with an in-built main library (NIST08.L). GC/MS was fortified with Elite -5MS (5% diphenyl/ 95% dimethyl poly siloxane), 30 x 0.25 mm x 0.25 μm df. For GC/MS detection, electron ionization energy of 70Ev was applied, with absolute helium gas as the carrier, at a fixed current flow of 1 ml/minute. An injection capacity of 2μl was engaged (split ratio 10:1) with an automatic temperature of 250 oC and ion source temperature maintained at 200 oC. The oven heat was modified from 110 ºC (isothermal for 2 min), with a rise of 10 oC /minute, to 200 oC, then 5 oC/ minute to 280 oC for a 9-minute isothermal time. Mass spectra were taken at 70 eV; a scan interval of 0.5 sec and pieces from 45-450 Da were used. The complete GC/MS operating time was 36 minutes. The corresponding percentage of every integral was estimated by contrasting its average top area with the total areas, utilizing software modified to deal with mass spectra and chromatograms.
Total phenolic content (TPC)
A gram of dried sample was reconstituted with 25 ml of methanol (70%) (v/v). The suspensions were agitated actively in a shady container for 100 minutes at 100 rpm, followed by centrifugation for 10 minutes at 3,225 g. The supernatant was collected and the pellet was re-extracted doubly with 15 ml methanol (70%) for determination of TPC and antioxidant activity. The extract was saved in the dark at – 20 oC and analysis was done within 48h to avoid oxidation. The TPC was measured according to the Folin-Ciocalteu spectrophotometric analysis, and the obtained results were linked to a standard curve of previously organized gallic acid (GA) solution (Lu et al., 2007). TPC was shown as mg of gallic acid equivalents (GAE) per gram of dried powder (mg of GAE g –1 dw).
The DPPH scavenging activity assay
The DPPH reagent (1, 1-dipheny1-2-picrylhydrazyl) was utilized to determine the fundamental scavenging activity for the dried sample extract using a modification of the method by Lu et al. (2007). 0.1 ml of the sample was mixed with 2.9 ml of 6x10-5 mol of DPPH (methanolic solution). The mixture was kept in a dark place for one hour, and the absorbance was read at 517 nm. The Trolox calibration curve was constructed as a function of DPPH radical scavenging activity and expressed in %. The obtained data were shown as µmol of Trolox equivalent (TE) per gram of sample (µmol TE g-1dw).
In-vitro carbon clearance assay
This assay was utilized to assess the phagocytic activity on the last day of the experiment (day 42). 1.5 ml of blood from each animal in the three groups was collected in heparin-containing tubes (50 IU/ml). 6 µl of the supernatant portion of India ink (Pelikan AG D-3000, Hanover, Germany) was injected in each sample. After mixing, each sample was split into 3 equal aliquots, which were kept at 37°C for either 20 or 40 mins. Following incubation, 150 µl of each aliquot was mixed with 2 ml of PBS and spun at 500 g for 4 mins. The supernatant fraction was examined spectrophotometrically at 535 nm, with the background considered as zero. The absorbance levels declined with time as carbon was phagocytosed. Optical density (OD) values were transformed into a log2 scale, and the phagocytic index was calculated as the negative of the slope of the regression of optical density (log2) on time (h).
Glucose consumption test (Lymphocytes proliferation)
To purify leukocytes, red blood cells (RBCs) in whole blood were lysed using a lysis buffer composed of 90g of NH4Cl (0.155M), 10g of KHCO3 (0.01M), and 370 mg of EDTA (0.1mM) dissolved in 1 liter of double-distilled water, filtered with a 0.22-micron filter, and used at a dilution of 1:10. 2 ml of the lysis buffer was added to 200 µl of whole blood and kept at RT for 5 mins, succeeded by spinning at 300 x g for lysis buffer removal. Lymphocyte proliferation was assessed using the glucose consumption test, as previously reported by Kosti et al. (2010). Phytohemagglutinin (PHA) was used as a T cell-specific mitogen. Using 24-well plates, lymphocytes were seeded in triplicate in the absence or presence of 5 μg/ml PHA. Each well had 200µl of culture medium (RPMI, Sigma Aldrich) containing 2 × 106 cells. The cultures were incubated at 37oC in a humidified 5% CO2 incubator for 72 hours. Using commercial kits (Human GmbH, 65205 Wiesbaden, Germany), glucose was quantified in the medium by assessing changes in the optical density at 500 nm due to NAD reduction following glucose consumption by hexokinase. Lymphocyte proliferation was estimated as the quantity of glucose (mg/dl) utilized minus the glucose concentration of stimulated cell culture of control samples.
Blood parameters and hematological measurements
Blood samples were collected from 3 birds/ group at days 14, 28 and 42 via the jugular vein into plain tubes. The collected blood was centrifuged at 900 xg for 10 minutes and left overnight at room temperature to clot. The separated serum was collected and saved in clean sterile tubes at -20°C until required for use.
Serum activities of total antioxidant capacity (tac), superoxide dismutase (sod) and catalase (cat)
Assessment of the antioxidant activities was performed via quantification of the serum levels of TAC, SOD, and CAT using commercially available kits (Biodiagnostic kits, Cat. No. 2376 and 2563 and 2552, respectively).
Antibody titers
The titers of total IgM and IgG were quantified using commercial ELISA Kits following the manufacturer’s instructions (SunLong Biotech Co., LTD).
Leukogram
Heterophil % (H), lymphocyte % (L), and monocyte % (M), in addition to calculated Heterophil/lymphocyte ratio (H/L), were counted by the cross-sectional method on blood smears, as described by Cray and Zaias (2004). The number of leucocytes counted/slide was 100, and the counted cells were presented in percentages. The total leucocytes count (TLC) was counted in blood collected in heparinized tubes by improved Neubauer hemocytometer using Natt and Herrick solution (1:200 dilution), as described by Campbell and Ellis (2007).
Spleen and bursa fabrica histology
Spleen and bursa Fabrica tissue specimens were obtained and fixed in neutral buffered formalin (10%) and embedded in paraffin wax. Thin sections of 4-5µm in thickness were mounted on slides that were stained with hematoxylin and eosin (H and E) for the microscopic examination of changes, according to the method defined by Guo et al. (2018).
Statistical analysis
Obtained results were assigned for statistical analysis using GraphPad Prism version 5 software. One-way ANOVA was utilized for the detection of significant variations among the groups. In case of significant differences, the Student-Newman-Kuels test was done. Differences were considered to be significant when p < 0.05. All data were recorded separately.
RESULTS AND DISCUSSION
Chemical composition of r. epapposum and lycium shawii
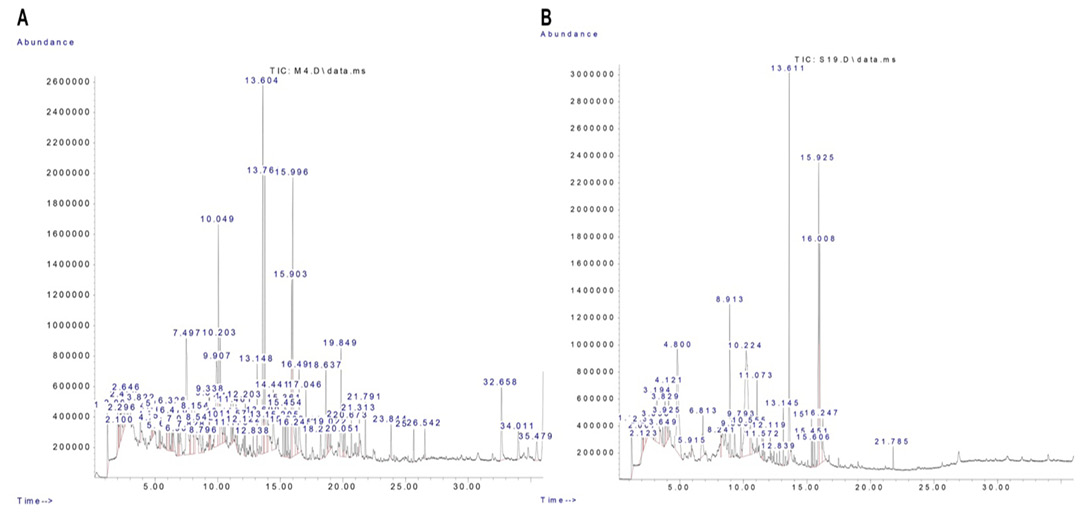
Signal peaks associated with segregated components were detected by GC-MS in the R. epapposum and Lycium shawii extracts (Figure 1). Noteworthy constituents are recorded in Table 2.
The total phenolic content (TPC) and antioxidant activity
The TPC of the Lycium shawii and R. epapposum (Arfag) extracts were quantified, and results are shown in Table 3. The TPC of the methanolic extract of Lycium shawii was 166 mg GAE 100 g-1 dw, which corresponds to observations reported in previous studies. The type of solvent used in the extraction process has been shown influence the TPC, with the methanol extract having a significantly elevated level of phenolics compared to ethanol, ethyl acetate, and water extracts. In contrast, the TPC of the R. epapposum extract was significantly elevated and reached up to 404 mg GAE 100 g-1 dw.
Table 2: Compounds detected by area from R. epapposum and Lycium shawii extracts
| Lycium shawii | R. epapposum | ||||
| Name | Peak % area | RT | Name | Peak % area | RT |
| Guanethidine | 6.44 | 3.19 |
Benzenepropanoic acid, β-amino-4-methoxy- |
6.12 | 7.49 |
| 2-Cyclohexylpiperidine | 2.87 | 6.81 | 1-Dodecanol, 3,7,11-trimethyl- | 5.64 | 9.90 |
| Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- | 4.57 | 8.91 | 1.4-Isopropyl-1,6-dimethyl1,2,3,4,4a,7,8,8a-octahydro-1naphthalenol | 6.00 | 10.04 |
| D-Streptamine, | 19.6 | 10.2 | n-Hexadecanoic acid | 8.44 | 13.6 |
| n-Hexadecanoic acid | 11.5 | 13.6 | 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one | 6.60 | 13.7 |
| 9,12-Octadecadienoic acid (Z,Z)- | 11.2 | 15.9 | 9,12-Octadecadienoic acid (Z,Z)- | 4.84 | 15.9 |
| 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | 9.88 | 16.0 | 9,12,15Octadecatrienoic acid, (Z,Z,Z)- | 9.89 | 15.9 |
| Oxiraneoctanoic acid, 3-octyl-, cis- | 1.85 | 16.2 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester, trans- | 3.63 | 19.8 |
Table 3: Total phenolic compounds (TPC) and antioxidant activity of R. epapposum or Lycium shawii extracts.
| Item | Lycium shawii | R. epapposum |
|
TPC [mg GAE 100 g-1 dw] |
166 ±0.28 | 404 ±2.91c |
|
Antioxidant activity [μmol TE 100g-1 dw]* |
71.5 ±1.01 | 122 ±14.19c |
Values within the same raw having the letter c are significantly different from other value at P<0.001. dw: dry weight, *: DPPH radical scavenging activity (BPPH-RSA). (mean±SE).
Antioxidant enzymes
As shown in Table 4, the activities of the serum antioxidant enzymes were determined in all the chicken groups. In comparison with the control group, our data showed a significant increase in the serum activities of CAT (P < 0.01) and SOD (P < 0.05) in the chickens supplemented with R. epapposum extract for 14 days. In contrast, treatment with Lycium shawii extracts for the first 14 days of life resulted in a significant increase in the activity of SOD only (P < 0.05). At day 28 of age, sera samples obtained from the R. epapposum supplemented/SRBC challenged group exhibited a significant increase in the activities of CAT (P < 0.05), SOD (P < 0.05), and total antioxidant capacity (P < 0.05) compared to the control group, while those of the Lycium shawii supplemented/SRBC challenged group was no different than the control group. However, the activities of the serum CAT and SOD appeared to be significantly elevated in 28-day-old chickens that received no extract treatment, but were challenged with SRBC, as compared to the control group (P < 0.05). At the end of the experiment, on day 42, the serum activities of the three examined antioxidant parameters were significantly higher in the R. epapposum group compared to the control group (P < 0.05). In contrast, only the SOD enzyme activity and the total antioxidant capacity were significantly elevated in the Lycium shawii recipient group (P < 0.05). Likewise, both SOD and the total antioxidant capacity were significantly higher in the SRBC group at day 42 of age compared to the control group (P < 0.01 and P < 0.05, respectively).
Phagocytic activity
As shown in Table 5, the in-vitro phagocytic index of chickens supplemented with either Lycium shawii or R. epapposum extracts and challenged with SRBC was determined at the time points 14, 28, and 42 days post-treatment. Our data showed a significant increase in the in-vitro phagocytic index (P < 0.05) of chickens at day 14 post-Lycium shawii or R. epapposum extract supplementation compared to the control group. On day 28, the phagocytic index was significantly higher (P < 0.01) relative to the control group only in chickens supplemented with R. epapposum extract, whereas the Lycium shawii and SRBC groups were no different than the control group. On the last day of the experiment, day 42, the in-vitro phagocytic index was significantly higher than the control group only in the the R. epapposum and SRBC groups (P < 0.01), while the Lycium shawii group did not show any significant differences.
Lymphocyte proliferation
Assessment of the effects of examined plant extracts on lymphocyte proliferation was done using the glucose consumption test. As shown in Table 6, our results demonstrated that the lymphocytes of chickens in the R. epapposum and Lycium shawii groups consumed significantly higher amounts of glucose in comparison to those of the control group (P < 0.01 and P < 0.05, respectively). Nevertheless, glucose consumption was significantly elevated in the SRBC group (P < 0.05) compared to the control group.
Table 4: Serum antioxidant enzymes of chickens supplemented with R. epapposum or Lycium shawii and challenged with SRBC.
| Sampling day | Groups | Catalase ul/l | Total antioxidant capacity mM/l | Superoxide dismutase U/ml |
| 14 | Control | 281 ±5.36 | 2.11±0.56 | 345 ±4.65 |
| R. epapposum | 345 ±7.18b | 2.74 ±0.47 | 399 ±5.11a | |
| Lycium shawii | 296 ±5.11 | 2.32 ±0.43 | 385 ±3.77 a | |
| 28 | Control | 204 ±3.75 | 2.33 ±0.23 | 296 ±4.11 |
| SRBC | 314 ±9.01a | 2.64 ±0.43 | 351 ±3.98a | |
| R. epapposum + SRBC | 342 ±11.32a | 3.44 ±0.22a | 377 ±3.77a | |
| Lycium shawii + SRBC | 222 ±6.43 | 2.73 ±0.43 | 284 ±2.42 | |
| 42 | Control | 258 ±7.09 | 2.88 ±0.17 | 285 ±4.11 |
| SRBC | 272 ±3.74 | 3.53 ±0.29a | 381 ±3.76b | |
|
R. epapposum + SRBC |
311 ±2.43a | 3.66 ±0.41a | 342 ±2.64a | |
| Lycium shawii + SRBC | 229 ±3.54 | 3.73 ±0.32a | 333 ±2.43a |
Values within the same day having the letters a or b are significantly different from the control value at P<0.05 and P<0.01, respectively. Mean± Standard error.
Table 5: In vitro phagocytic index of chickens supplemented with R. epapposum or Lycium shawii and challenged with SRBC.
| Sampling day | Groups | Phagocytic index |
| 14 | Control | 0.0045±0.0002 |
| R. epapposum | 0.0095±0.0001a | |
| Lycium shawii | 0.0089±0.0001a | |
| 28 | Control | 0.0095±0.0001 |
| SRBC | 0.0080±0.0002 | |
|
R. epapposum + SRBC |
0.0330±0.0004b | |
|
Lycium shawii + SRBC |
0.0086±0.0008 | |
| 42 | Control | 0.0006±0.0001 |
| SRBC | 0.0125±0.0005b | |
|
R. epapposum + SRBC |
0.0330±0.0008b | |
|
Lycium shawii + SRBC |
0.0077±0.0012 |
Values within the same day having the letters a or b are significantly different from the control value at P<0.05 and P<0.01, respectively. Mean± Standard error.
Table 6: Glucose consumed (mg/dl) by lymphocytes stimulated With PHA of chickens pretreated with the R. epapposum or Lycium shawii extract and challenged with SRBC.
| Groups | Glucose in the medium (mg/dl) after 72hrs of incubation | ||
| Without PHA | With PHA | Glucose consumed | |
| Control | 37.9 ±3.43 | 29.3 ±2.76 | 8.53 ±1.04 |
| SRBC | 34.1 ±4.65 | 46.4 ±5.99 | 13.6 ±1.32a |
|
R. epapposum + SRBC |
35.7 ±2.44 | 52.4 ±2.64 | 18.6 ±3.54b |
|
Lycium shawii + SRBC |
36.6 ±1.85 | 55.7 ±3.76 | 16.5 ±2.00a |
Values the letters a or b are significantly different from control value at P<0.05 and P<0.01 respectively. Mean± Standard error.
Serum levels of total IgM and total IgG
The total levels of IgM and IgG in the examined groups are shown in Table 7. Serum samples were obtained from each group at 14, 21, 28, and 42 days of age for quantifying the levels of total IgM and IgG using an isotype-specific Elisa kit. Lycium shawii extract-pretreated chickens aged 14 days had significantly higher levels of IgM (P<0.05) compared to the control group. The IgM levels remained significantly elevated on day 21 of age in the Lycium shawii group (P<0.05), with the total IgG levels being significantly higher in the chickens pretreated with either extract, as well as in the SRBC group (P<0.01). On day 28, only Lycium shawii recipient chickens had significantly higher titers of total IgM and total IgG (P<0.05), with the IgG levels being significantly elevated until the end of the experiment (day 42) (P<0.01).
Table 7: Concentrations of total IgM and total IgG in serum samples obtained from chickens pretreated with the R.epapposum or Lycium shawii extract and challenged with SRBC
| Sampling day | Groups | IgM ug/ml | IgG ug/ml |
| 14 | Control | 3.24 ±0.43 | 2.91 ±0.41 |
| R. epapposum | 3.66 ±1.32 | 2.54 ±1.05 | |
| Lycium shawii | 6.42 ±0.65a | 3.43 ±1.11 | |
| 21 | Control | 3.88 ±0.54 | 3.62 ±0.71 |
| SRBC | 4.46 ±0.88 | 9.42 ±1.21b | |
| R. epapposum | 4.53 ±0.52 | 8.42 ±1.19b | |
| Lycium shawii | 6.63 ±0.32a | 9.54 ±1.01b | |
| 28 | Control | 4.32 ±0.87 | 6.67 ±1.43 |
| SRBC | 3.67 ±1.32 | 6.90 ±2.31 | |
| R. epapposum | 3.75 ±1.90 | 6.55 ±2.43 | |
| Lycium shawii | 6.44 ±1.12a | 9.27 ±1.43a | |
| 42 | Control | 2.66 ±1.09 | 3.78 ±1.82 |
| SRBC | 3.94 ±1.81 | 3.16 ±2.43 | |
| R. epapposum | 4.51 ±1.43 | 3.54 ±1.08 | |
| Lycium shawii | 3.53 ±1.33 | 8.54 ±0.32b |
Values within the same day having the letters a or b are significantly different from control value at P<0.05 and P<0.01 respectively. Mean± Standard error.
Body weight gain and feed conversion
As shown in Table 8, chickens supplemented with either R. epapposum or Lycium shawii and challenged with SRBC exhibited a significant increase (P < 0.05) in body weight gain compared to the control group. However, no significant differences in the rate of feed conversion were observed among the examined groups.
Table 8: Bodyweight gain, individual feed consumption, and feed conversion of chickens pretreated with the R.epapposum or Lycium shawii extract and challenged with SRBC.
| Groups | initial body weight (gm) | Final body weight (day 42) | Δ body weight (body gain) | Individual feed consumption | Feed conversion |
| Control | 45.0 ±2.54 | 3111 ±8.22 | 3066 ±5.76 | 4405 ±8.97 | 1.44 ±0.33 |
| SRBC | 45.7 ±3.11 | 3191 ±7.13 | 3145 ±6.23 | 4414 ±4.08 | 1.40 ±0.39 |
| R. epapposum + SRBC | 45.5 ±1.99 | 3390 ±4.11 | 3352 ±5.99a | 4441 ±4.81 | 1.33 ±0.12 |
| Lycium shawii + SRBC | 47.5 ±2.73 | 3284 ±4.60 | 3221 ±4.81a | 4432 ±3.51 | 1.34 ±0.19 |
Values with the letter a are significantly different from control value at P<0.05. Mean± Standard error.
Relative weights of spleen and bursa
Table 9 shows the relative weights of spleen and bursa of chickens challenged with SRBC following treatment with either R. epapposum or Lycium shawii. The spleen and bursa weights of the 14-day-old chickens supplemented with R. epapposum was significantly increased (P < 0.05) over the control group, whereas Lycium shawii treated-chickens of the same age were no different than the control group. Similar observations were noted on day 28, at which spleen and bursa weights were significantly increased (P < 0.05) only in the R. epapposum + SRBC group compared to the control group. On day 42, the bursa weights were significantly higher (P < 0.05) in both of the extract recipient groups, whereas the spleen weight increased significantly in the R. epapposum treated-group only (P < 0.05).
Table 9: Relative weights of spleens and bursa of chickens challenged with SRBC following pretreatment with the R. epapposum or Lycium shawii extract.
| Sampling day | Groups | Spleen weight (%) | Bursa weight (%) |
| 14 | Control | 0.155 ±0.01384 | 0.234 ±0.0163 |
| R. epapposum | 0.205 ±0.0636a | 0.285 ±0.0165a | |
| Lycium shawii | 0.164 ±0.0550 | 0.298 ±0.0241a | |
| 28 | Control | 0.133 ±0.0354 | 0.265 ±0.0254 |
| SRBC | 0.138 ±0.0940 | 0.236 ±0.0166 | |
| R. epapposum + SRBC | 0.264 ±0.0253a | 0.281 ±0.0123a | |
| Lycium shawii + SRBC | 0.263 ±0.0431 | 0.254 ±0.0423 | |
| 42 | Control | 0.126 ±0.0265 | 0.235 ±0.0176 |
| SRBC | 0.122 ±0.0365 | 0.254 ±0.0162 | |
| R. epapposum + SRBC | 0.182 ±0.0265a | 0.286 ±0.0251a | |
| Lycium shawii + SRBC | 0.128 ±0.0213 | 0.294 ±0.0333a |
Values within the same day having the letter a are significantly different from control value at P<0.05. Mean± Standard error.
Leukocyte counts
As shown in Table 10, the data revealed a significantly higher L% (P < 0.05) and lower H/L ratio (P < 0.05) in chickens aging 14 days treated with either R. epapposum or Lycium shawii extracts relative to the control group. We noted similar observations on day 28 of age, at which the L% was significantly increased (P < 0.05), and the H/L ratio was significantly reduced (P < 0.05) in the extract-treated groups compared to the control group. However, on day 28, the total leucocyte count (TLC) and H% were significantly elevated (P < 0.05) in the SRBC group relative to the control group. On day 42, a significant increase in the L% (P < 0.05) and a significant reduction in the H/L ratio (P < 0.05) were recorded only in the R. epapposum group, whereas the TLC, H, and M% were significantly higher in the SRBC group compared to the control group.
Histology of spleen and bursa
At the end of the experiment (Day 42), a histological evaluation of spleen and bursa was performed. Bursa of control and challenged chickens showed lymphoid follicles with scattered evacuation, depletion and medium condensation of lymphocytes in cortical and medullary regions (Figure 2A and 2B, respectively). Bursa of chickens challenged with SRBC post-treatment with Lycium shawii exhibited lymphocyte depletion, while bursa of chickens pretreated with the R. epapposum extract showed condensation of cortex with lymphocytes with large empty spaces (Figure 2B and 2C, respectively). On day 42, the spleens of the control chickens had normal splenic histology and architecture (Figure 3A). The spleens of challenged chickens (SRBC group) showed decreased red pulp with a few sections of white pulp (Figure 3B). Spleen of chickens challenged with SRBC post-treatment with Lycium shawii or R.epapposum exhibited mild and severe increases in white pulp (Figure 3C and 3D).
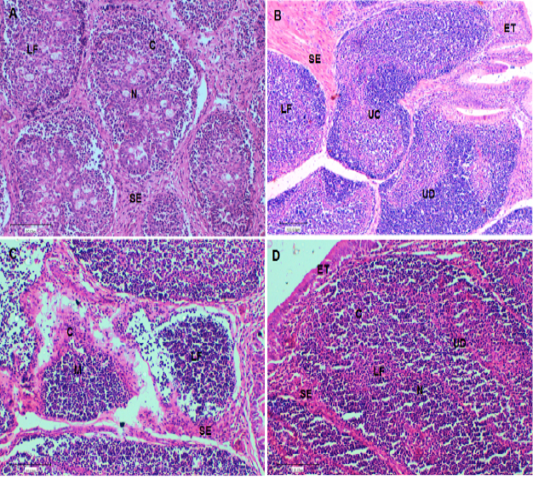
Figure 2: (A). Bursa of control chickens on day 42 showing lymphoid follicles with scattered evacuation and medium condensation of lymphocytes in cortical regions. The demarcation between cortex and medulla was disappeared. (B). Bursa of challenged chickens with sheep erythrocytes (SRBC) on day 42 showing depletion of medulla from lymphocytes with smaller lymphoid follicles. (C). Bursa of chickens challenged with SRBC post-treatment with Lycium shawii showing depletion of cortex from lymphocytes with large empty spaces. (D). Bursa of chickens challenged with SRBC post-treatment with R. epapposum showing condensation of cortex with lymphocytes and medulla with extensive population of lymphocytes with clear demarcation between them. H and E (X40). SI: Space interfollicular, C: Cortex, M: Medulla, LF: Lymphoid Follicle, ET: Epithelial Tissues, UC: Undifferentiated Cells, BC: Blood capillaries.
Table 10: Total leucocyte count (TLC 103/mm³); Heterophils (H %); Lymphocyte (L %), Monocyte (M%) and H/L ratio of chickens challenged with SRBC after treatment with the R.epapposum or Lycium shawii extract.
| Sampling day | Groups | (TLC103/μl) | H % | L% | H/L ratio | M% |
| 14 | Control | 12.5 ±1.85 | 32.1 ±2.73 | 49.3 ±1.66 | 0.66 ±0.01 | 17.9 ±1.43 |
| R. epapposum | 13.6 ±2.88 | 31.5 ±1.09 | 55.3 ±2.08a | 0.53 ±0.03a | 18.3 ±2.31 | |
| Lycium shawii | 12.8 ±2.10 | 30.7 ±1.53 | 58.7 ±2.53a | 0.51 ±0.02a | 18.2 ±2.66 | |
| 28 | Control | 12.1 ±1.04 | 30.5 ±2.23 | 48.9 ±2.56 | 0.62 ±0.04 | 16.4 ±1.03 |
| SRBC | 14.9 ±0.65a | 36.8 ±1.65a | 50.3 ±2.03 | 0.52 ±0.03 | 19.9 ±1.02 | |
| R. epapposum + SRBC | 11.3 ±1.45 | 30.2 ±1.21 | 60.5 ±1.43a | 0.51 ±0.03a | 18.3 ±3.10 | |
| Lycium shawii + SRBC | 11.9 ±2.18 | 31.6 ±2.61 | 61.3 ±2.13a | 0.50 ±0.02a | 17.9 ±1.82 | |
| 42 | Control | 11.3 ±1.21 | 31.9 ±1.72 | 50.4 ±1.48 | 0.62 ±0.01 | 16.2 ±1.20 |
| SRBC | 14.9 ±0.53a | 35.6 ±1.38a | 51.9 ±2.72 | 0.68 ±0.02 | 19.4 ±1.11a | |
| R. epapposum + SRBC | 11.4 ±1.62 | 30.4 ±1.53 | 59.3 ±1.31a | 0.49 ±0.03a | 16.4 ±1.52 | |
| Lycium shawii + SRBC | 12.8 ±1.04 | 30.6 ±1.22 | 51.3 ±1.04 | 0.51 ±0.02 | 18.2 ±2.22 |
Values within the same day having the letter a are significantly different from control value at P<0.05. Mean± Standard error.
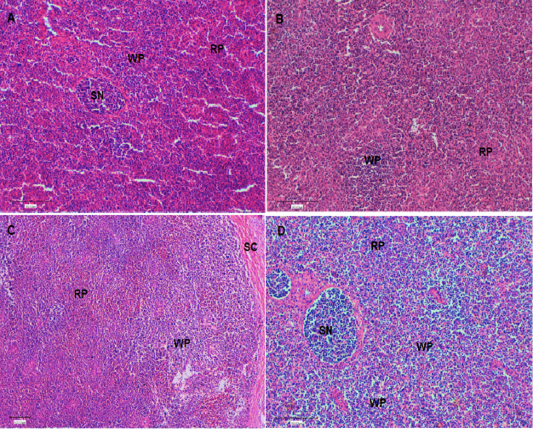
Figure 3: (A). Spleen of control chickens on day 42 showing normal splenic histology and architecture. In addition, different types of red pulp and white pulp were easily visible. (B). Spleen of challenged chickens with sheep erythrocytes (SRBC) on day 42 showing decrease of red pulp with a few white pulp. (C). Spleen of chickens challenged with SRBC post-treatment with Lycium shawii on day 42 showing mild increase in white pulp. (D). Spleen of chickens challenged with SRBC post-treatment with R.epapposum on day 42 showing sever increase in white pulp and condensation of lymphocytes. Differentially types of splenic nodules were easily visible. HandE (X40). RP: red pulp; WP: white pulp, SN: splenic nodule; Sc: splenic capsule.
The current study aimed to investigate the effects of the addition of either R. epapposum or Lycium shawii methanolic extracts in the drinking water of broilers for the first two weeks of life. The general performance of broilers was evaluated, with a particular interest in the performance of the immune system. Major observations included significant increases in the levels of antioxidant enzymes, as well as IgM and IgG concentrations in the serum samples of extract recipient chickens. In addition, the data showed that supplementation with either R.epapposum or Lycium shawii extracts enhanced cellular immunity, as demonstrated by the significant increases in in-vitro phagocytic activities and lymphocyte proliferation.
The antioxidant activities of plant extracts are associated with their phenolic compounds. Hence, quantification of the total phenolic content (TPC) is important to evaluate its contribution to antioxidant activities. The antioxidant properties of phenolics are associated with their ability to act as metal chelators, hydrogen donors, and reducing agents (Attia et al., 2017a, 2017b, 2018). The total phenolic content of the methanolic extracts of Lycium shawii and R. epapposum were 166.33 and 404.00 mg GAE 100 g-1dw, respectively. These findings correspond to observations reported by previous studies (Aldoweriej et al., 2016; Ali et al., 2020b). The TPC has been shown to be affected by the type of solvent used in the extraction process, with the methanol extract having significantly elevated levels of phenolics compared to the ethanol, ethyl acetate, and water extracts (Ali et al., 2020b). The DPPH scavenging activity of the methanolic extracts of Lycium shawii and R. epapposum were 71.57 and 122.14 μmol TE 100g-1 dw, respectively. This can be credited to the higher content of phenolic and flavonoid substances. Similar antioxidant activities for other plant species by the DPPH assay have been reported (Lin et al., 2018; Rachitha et al., 2018; Attia et al., 2017b).
As the chickens advanced in age, the bursa and spleen weights increased significantly only in the R. epapposum pretreated group as compared to the control group. The body weight gain increased significantly in the extract-supplemented chickens relative to the control group. The feed conversion rate tended to be lower in the control group compared to the groups pretreated with the extracts or the SRBC group. To the best of our knowledge, no study has investigated the possible role of Lycium shawii or the R. epapposum extracts as promoters of growth in broilers.
In the current investigation, cellular immunity was examined through evaluation of the in-vitro phagocytic index and lymphocyte proliferation. The glucose consumption test is one of multiple assays that can be utilized to measure lymphocytes proliferation in-vitro. In this assay, leukocytes were stimulated with phytohaemagglutinin (PHA), a well-known mitogen that selectively activates T cells through crosslinking their T-cell receptors (TCRs) (Movafagh et al., 2011; Abo-Aziza et al., 2021). The data showed significant enhancements in lymphocyte proliferation in extract-recipient chickens as well as SRBC-challenged chickens. However, the increase in lymphocyte proliferation was remarkably higher in chickens pretreated with the methanolic extract of R. epapposum. This could be partially attributed to the TPC of the extract, since lymphocyte proliferation in-vitro has been shown to correlate positively with the TPC of multiple fruits and vegetables extracts (Lin and Tang, 2007). The phagocytic index of the Lycium shawii group was significantly elevated on day 14 of age only, while the phagocytic index of the R. epapposum group significantly increased at all the examined ages. Cells in the immune system are known to possess various polyphenol receptors that enable their polyphenol uptake, which in turn activates multiple signaling pathways, leading to induction of immune responses (Ding et al., 2018). Several phytophenols have been shown to activate mononuclear cells and augment their phagocytic activities, mainly by affecting the nuclear factor- κB and MAPK signaling pathways (Grigore, 2017).
The present studies prefer the bursa Fabricious and spleen to be examined histologically by hematoxylin-eosin stains (Figures 2 and 3, respectively). These organs are well-known as the essential avian lymphoid organs and the main way antigens are recognized by the immune system (Abdul-Aziz et al., 2016). The items used in the measurement of the spleen to be used as an indicator of immune status are the degree of distribution of white and red pulps and splenic nodules. The splenic nodules are the site of proliferating and differentiating B lymphocytes. On day 42, the spleens of control chickens showed normal splenic histology and architecture. In addition, different types of red pulp and white pulp were easily visible. On day 42, the spleens of challenged chickens with SRBC showed decrease of red pulp, with a few patches of white pulp. This picture revealed that the immune organs of birds are a complex impression that is affected by different stress factors (Luskin and DeAngelo, 2019; Attia et al., 2017). The spleens of chickens challenged with SRBC post-treatment with Lycium shawii showing mild increases in white pulp on day 42, while those challenged post-treatment with R.epapposum showed severe increases in white pulp and condensation of lymphocytes. Different types of splenic nodules were easily visible.
The bursa of chickens develops quickly after hatching and reaches extreme size between 1 and 4 months of age to control humeral immunity (Abdul-Aziz et al., 2016). It composed of high numbers of lymphoid or bursal follicles. Each follicle is composed of a cortex and medulla, separated by a corticomedullary border of blood capillaries and undifferentiated epithelial cells, which provide the body with antibodies producing B lymphocytes (Cooper, 2015; Abdul-Aziz et al., 2016). The cortex of the lymphoid follicles is covered by connective tissue and epithelial cells and it contains blood capillaries. The lymphoid or bursal follicles are the region for synthesis, proliferation, and final differentiation in addition to the antibody production of B-lymphocyte. The history of the recognition of the lymphoid follicles as an immunologically major organ was studied by Taylor and McCorkle (2009). Histological examination of the bursa of control chickens on day 42 showed lymphoid follicles with scattered evacuation and medium condensation of lymphocytes in cortical regions. The demarcation between cortex and medulla had disappeared. On day 42, the bursa of chickens challenged with SRBC showed depletion of medulla from lymphocytes with small lymphoid follicles. The bursa of chickens challenged with SRBC post-treatment with Lycium shawii showed depletion of cortex from lymphocytes with large empty spaces, while those challenged post-treatment with R. epapposum showed condensation of the cortex with lymphocytes and medulla, with extensive population of lymphocytes and clear demarcation between them.
The effect of the extract treatment on humoral immunity was evaluated via quantification of the serum levels of total IgM/IgG following the SRBC challenge. Inoculation with sheep erythrocytes (SRBCs) is a procedure used for the purpose of examining variations in the responses of the immune system without compromising the animal wellbeing (Seidavi et al., 2017). This type of challenge has been used in various species, particularly for measuring changes in antibody titers in response to foreign RBCs (Seidavi et al., 2017). The RBCs challenge has been utilized to quantify IgM and IgG titers in mice (Liu et al., 2018), and rabbits (El-Gindy et al., 2020). The data showed that only Lycium shawii-treated chickens had significantly elevated levels of IgM antibodies. The IgG levels significantly increased in extract-recipient chickens as well as the SRBC group, with the IgG levels remaining significantly elevated until the end of the experiment only in the Lycium shawii group. The IgM response increases rapidly after the first exposure to an antigen; however, the IgM titer declines following the secondary exposure to the same antigen. The IgG is the predominant isotype in the serum produced after the IgM isotype in the primary humoral response, and it is the main isotype in the succeeding humoral responses (Abo-Aziza et al., 2019). It is well known that plant extracts with high contents of polyphenols have a stimulatory effect on IgM and IgG production, with some plant phenolic compounds being used as vaccine adjuvants (Grigore, 2017; Reyna-Margarita et al., 2019; Saybel et al., 2020).
CONCLUSIONS AND RECOMMENDATIONS
The addition of 18 mg / chick extracts from the aerial pieces of the two plants examined in the present study in the drinking water of broilers for the first 2 weeks of life resulted in enhanced cellular and humoral immune response. This was especially true in the case of supplementation with R. epapposum. The data reported here highlight the potential utilization of these plants for therapeutic and preventive purposes. Further research is required to isolate their active composites and examine their mode of action against diseases.
ACKNOWLEDGMENTS
The researcher would like to thank the Deanship of Scientific Research, Qassim University, for the support of this project. Thanks, are extended to Alwatanya company for poultry, Qassim, Saudi Arabia, for kindly providing the birds and their feeds.
NOVELTY STATEMENT
To the best of our knowledge, no study has examined the effects of Lycium shawii or Rhanterium epapposum supplementation on broiler chickens performance, particularly the immune system. Data of the present study provided some evidence that supplementation with the methanolic extract of these plants enhances humoral and cellular responses as well as broilers’ performance in general.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES