Advances in Animal and Veterinary Sciences
Research Article
Effects of Palm Oil Mill Effluent on Reproductive Hormone of Female Nile Tilapia, Oreochromis niloticus (Linnaeus 1758)
Muliari Muliari1, Ilham Zulfahmi2*,Yusrizal Akmal1, Ni Wayan Kurniani Karja3, Chairun Nisa4, Kizar Ahmed Sumon5
1Department of Aquaculture, Faculty of Agriculture, Almuslim University, Indonesia; 2Department of Biology, Faculty of Science and Technology, Ar-Raniry Islamic State University, Indonesia; 3Department of Veterinary Clinic, Reproduction and Pathology, Faculty of Veterinary Medicine, IPB University, Indonesia; 4Department of Anatomy Physiology and Pharmacology, Faculty of Veterinary Medicine, IPB University, Indonesia; 5Department of Fisheries Management, Bangladesh Agricultural University, Bangladesh.
Abstract | Exposure to Palm Oil Mill Effluent has led to a disturbance on fish health, especially in reproduction. However, information related to the negative effect of Palm Oil Mill Effluent on fish reproduction performance is still rare. We aimed at investigating the reproductive hormone performances (estradiol, testosterone and progesterone) of female Nile Tilapia (Oreochromis niloticus Linnaeus 1758) exposed to Palm Oil Mill Effluent. A chronic test was carried out by using a completely randomized design consisting of four treatments including five replicates: control (0 mg/L), treatment A (1.565 mg/L), treatment B (2.347 mg/L), and treatment C (3.130 mg/L). Exposure to Palm Oil Mill Effluent triggered a significant decrease in estradiol and testosterone hormone (P < 0.05) but such a significant decrease was not observed in progesterone hormone (P > 0.05). Estradiol hormone decreased significantly from 4.42 ± 1.92 ng/mL in control to 1.35 ± 0.03 ng/mL in treatment C, while testosterone hormone decreased significantly from 6.63 ± 2.62 ng/mL in control to 1.18 ± 0.11 ng/mL in the treatment C. The significant decreasing of total lipid content in gonads also observed in treatment C. The disruption of synthesis of lipid can also be indicated from the elevated values of relative bile volume exposed to Palm Oil Mill Effluent.
Keywords | Palm Oil Mill Effluent, Estradiol, Progesterone, Testosterone, Nile Tilapia
Received | June 25, 2019; Accepted | August 19, 2019; Published | November 01, 2019
*Correspondence | Ilhan Zulfahmi, Department of Biology, Faculty of Science and Technology, Ar-Raniry Islamic State University, Indonesia; Email: [email protected]
Citation | Muliari M, Zulfahmi I, Akmal Y, Karja NWK, Nisa C, Sumon KA (2019). Effects of palm oil mill effluent on reproductive hormone of female nile tilapia, oreochromis niloticus(linnaeus 1758). Adv. Anim. Vet. Sci. 7(11): 1035-1041.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.11.1035.1041
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Muliari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Indonesia has become the largest palm oil producer all over the world. Food and Agriculture Organization (FAO) noted that the total palm oil production of Indonesia in 2014 reached 29.27 million metric tons (FAO, 2016). Together with Malaysia, Indonesia was able to produce more than 85% of the world’s palm oil (Jayed et al., 2011; Fitzherbert et al., 2008). One of the negative impacts arising from the existence of palm oil mill industry is the increased pollution from Palm Oil Mill Effluent (POME), which can affect the aquatic environment (Alhaji et al., 2016). POME contains a high concentration of chemical oxygen demand (COD) ranging from 45,000–65,000 mg/L, biochemical oxygen demand (BOD) ranging from 21,500–28,5000 mg/L and high total suspended solids (TSS) ranging from 15,660–23,560 mg/L (Wong et al., 2009; Chan et al., 2013). Bello et al. (2013) reported that oil and grease content in POME being as high as 4,000 mg/L with a pH between 3.4 and 4.7.
High concentrations of COD and BOD harm aquatic organisms, for instance, decrease of oxygen consumption and cause of hypoxic condition (Maygaonkar et al., 2012; Verberk et al., 2016; Vithana et al., 2019). Oil and grease are considered as hazardous pollutants, particularly in the aquatic environments and can completely damage the ecology of the aquatic ecosystems (Bala et al., 2015). One previous study by Muliari and Zulfahmi (2016) revealed that contamination of POME had resulted in negative effects on the phytoplankton community. Furthermore, exposure to POME has led to a disruption of the fish liver and gill performances which can be signalled by histopathological changes in the tissue (Zulfahmi et al., 2017a; Muliari et al., 2018).
Nile Tilapia (Oreochromis niloticus) is a species of fish that is potentially impacted by POME. Contamination occurs due to several eco-biological characteristics such as their extensive distribution in aquatic environments and their availability at various stages throughout the season (Zulfahmi et al., 2014). Nile Tilapia has been used to assess the effects of several toxicants such as treated leachate (Gallão et al., 2019), copper oxide (Abdel-Khalek et al., 2015), and zinc oxide nanoparticles (Alkaladi et al., 2015).
Mekkawi and Hasan (2011) state that the disturbance of fish reproductive functions can potentially affect the dynamics of fish populations and has direct implications on sustainable fishery management. The presence of pollutants in a water body can result in a dangerously compromised fish reproduction system which potentially generates a scarcity of stock and the increased price of Nile tilapia. Al-Rawi (2015) proved that pollution can affects the physicochemical characteristics of the water, sediment and other biological components, so harms the quality and quantity of fish stocks
Reproductive hormones play an important role in supporting the success of fish reproductive process (Kime et al., 1996; Rurangwa et al., 1998). The disturbances of reproductive hormones affects the gonadosomatic index (GSI), fecundity, egg diameter, and vitellogenesis of fish (Hecker et al., 2002). Several studies have reported that pollutants have an impact on reducing the concentration of reproductive hormones. Ebrahimi and Taherianfard (2011) reported a decrease in the concentration of estradiol, progesterone and testosterone hormones in Cyprinus carpio and Capoeta sp. due to heavy metal exposure. Luo et al. (2015) reported that cadmium exposure had caused a decrease in the concentration of estradiol in Nile Tilapia. To date, we lacked the information on the exposure of POME to the performance of fish reproductive hormones. Hence, the objectives of this study were to elucidate the toxic effects of POME on the performance of reproductive hormones (estradiol, progesterone, and testosterone) of female Nile Tilapia.
MATERIALS AND METHODS
Study Area, Collection and Acclimatization of Fish
Fish (N = 300; weight: 9.46 g ± 1.16; length: 7.75 cm ± 1.67) were obtained from the Department of Fish Hatchery Center (BBI) Batee Iliek, Bireuen Regency Aceh, Indonesia. All procedures for fish treatment were conducted according to the international standards of animal welfare (Canadian Council on Animal Care, 1993). Fish were acclimatized over a period of one week.
Raw POME was collected from the Syaukat Sejahtera Palm Oil mill factory in a sterile container and brought back to the laboratory. Thirty liters (30 L) of stock solutions of POME (100 mg/L) was prepared and diluted to achieve the required concentration for the chronic test. Tanks (50 x 30 x 40 cm3) were used as fiberglass containing 25 L of water. Water was taken from an artesian well and aerated for 24 hours before being used. Fish rearing and chronic test were carried out at the Aquaculture Laboratory, Faculty of Agriculture, University of Almuslim (5°.19’47.54” N, 96°.78’72.44” S).
Experimental Design
The chronic test was conducted by using completely randomized design. The concentration of POME in each treatment was selected based on the 96-h LC50 of the POME on Nile Tilapia obtained from a previous study, this being 15.65 mg/L (Zulfahmi et al., 2017b). Treatments were designed as follows: control (0% palm oil mill effluent: 0 mg/L), treatment A (10% of the 96-h LC50: 1.565 mg/L), treatment B (15% of the 96-h LC50: 2.347 mg/L), and treatment C (20% of the 96-h LC50: 3.130 mg/L). Ten fish were stocked into each of the 20 fiberglass tanks. Each of the control and treatment was maintained in five replicates. Fish were fed with commercial feed at 5%/kg of body weight twice a day. Exposure period of POME lasted for 45 days. Fish faeces and excess food were stripped every day using the siphon method. Water was completely changed every 15 days, and new concentrations of POME were used.
Measurements of Hormone, Total Lipid Content and Relative Bile Volume
At the end of the exposure period of 45 days, ten fish from each replicate was collected and sacrificed by using clove oil for hormone analysis. Hormone measurements were performed by ELISA method at Veterinary Clinic, Reproduction and Pathology of the Faculty of Veterinary Medicine, Bogor Agricultural University. One mL blood sample from each fish was taken by using syringe from the caudal vessels and then inserted into microtube to be centrifuged for 15 minutes at 4000 RPM. The serum and blood plasma were separated and stored at – 20° C until use. The DRG Instruments GmbH, Marburg, Germany (REF EIA-2693 for Estradiol, REF EIA-1561 for Progresteron, REF EIA-1559 for Testosterone) was used for the analysis. The intensity of Color assay solution, was scanned and analyzed by using ELISA reader (BIORAD xMark Microplate Spectrophotometer) at a wavelength of 450 nm. The concentrations of estradiol, testosterone, and progesterone hormones were calculated by using MPM 6 program.
The relative bile volume was measured based on the equation described in Zulfahmi et al. (2014):
RBV = BV/LV * 100
Where RBV: relative bile volume (%), BV: bile volume (mL), LV: liver and bile volume (mL). Measurements of total lipid content in gonads and liver were performed by the Soxhlet method (AOAC 2005).
Statistical Analysis
SPSS 22 (SPSS Inc., Chicago, IL) for Windows software was used for the statistical analysis of reproductive hormone concentration, relative bile volume and total lipid content in each treatment by using one way analysis of variance (ANOVA). Least significant difference (LSD) was performed to test differences value of the parameter between treatments. P values ≤ 0.05 were considered significant.
RESULTS
Reproductive Hormone
Results showed that exposure to POME caused a decrease in the concentration of estradiol and testosterone in female Nile Tilapia. The highest estradiol concentration observed in treatment B while the lowest observed in treatment C were 6.31 ± 1.65 ng/mL and 1.35 ± 0.03 ng/mL, respectively. There was no significant difference in the concentration of estradiol hormone in treatment A and treatment B. However, a significant decrease in the concentration of estradiol hormone was observed in treatment C (p < 0.05) (Figure 1). The testosterone concentration was highest in control and lowest in treatment C were 6.63 ± 2.62 ng/mL and 1.18 ± 0.11 ng/mL, respectively. Statistically, testosterone concentration decreased significantly in treatment C (p < 0.05) (Figure 2). Exposures to POME had no significant effect on the concentration of progesterone hormone (p > 0.05) (Figure 3).
Total Lipid Content in Gonad and Liver
The highest value of total lipid content in gonads observed in control while the lowest observed in treatment C were 6.50 ± 0.60% and 2.12 ± 0.24%, respectively. Statistically, There were no significant differences in the total lipid content in gonads in treatments A and B. Nevertheless, a significant decrease in total lipid content in gonad was observed at treatment C (p < 0.05) (Table 1). There were no significant differences observed in the total lipid content in liver between treatment (p > 0.05) (Table 1). Furthermore, results showed a positive correlation between the decrease of total lipid content ratio between gonad and liver. A significant difference in total lipid content ratio in control and treatment C was observed (p < 0.05). However, the lowest ratio (0.75) was detected in treatment C (Figure 4).
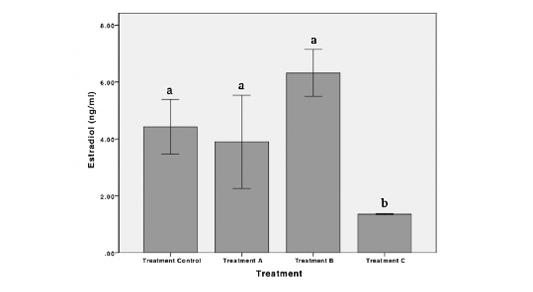
Figure 1: Concentration of estradiol hormone of female Nile Tilapia in each treatment. *The different letters indicate significant differences (p < 0.05).
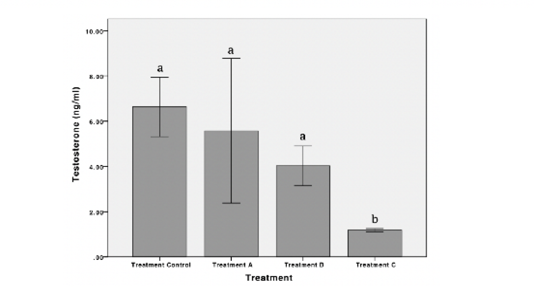
Figure 2: Concentration of testosterone hormone of female Nile Tilapia in each treatment. *The different letters indicate significant differences (p < 0.05).
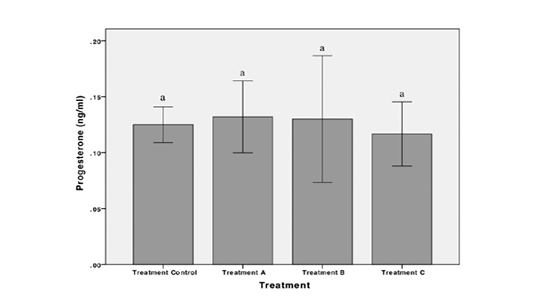
Figure 3: Concentration of progesterone hormone of female Nile Tilapia in each treatment. *The different letters indicate significant differences (p < 0.05).
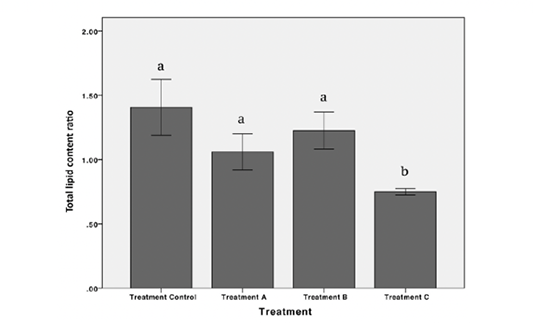
Figure 4: Total lipid content ratio between gonad and liver of female Nile Tilapia in each treatment *The different letters indicate significant differences (p < 0.05).
Table 1: Total lipid content in gonad and liver of female Nile Tilapia in each treatment.
| Total Lipid Content | Treatments | |||
| Control | A | B | C | |
| Gonad (%) |
6.50± 0.60 a |
4.13± 0.86 a |
4.47± 1.31 a |
2.12± 0.24 b |
| Liver (%) |
4.84± 1.39 a |
4.00± 1.54 a |
3.77 ±1.68 a |
2.27± 0.33 a |
*The different letters indicate significant differences (p < 0.05).
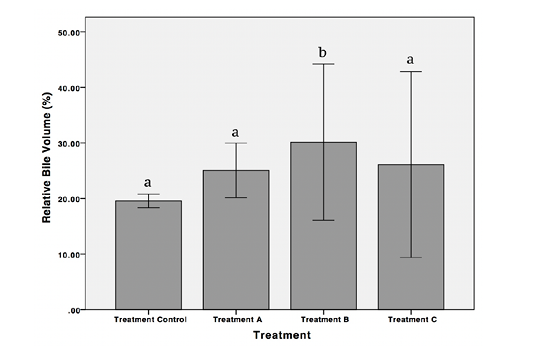
Figure 5: Relative bile volume of female Nile Tilapia in each treatment *The different letters indicate significant differences (p < 0.05).
Relative Bile Volume
Results showed a significant increase in the value of relative bile volume in treatment B compared to the control (p < 0.05). Nevertheless, the value of relative bile volume decreased significantly in treatment C (p < 0.05) (Figure 5).
DISCUSSION
Fish reproductive system is potentially affected by environmental factors such as pollutants. Previous research have reported that exposure to POME cause the decrease in gonadosomatic index value, and shrinking oocyte diameter of female Nile Tilapia (Zulfahmi et al., 2018). The decrease in reproductive performance is strongly associated with the disturbances of reproductive hormones. Carbofuran exposure reported a significant reduction of estradiol hormone and led to inhibition of the development of ovarian follicles in Clarias gariepinus and Heteropneustes fossilis (Ibrahim et al., 2014; Chatterjee et al., 2001). Ghazala et al. (2014) reported that the decreasing of hormones levels due to pollution helps to provide valuable biomarkers and useful tools in monitoring the impacts of stressors on fish.
In our study, estradiol hormone concentrations of the female Nile Tilapia decreased significantly after exposure to POME. Sullivan and Yilmaz. (2018) stated that estradiol hormone plays an important role in inducing calcitonin, which affects vitellogenin secretion and development of oocytes. The decrease of estradiol concentration in different fish has also been reported when they were exposed to heavy metals (Pb, Hg, Cd & As) (Ebrahimi and Taherianfard, 2011), dioxin (Heiden et al., 2008), and polycyclic aromatic hydrocarbons (Thomas et al., 1995). The disruption of reproductive hormone production is caused by disruption of the hypothalamic-pituitary system of fish. Kime (1995) stated that pollutants could negatively regulate estrogen signalling by inducing oxidative metabolism of estrogens, suppressing and efficacy of the estrogen receptors, and inhibiting estradiol-regulated gene expression.
Testosterone hormone plays an active role in the maturation stage of the gonads at the end of the reproductive cycle and acts as an intermediate product in synthesizing estradiol (Barannikova et al., 2002). In the present study, testosterone hormone in Nile Tilapia decreased significantly after exposure to POME at 3.130 mg/L. A similar finding was also observed in different fish species when they were exposed to bisphenol S (Ji et al., 2013) and cadmium (Gárriz et al., 2019). The reduced testosterone hormone concentration is due to the disruption of enzymatic reactions that synthesize the hormone. Zhang et al. (2014) reported that two specific aromatase enzymes (CYP19a and CYP19b) that are responsible for catalyzing testosterone hormone production. CYP19a is mainly expressed in the gonads and CYP19b is mostly found in the SEP brain. Carnevali et al. (2018) reported that the concentration of estradiol hormone in fish is the result of synthetization of testosterone hormone; thus the decreased testosterone level can cause the decreased level of estradiol (Carnevali et al., 2018). Testosterone is converted into estradiol within granulosa cells via aromatization, where, aromatase is the enzyme that converts testosterone into estradiol (Huffman et al., 2013).
To date, information related the effect of pollutants to hormone progesterone level in fish is still not widely studied. This study indicates that exposures to POME have no significant effect on the concentration of progesterone hormone of Nile tilapia. According to Kime (1995), unlike estradiol and testosterone, the induction of progesterone hormone tends to take place during the oocyte final maturation process. In this study, we assume that until the end of the exposure period, the test fish did not yet enter the oocyte final maturation process. Thus, the effect of POME on the progesterone hormone tends to not detected. Besides that, Barannikova et al. (2002) also stated that the hormone progesterone tends to perform in gametogenesis than oogenesis development. Amutha & Subramanian (2013) reported that there was no significant effect on the concentration of progesterone hormone in female tilapia exposed to cadmium. Otherwise, the decreased concentration of the progesterone hormone in male Nile tilapia was detected.
The presence of pollutant exposure results in reproductive hormone dysfunction, which impacts the disruption of vitellogenin receptors in oocytes (Kime, 1999). Disruption of vitellogenesis is often associated with damage to liver tissue (Zulfahmi et al., 2015; Paraso et al., 2017). It is known that one of the products of the vitellogenesis is the availability of an adequate amount of egg yolk as a reserve food for the embryo. The fish liver is responsible for synthesizing lipid into egg yolks through the vitellogenesis. In the present study, total lipid content in the gonad of Nile Tilapia decreased significantly after exposure to POME at 3.130 mg/L. A similar result was reported in Danio rerio when exposed to di-isononyl phthalate (Carnevalli et al., 2018). Zulfahmi et al. (2017a) revealed that POME exposure could cause hemorrhage, congestion, inflammatory cell infiltration, hydrophilic degeneration, and necrosis in the liver tissue of Nile Tilapia, so that the liver performance is decreased. The decrease in liver performance is thought to be the cause of the decrease in the ratio of lipid content in the gonad.
The results of the present study revealed that exposure of POME at 2.347 mg/L increased relative bile volume of Nile Tilapia; however, in higher concentrations of POME the relative bile volume value decreased significantly. Zulfahmi et al. (2014) also revealed that there was an increase in the value of relative bile volume of Nile Tilapia when exposed to mercury. Several studies have shown that many pollutants are subsequently excreted through the bile as conjugated metabolites (Neves et al., 2012; Blanco et al., 2019). In the present study, this may reflect the increase of relative bile volume in treatment B (2.347 mg/L). However, in higher concentration or more extended exposure period of pollutants, the alteration of liver cells is increased; thus the synthesis of pollutants in bile becomes disrupted and reduced the relative bile volume.
CONCLUSION
The present study revealed that exposure to POME had significant impacts on estradiol and testosterone hormone of female Nile Tilapia. The occurrence of decreased hormones resulted in a decrease in total lipid content in fish gonad. The disturbance to lipid synthesis can also be indicated from the elevation values of relative bile volume in fish exposed to POME. Further research is needed to analyze the effect of POME on vitellogenesis and the performance of the hypothalamic-pituitary-gonadal system of Nile Tilapia.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The study was funded by a Research Grant from the Directorate of Research and Community Service, the Ministry of Research, Technology, and Higher Education Republic of Indonesia under the “PKPT” Scheme, Grant no. 0045/E3/LL/2018.
REFERENCES






