Advances in Animal and Veterinary Sciences
Research Article
Total Crude Protein and Fat in Meat of African Catfish (Clarias gariepinus) Fed a Diet Containing Dried Larvae of Black Soldier Fly (Hermetia illucens)
Era Hari Mudji1*, Jeny Yunita Ningsih2, Heni Arista3
1Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia, 60115; 2Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia, 60225; 3Agency of East Java Animal Husbandry, Surabaya, Indonesia, 60235.
Abstract | The development of fisheries in Indonesia has been increased for several decades. However, the public interest in utilizing fisheries products still low compared to the livestock. It because they think that the protein from fish is lower than the livestock. This study aimed to analyze the potency of feed formulation using larvae of black soldier fly (BSF/Hermetia illucens) on total crude protein and fat in the meat of African catfish (Clarias gariepinus). This study used 120 African catfish seeds as animal models. They were separated into four groups as follows: P0 = control, P1 = 5% dried larvae of BSF, P2 = 7% dried larvae of BSF, and P3 = 9% dried larvae of BSF. The feed was given four times a day for 30 days. The meat of African catfish was collected on day 8 and its total crude protein, fat, and ratio of crude protein/fat were tested. The data was analyzed using SPSS version 16 with a probability value (p≤0.05). The result showed that utilization of BSF larvae in African catfish decreased the total crude fat compared to the control (p≤0.05), however not regarding the total crude protein (p≥0.05). Another result showed that there is a significant increase in the ratio of crude protein/fat in the treated group compared to the control (p≤0.05). There is no significant difference regarding total crude fat and ratio of crude protein/fat between the treated group with 5%, 7%, and 9% of BSF larvae in this study. It indicated that feed formulation using larvae of BSF maintained the protein content and depressed fat formation inside the muscle tissue. In conclusion, the feed formulation using 5% of BSF larvae could be used as the alternative fish feed with a better result in the depressing fat formation and increase of the ratio of crude protein/fat compared to the commercial feed.
Keywords | African catfish, Black soldier fly, Fat, Feed, Protein.
Received | March 12, 2021; Accepted | March 17, 2021; Published | September 05, 2021
*Correspondence | Era Hari Mudji, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia, 60115; Email: [email protected]
Citation | Mudji EH, Ningsih JY, Arista H (2021). Total Crude Protein and Fat in Meat of African Catfish (Clarias gariepinus) Fed a Diet Containing Dried Larvae of Black Soldier Fly (Hermetia illucens). Adv. Anim. Vet. Sci. 9(10): 1705-1709.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1705.1709
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mudji et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The development of aquaculture in Indonesia has been increased for several decades. It because of the potencies of aquaculture on the fulfillment of the protein for Indonesian citizens. However, the Indonesian public interest in utilizing aquaculture products still very low compared to the livestock products. Advanced strategies are needed to guarantee that the protein sources from fisheries have a similar quality compared to the livestock. One of the fish that have been widely consumed is African catfish (Clarias gariepinus) (Muchlisin and Siti-Azizah, 2010).
African catfish is the most common farming fish in Indonesia. It is due to the high performance of production, high tolerance, and hardy in nature (Ibrahim et al., 2016). Because of its ability, the African catfish can be cultivated either in conventional and modern aquaculture, (Dunham and Elaswad, 2018). African catfish can consume varieties of feed types including commercial feed and natural feed. The natural feed for fish is commonly sourced from insect larvae such as black soldier fly (BSF/Hermetia illucens) (Schmitt et al., 2019).
Larvae of BSF serve a high amount of protein (49%) and fat (29%) that higher compared to the other insect (Wang and Shelomi, 2017). A previous study describe that there is no significant differences on fish growth and vision regarding the utilization of BSF as feed additive in rainbow trout (Sealey et al., 2011). Another study reported that larva of BSF improves the health status in marron via up-regulating the cytokine gene in the intestinal tissue (Foysal et al., 2019).
It proves that BSF larvae potentially increase the performance of fish and marron with a similar result as good as a commercial fish food. However, the utilization of BSF larvae for a feed formulation in African catfish has not been conducted before. This study aimed to analyze the potency of feed formulation using larvae of BSF on total crude protein and fat in the meet of African catfish (Clarias gariepinus).
Methods
Ethics approval
This study has been approved by the local ethical clearance committee from the Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, East Java, Indonesia with registration number: 18/E.C/FKH-UWKS/June/2020. The committee has been monitored all the experimental procedures during the study.
Time and place of study
The study was carried out from July 2020 until February 2021. The maintenance of experimental animals was conducted in a local farmer pond in Surabaya. The laboratory tests were conducted in the Unit of Veterinary Testing and Feed Analysis, Faculty of Veterinary Medicine, University of Airlangga, East Java, Indonesia.
Larvae of black soldier fly (BSF) preparation
The egg of BSF was obtained from the Laboratory of Parasitology, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, East Java, Indonesia. The egg was stored in a jar and incubated inside the chamber at 29ºC of temperature, 60% of humidity, and 16/8 light/dark (daily) for three days (Miranda et al., 2020). The newly-hatched larvae was placed in an acrylic container with a lid. Further, they were fed with mixed vegetables including cabbage, carrots, and tomatoes (1:1:1) as much as 0.5 kg/day for 15 days.
Feed formulation
This study used two types of feeds, such as commercial fish feed (Performa 3, Superindo, Indonesia) and natural fish feed using larvae of BSF. Before the formulation, the larvae of BSF was dried using a combination of solar-drying and oven-drying (Nyangena et al., 2020). After the drying, the dried larvae was cooled at room temperature for 30 minutes and was mashed using an electric blender. The powder of dried larvae was stored inside the zip-lock plastic bag. Further, the commercial feed was mixed with the powder of dried larvae of BSF in 5%, 7%, and 9% from the total body weight of catfish seeds (Putra et al., 2017).
Animal models and design
A total of 120 African catfish seeds (Clarias gariepinus), 60-days-old, weighed 20 grams, length 15 cm, were used as animal models in this study. The catfish seeds were obtained from Modern Fish Market, Gunungsari, Surabaya, East Java, Indonesia. The catfish seeds were transported to the local farmer pond in Surabaya. Further, they were adapted for a day in the pond to decrease the stress after transportation.
In advances, they were separated into four groups as, P0 (control) = treated with commercial fish feed; P1 = were treated with a combination of commercial feed + 5% of a dried larvae of BSF; P2 = were treated with a combination of commercial feed + 7% of a dried larvae of BSF; P3 = were treated with a combination of commercial feed + 9% of the dried larvae of BSF. The treatment was given four times a day for 30 days.
Sample collection
On day 8, the African catfish were euthanized using rapid cooling (Wallace et al., 2018), and the catfish meat samples were collected. The catfish meat samples were collected using an aseptic procedure. The collected samples were stored inside the zip-lock plastic bag. The sample was kept inside the cooler box and were transported into the laboratory.
Crude protein analysis
The crude protein of the catfish meat was measured by the Dumas method using LECO FP 528 (LECO FP 528, USA). This method is used to determine the value of nitrogen content of the sample as the conversion factor for measure the protein content (Thompson et al., 2002). Finally, the crude protein was measured using the following formula:
Protein (%) = nitrogen (%) × 6.25 (1).
Crude fat analysis
Prior to fat analysis, the catfish meat was weighed as much as 2 grams. The meat was inserted into the test tube and mixed with 2 ml of ethanol and 10 ml of HCl. The mixture was boiled for 30 minutes. The tube was cooled at room temperature. The 25 ml of diethyl ether and 25 ml of petroleum ether were added into the tube and it was shaken for a minute, and these procedures were repeated twice. The upper portion of the mixture was poured on the fat-cup and was placed on the sand bath at 30ºC for 45 minutes until the solvent is evaporated. The crude fat was measured using the following equation:
Fat (%) = (mass of cup+fat)/mass of sample x 100 (2).
Ratio of protein/fat
The ratio of crude protein/fat was measured after the value of crude protein and fat has been analyzed. A higher ratio of protein/fat indicated a better diet. The ratio of crude protein/fat was measured using the simple equation below:
Ratio protein/fat = crude protein (%)/ crude fat (%) (3).
Analysis data
The collected data were crude protein, fat, and the ratio of protein/fat from the catfish meat. The types of data were numerical data and it is appropriate to be analyzed using a parametric test. Further, the data were analyzed using analysis of variance (ANOVA) and post hoc test. All the statistical analysis was conducted using SPSS version 16 with a probability value in (p ≤ 0.05).
Results and discussion
The result showed that there are no significant differences between the utilization of dried larvae of BSF on the crude protein of African catfish compared to the control (p≥0.05) (Table 1). In contrast, the utilization of dried larvae of BSF decreased fat total on African catfish meat compared to the control (p≤0.05) (Table 2). However, there are no differences between the low concentration of dried larvae of BSF with the higher concentration (p≥0.05) (Table 2). This result indicated that a low concentration of feed formulation using dried larvae of BSF (5%) decreased the total of fat as similar as higher concentration (7% and 9%). Another result showed that utilization of dried larvae of BSF as feed formula in African catfish increased the ratio of protein/fat (p≤0.05) (Figure 1). The increasing ratio of protein/fat showed higher and higher along with the increased concentration of BSF in the feed.
The development of fish muscle is involving various factors including aquaculture models, genetics, condition, temperature, and feed (Gisbert et al., 2016). Feed formula is the
Table 1: Total of crude protein in meat of African catfish fed a diet containing dried larvae of BSF
| Group | Mean (%) ± standard deviation |
| P0 (control) |
18.68 ± 1.99a |
| P1 (5% of dried BSF larvae) |
17.92 ± 0.82a |
| P2 (7% of dried BSF larvae) |
18.91 ± 0.50a |
| P3 (9% of dried BSF larvae) |
18.95 ± 1.24a |
a,b different superscript indicated significant differences (p≤0.05).
Table 2: Total of crude fat in meat of African catfish fed a diet containing dried larvae of BSF
| Group | Mean (%) ± standard deviation |
| P0 (control) |
2.67 ± 0.86a |
| P1 (5% of dried BSF larvae) |
0.61 ± 0.17b |
| P2 (7% of dried BSF larvae) |
0.63 ± 0.34b |
| P3 (9% of dried BSF larvae) |
0.62 ± 0.15b |
a,b different superscript indicated significant differences (p≤0.05).
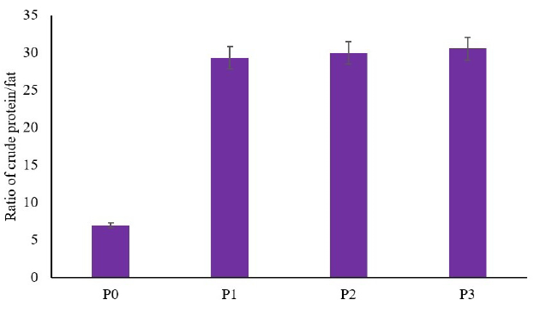
Figure 1: Ratio of crude protein/fat in meat of African catfish fed a diet containing dried larvae of BSF.
main factor that potential to influence muscle formation in fish. A previous study described that utilization of differently sourced feed changed the characteristics of muscle fiber (protein content, fat content, and rapamycin complex 1), size of the fiber, and the content of the muscle either in mammals (Li et al., 2016) and fish (Cruz-Garcia et al., 2011). The utilization of modified feed in the fish potential to promote tissue synthesis rather than for energy use (Kim et al., 2012). It is supported by the result of this study that demonstrated the changing of crude fat content within the muscle tissue rather than the protein through the utilization of dried larvae of BSF.
A previous study described that utilization of BSF larvae as a feed ingredient in fish feed decreases the fat formation rather than protein due to the balancing of amino acid content inside the BSF (Rawski et al., 2020). The amino acids within the BSF larvae increase the absorption rate of feed that promotes the increase of energy within the muscle. However, the energy is not depositing as fat because of an increase of thermal and mechanical energy within the muscle tissue. Further, the digestibility of dried BSF larvae as a feed is higher and well-tolerated by the recipient (Freel et al., 2021). Well-tolerated means that the utilization of BSF larvae as fish meal increases the normalization of the liver activity via the up-regulation and minimalize of fatty deposition inside the hepatocytes (Belghit et al., 2019). So that, the improvement of liver tissue increase the performance of the muscle tissue.
High digestibility of feed promotes the protein content increasing within the tissue that depresses the fat formation. It was proved by the result of this study that demonstrated a higher ratio of crude protein/fat compared to the control. Another factor that affects the decrease of crude fat within the muscle is the ability of African catfish to convert the shorter-chain precursor into highly unsaturated fatty acids. That mechanism occurs through the activation of specific elongase and desaturase (Tocher, 2010). The utilization of BSF larvae as feed formulation for African catfish promotes sustainable energy for green technology in this era. In advances, this study provides something needed by the societies, through the development of alternative feed for fisheries that potential in promoting the formation of high ratio of crude protein/fat in catfish meat product and maybe the other aquaculture products.
Conclusions
The feed formulation using BSF larvae could be used as the alternative fish feed with a better result in the depressing fat formation and increase of the ratio of crude protein/fat compared to the commercial feed. Further, the result of this study can be applied in producing fish meat products with a high ratio of protein/fat. Advances study is needed with larger parameters that include the molecular analysis of the utilization of BSF larvae as feed formulation in fish.
Author’s contributions
EHM and JYN performed the experiment, statistical analysis and drafted the manuscript. HA participated in the design of the study. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflict of interest.
References






