Advances in Animal and Veterinary Sciences
Research Article
Monthly Variations of the Prevalence of Bovine Brucellosis in Benin
Nestor Dénakpo Noudeke1*, Luc Gilbert Aplogan2, François Dossa1, Issaka Youssao1, Souaïbou Farougou1
11Département de Production et Santé Animales, Ecole Polytechnique d’Abomey-Calavi, Université d’Abomey-Calavi, Cotonou, République du Bénin; 2Laboratoire de Diagnostic Vétérinaire et sérosurveillance des maladies animales, Ministère de l’Agriculture, de l’Elevage et de la Pêche, Bénin.
Abstract | A longitudinal study was conducted from February 2012 to January 2013 in Benin to determine monthly prevalence of bovine brucellosis. Five herds were sampled at a rate of two in the North (Gogounou and Okpara) and three in the South (Athiémé, Kpinnou and Ouidah). Twelve animals were selected and identified in each of these herds. Blood samples were collected every month from the animals and their sera were analyzed with Rose Bengal and indirect ELISA tests. Space-time analyses were performed with SaTScan ™ software and monthly seroprevalence was compared in pairs using Fisher Exact Test in R software. Overall, 105 animals were detected positive from the 626 analyzed samples. The prevalence differed significantly from one month to another between the North and the South (p <0.05). Animals were 5.65 times more likely to be infected in the North, while in the South this risk was 4.72 times. The periods from October to December and August to September were those at risk in the North and South, respectively. This information alongside the identification of circulating Brucella strains can lead to the establishment of an effective vaccination strategy.
Keywords | Brucellosis, Bovine, Prevalence, Variation, Benin
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 08, 2016; Accepted | January 18, 2017; Published | January 22, 2017
Correspondence | Nestor Dénakpo Noudeke, Laboratory of Research in Applied Biology, Polytechnic School of Abomey-Calavi, University of Abomey, Calavi; Email: [email protected]
Citation | Noudeke ND, Aplogan LG, Dossa F, Youssao I, Farougou S (2017). Monthly variations of the prevalence of bovine brucellosis in Benin. Adv. Anim. Vet. Sci. 5(1): 23-29.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.1.23.29
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Noudeke et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Brucellosis is a contagious bacterial disease of zoonotic importance and endemic in many African countries where it causes heavy damages to reproductive performances of animals mainly cattle regardless of the production system (Cutler et al., 2005; Gebretsadik et al., 2007; Bronsvoort et al., 2009). It is classified among “neglected zoonoses” because of a lack of knowledge and sensitization of the public (McDermott and Arimi, 2002; Tsehay et al., 2014). Effective control of human Brucellosis is highly dependent on its effective control in animal populations (Ibironke et al., 2008). However, this control requires a thorough understanding on the evolution of the infection.
In West Africa, the prevalence of bovine brucellosis varies significantly between countries, regions and within the same country. It can also vary within herds of the same region where there are no control measures in place (Akakpo and Ndour, 2013; Cadmus et al., 2013). Therefore, the infection is not static and it is generally admitted that the prevalence of brucellosis is higher in natural grazing systems than in urban and peri-urban systems, where the size of the herds is smaller (Cadmus et al., 2006; Kang’ethe et al., 2007; Makita et al., 2011; Megersa et al., 2011).
In Benin, the first studies on bovine brucellosis were conducted by Akakpo et al. (1984) who reported a national seroprevalence of 10%. From 2000 to 2005, a number of cross-sectional studies were carried out in selected areas of the country and reported up to 15.21% as seroprevalence of brucellosis (Noudèkè, 2002; Koutinhouin et al., 2003; Adehan et al., 2005). However the evolution of the prevalence is not documented. The present study was then carried out to assess the variability of monthly prevalence of bovine brucellosis between the North and the South of the country in order to establish an effective control strategy
Material and Methods
Study Area
Republic of Benin is a West African country, located between 6° 10’ N and 12° 25’ N and between 0° 45’ E and 3° 55’ E. It covers a surface of 114.763 km² and is limited at the Northwest by Burkina Faso, the North by Niger, the west by Togo, the East by Nigeria and the South by the Atlantic Ocean. The administrative card of Benin is constituted of 4 hierarchical levels that are: departments, Municipalities, districts and villages or Wards. Benin is part of the inter-tropical zone. Following the latitude, rainy seasons are combined in different manners to define particular rainfall regimes. In the South of the parallel 7° 45’, the bimodal regime comprises four seasons of which: a big rainy season (April to July), a small dry season (August to September), a small rainy season (October to November) and a big dry season (December to March). In the North of the parallel 8°30’, it’s a unimodal type of regime with two seasons: one dry and one rainy. The dry season covers November to early May and the rainy season ranges from May to October.
Methodology
Five farms were selected based on their location, production system and the availability of farmers to cooperate. Two farms were chosen in the North (Gogounou with 72 animals and Okpara with 110 animals), and three in the South (Kpinnou with 104 animals; Athiémé with 74 animals and Ouidah with 81 animals) (Figure 1). Among the two farms of the north, one was private and the other one was a state farm (Opkara). Likewise, in the South one of the chosen farms was a state/public farm (Kpinnou) while others were private. The state farms are involved in semi intensive production, while the private farms practice an extensive husbandry. Furthermore, the herds of Gogounou and Athiémé were in rural areas, while those of Okpara, Kpinnou and Ouidah were in periurban areas. In each herd, twelve animals were randomly sampled. They were identified by their identification earrings. Every month during the study period (February 2012 to the January 2013), whole blood samples were taken from these animals. For the entire investigation period, there was no introduction of a new animal in the herds. Sometimes animals retained for the study could not be caught for blood sampling. Moreover, in April 2012, no blood sample was collected. All collected blood samples were centrifuged and the sera were kept at -20°C at the laboratory of the Research Unit in Biotechnology of Animal Health and Production of the University of Abomey-Calavi. At the end of sample collection, sera were sent to the Laboratory of Serological Diagnosis of Parakou, where they were subjected to Rose Bengal (RB) test and indirect Enzyme Linked Immuno-Sorbent Assay (iELISA) performed with pools of 8 sera. Sera from positive pools were individually tested again using the iELISA. Positive samples were those that gave positive result to at least one the two tests (RB and iELISA).
Figure 1: Localization of the herds
Statistical Analyses
A time and space-time analysis was carried out with SaTScan™ software version 9.4.1 following the method described by Kulldorff and Nagarwalla (1995). The monthly seroprevalence in a particular area was calculated by dividing the number of positive cases by the total number of samples collected in that area in the concerned month. For regions, the numbers used were those of the entire region in question. These monthly seroprevalences were compared two by two using Fisher Exact test and the sensitivity of the two tests was compared with Chi-Square test using R software version 3.1.2.
Results
Overall, 626 sera were analysed of which 5 and 105 were
Table 1: Time and space-time analysis
|
Regions |
Time analysis |
Space-time analysis |
||||||
|
Period at risk |
Log likelihood |
Relative Risk |
P-value |
Location at risk |
Log likelihood |
Relative Risk |
P-value |
|
|
North |
August to January |
23.522861 |
5.65 |
0.001 |
Gogounou |
10.371181 |
3.16 |
0.000084 |
|
South |
June to September |
4.811775 |
4.72 |
0.023 |
Athiémé |
6.625149 |
7.53 |
0.014 |
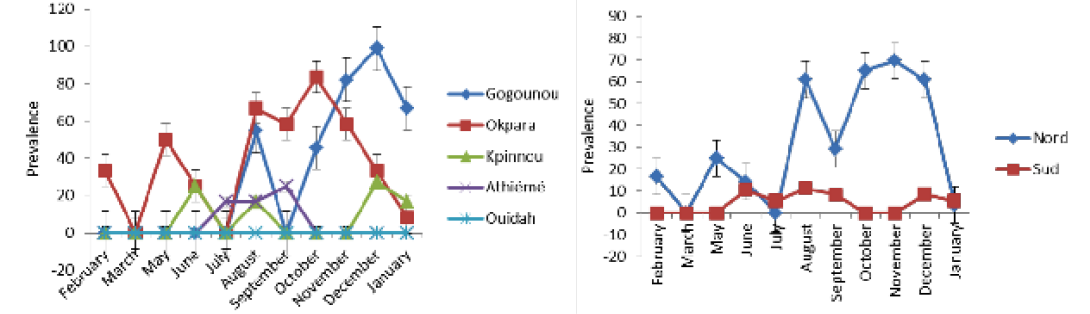
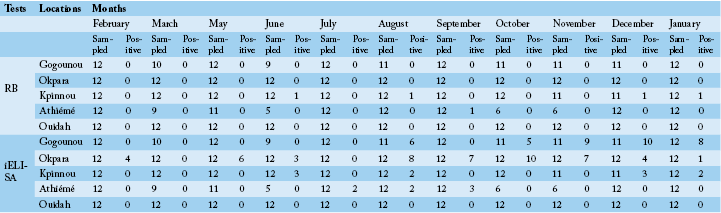
detected positive for Rose Bengal and indirect ELISA, respectively. All positive sera in RB were also positive with iELISA. The study revealed a seroprevalence that varied from 0.8% to 16.77%. Four herds out of the five presented at least one positive case during the study period. The general monthly seroprevalence (from the five farms) varied between 0% and 30.76% (p <0.05). In the North, it varied significantly (p <0.05) between 0% and 69.56%, while in the South it was between 0% and 11.11% without a significant difference (p>0.05). The monthly distribution of the prevalence of brucellosis is discontinuous with a strong variability within the herds (Figure 2A and B). Nevertheless, between regions the prevalence was steady in the South as opposed to the tendency in the North. The period of February to June presented a relatively low prevalence in the four infected herds in all regions. It was from July that high levels of prevalence were observed.
Table 1 shows the time and space-time distribution of brucellosis in the study populations. All clusters (zones at high risk: Gogounou in the North and Athiémé in the South) were significantly infected (p <0.05). Animals from Gogounou were 3.16 times more likely to be infected than those of Okpara. Similarly, animals of Athiémé were 7.53 times more at risk than those of Kpinnou and Ouidah. It was noticed that herds in extensive systems were more exposed than those in semi-intensive systems. In the North, brucellosis cases were more common during the period of August to January, while in the South it was from June to September. Furthermore, animals from the North were 5.65 times more likely to be infected as compared to the South where the risk was 4.72 times.
The sensitivity of the iELISA test deferred significantly with that of RB test (p <0.001) (Table 2). Apart from the farm of Ouidah where no positive case was detected, all the other farms presented at least one positive case. Moreover, some animals that were detected positive in a given month could become negative in the next month and then detected positive again in the following month.
Table 3 presents the monthly prevalence in the investigated herds. The prevalence varied between locations (P <0.005). In Gogounou, analyses revealed very significant monthly prevalences from August (p <0.05) until January except for September where no case was detected just like from February to July. They varied between 0% and 98.90%. In Okpara, important monthly prevalences were recorded from August to January. They varied between 0% and 58.33% without a significant difference (p>0.05). The monthly prevalences recorded in Kpinnou, Athiémé and Ouidah, did not present any significant difference throughout the year (p>0.05).
Table 4 shows the monthly prevalences in the North and the South. Prevalences of the period of February to July in the North presented a significant difference compared to the period of August to January except the month of September. However, no significant difference was observed in the South throughout the year.
These observations show that the distribution of the monthly prevalence varied significantly in the North, contrary to the South. There was a significant difference between the monthly rates recorded in the North as compared to those in the South. According to the monthly significance tests
Table 3: Monthly prevalence in locations
|
Locaations |
Months |
||||||||||
|
February |
March |
May |
June |
July |
August |
September |
October |
November |
December |
January |
|
|
Gogounou |
0a |
0a |
0a |
0a |
0a |
54.54b |
0a |
45.45b |
81.81b |
98.90b |
66.66b |
|
Okpara |
33.33a |
0a |
50ab |
25a |
0ac |
66.66ab |
58.33ab |
83.33ba |
58.33ab |
33.33abc |
8.33abc |
|
Kpinnou |
0a |
0a |
0a |
25a |
0a |
16.66a |
0a |
0a |
0a |
27.27a |
16.66a |
|
Athiémé |
0a |
0a |
0a |
0a |
16.66a |
16.66a |
25a |
0a |
0a |
0a |
0a |
|
Ouidah |
0a |
0a |
0a |
0a |
0a |
0a |
0a |
0a |
0a |
0a |
0a |
|
Monthly significance test |
S |
NS |
S |
NS |
NS |
S |
S |
S |
S |
S |
S |
|
Overall monthly prevalence |
6.66b |
0bc |
10.17bdf |
12bf |
3.33ef |
30.5df |
16.66df |
28.3af |
30.76a |
29.31a |
18.33abf |
NS : P>0.05; S : P<0.05; prevalences of the same row followed by the same letters do not differ significantly at 5%.
Table 4: Monthly prevalence in regions
|
Regions |
Months |
||||||||||
|
February |
March |
May |
June |
July |
August |
September |
October |
November |
Décember |
January |
|
|
North |
16,66a |
0a |
25ab |
14,28ac |
0ad |
60,87b |
29,16ab |
65,22b |
69,56b |
60,87b |
37,5ab |
|
South |
0a |
0a |
0a |
10,34a |
5,55a |
11,11a |
8,33a |
0a |
0a |
8,57a |
5,55a |
|
Monthly significant test |
S |
NS |
S |
NS |
NS |
S |
NS |
S |
S |
S |
S |
NS : P>0.05; S : P<0.05; prevalences of the same row followed by the same letters do not differ significantly at 5%.
(Table 3 and 4), period of October to December was the one with many cases of brucellosis in the North, whereas in the South it was from August to September.
Discussion
The significant difference observed between results of RB and iELISA tests was previously reported by several authors in Benin and elsewhere in Africa (Delafosse et al., 2002; Koutinhouin et al., 2003; Kouamo et al., 2010; Dean et al., 2013; Sanogo et al., 2013). The monthly variations of the prevalence are due to variations of the antibody titre. RB is actually used to identify IgGs and is therefore very useful in the diagnosis of acute bovine brucellosis. However, after the first clinical signs of the disease, IgMs are the first antibodies that appear and are detectable from the 10th day. IgGs are thereafter, detectable and the titre of the two antibodies (IgM and IgG) will rise together during the acute phase of the disease. In chronic cases, IgMs disappear while IgGs persist. In spite of these considerations, the dynamic of the different classes of antibodies is not absolute and vary from one individual to another (Maurin, 2005). This situation could explain the low numbers of positive cases recorded with RB test. Nevertheless, iELISA test is used to detect all the different classes of antibody (IgG, IgM and IgA). IgM types of antibodies usually disappear after 3 to 6 months; their presence reveals a recent infection (Chakroun and Bouzouaia, 2007). Therefore, the increase of positive cases between October and December in the North and August and September in the South is a sign of occurrence of new cases. This means that there were contaminations of new animals or reactivation of previously infected animals because Brucella species have a relatively weak immunogenic potential. Besides, in natural conditions, brucellosis leads to a reinfection despite a certain degree of immunity conferred by the first attack (Bula et al., 1987). This could be one of the reasons that justify the monthly variations of the seroprevalence of the animals. In fact, an adult cow contaminated during pregnancy will develop in more than 50% of the cases a short, spontaneous and curable infection. Likewise, young animals often get healed and only develop a discreet and transient serological reaction. The variation of the seroprevalence of brucellosis according to the variation of antibodies in the time was also observed by Kashiwazaki et al. (2012).
Furthermore, iELISA was used in several recent cross-sectional studies in Africa for the evaluation of the seroprevalence of brucellosis with reliable results (Boukary et al., 2013; Sanogo et al., 2013). However, in this longitudinal study, the overall seroprevalence of 16.77% cannot be extrapolated to the entire country because of the limited size of the investigated herds. Nevertheless, this prevalence is close to the one of Koutinhouin et al. (2003) who reported 15.21%. Since no control system of bovine brucellosis exists in Benin, the prevalence of this disease could be in permanent increase over time.
In this study, the period of October to December in the North was a high brucellosis infection time. It is also the period where many calving were recorded. However, in young infected cows, the serological reaction is only detectable after the first calving. Besides, Brucella species have the potential to resist the action of immune mechanisms and maintain themselves several years in some privileged sites, notably in lymph nodes. A reactivation can, therefore, occur during every pregnancy and the placental infection can provoke an abortion and/or an excretion of the bacteria during calving. This confirms that the period of October to December is a period at risk. In the South, with a low relative risk and seroprevalence as compared to the North, it is the period of August to September that is the most sensitive. This shows that apart from calving, there are many other risk factors like the production system that influences the occurrence of brucellosis (Ahmed et al., 2010; Gebretsadik et al., 2007; Boukary et al., 2013; Mai et al., 2012). Farms operating in extensive production systems such as Gogounou in the North and Athiémé in the South presented the highest monthly prevalence. However, the period of August-September in the South and October-December in the North correspond to dry seasons where animals make long distance especially in the North, before getting a pasture or water source that is shared by many other herds. This situation increases the risks of transmission of the disease (Gebretsadik et al., 2007). Moreover, Kadohira et al. (1997) reported that the North of Kenya which is a pastoral area is at higher risks of brucellosis infection as compared to other regions of that country.
In conclusion, the North and the South of Benin presented significantly different monthly prevalence of brucellosis. The main risk factor is calving and the periods at risk were October-December and August-September in the North and the South, respectively. For effective control of bovine brucellosis in Benin, mass vaccination campaigns should take in consideration these variations in order to decrease the frequency of the cases to the point that it can be possible, without serious economic losses, to conduct elimination of positive animals. However the eradication of brucellosis cannot be effective and efficient without being holistic by involving all concerned animal species: pets, cattle, goats, sheep, and pigs if needed.
Authors’ Contribution
IY and SF conceived the study and acquired the funding. NDN, and FD coordinated the study design and carried out the field work. NDN and LGA have done the analysis of sera. NDN has done the analysis of the information and wrote the manuscript. All authors were involved in revising the manuscript and approved the final manuscript.
Acknowledgements
Authors are grateful to Union Economique et Monétaire Ouest Africaine for financial support through PAES (Projet d’Appui à l’Enseignement Supérieur) Project.
Conflicts of Interest
Authors declare that there are no conflict of interest.
References