Advances in Animal and Veterinary Sciences
Research Article
Epidemiological Investigations and Ecological Impacts of Commensal Rodents at the North Sinai, Egypt
Doaa S. Farid1, Ali A. El-Sebae1, Ahmed I. Youssef2*
1Arish University, Department of Environmental Protection, Faculty of Environmental Agricultural Sciences, North Sinai, Egypt; 2Suez Canal University, Departments of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, Ismailia, Egypt.
Abstract | Rodents have adverse effects on the natural environment and can threat public health by transmitting many zoonotic diseases. This study aimed to identify the types of commensal rodent species and their populations in three localities of the North Sinai Governorate, Egypt, and to determine the impacts of locality, gender, and season on the pattern of the distribution. The rodents were captured using wire-live traps from December 2016 to November 2017 at three localities (Bir El`Abd, Rabaa, and Qatia). A fixed number of baited traps were applied during the night once monthly. A total of 119 rodents were trapped and transported to the laboratory. Four rodent species belong to the family Muridae were classified according to reference keys. The most common captured rodent species was R. norvegicus (68.91%), and the least common species was M. musculus (2.52%). The highest rodent prevalence was recorded in Bir El-`Abd (63.87%), and the highest rodent index was recorded in the fall season (index=0.175). In conclusion, this study identified three rat species (R. norvegicus, R. r. frugivorus, and R. r. alexandrinus), and one mouse species (M. musculus) captured from three locations in North Sinai, Egypt. These rodents could cause economic impacts and health hazards to humans and animals.
Keywords | Ecology, Rattus norvegicus, Rattus rattus, Mus musculus, Epidemiology
Received | February 04, 2021; Accepted | June 04, 2021; Published | August 15, 2021
*Correspondence | Ahmed I. Youssef, Suez Canal University, Departments of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, Ismailia, Egypt; Email : [email protected]
Citation | Farid DS, El-Sebae AA, Youssef AI (2021). Epidemiological investigations and ecological impacts of commensal rodents at the North Sinai, Egypt. Adv. Anim. Vet. Sci. 9(10): 1511-1516.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1511.1516
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Farid et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Rodents are the most frequent mammals worldwide. They consist of 30 families including 2700 species (Aplin et al., 2003). Rodents are classified into two groups: Commensal rodents, which belong to Muridae family of Myomorpha, and wild rodents, which belong to Cricetidae family of Myomorpha (Etemad, 1979). Worldwide, the most dominant commensal rodent species are Rattus norvegicus, Rattus rattus, and Mus musculus. These rodents have great adaptability to varying environmental conditions and are commonly distributed in the urban environment (Puckett et al., 2016; Schweinfurth, 2020). From economic perspectives, it is estimated that approximately 20% of the world’s food supply is consumed or damaged by rodents (Ehrlich et al., 1993). The commensal rodents can threaten public health by spreading numerous diseases to humans and animals, especially in low hygienic levels, densely populated areas, and without adequate infrastructures. It has been well documented that the commensal rodents, including rats and mice, are vehicles or reservoirs of pathogens to humans and animals and have a primary role in transmitting viral, bacterial, rickettsial, and parasitic diseases to humans and animals by direct or indirect ways (Panti-May et al., 2017; Williams et al., 2018). Rodents are reservoirs for many diseases such as salmonellosis, campylobacteriosis, and plague (Garber et al., 2003; Anon, 2010), Weil’s disease (Bunnell et al., 2000), leishmaniasis (Brandao-Filho et al., 2003), etc. The human and animal foods and food storage areas and facilities could be contaminated by rodents’ fur, urine, and droppings that contaminate the stored food and water supplies and transmit disease through contamination, bites to humans, and vectoring zoonoses (Han et al., 2015).
On a global level, the distribution of rodent species could be influenced by several factors such as the rapid development of the industry and agriculture investments, changing human settlement patterns, urbanization trends, and climate change (Rabiee et al., 2018). Therefore, studying rodent ecology is essential to determine ecological impacts and pathogen transmission dynamics within rodent populations and estimate zoonoses’ risk from these rodents to humans and animals (Himsworth et al., 2013). The high abundance of rodent populations expected to increase the risk of more contact between humans and rodents, which in turn, resulting in rat-borne zoonoses (Davis et al., 2005).
In Egypt, rats and mice are ubiquitous in many Governorates (Shoukry et al., 1987). Previous studies have been conducted for studying the prevalence of different species of rats and mice and estimating their harmful impacts on the environment in North Sinai (Shoukry et al., 1986, 1993; Younis et al., 1995; El-Kady et al., 1998; Mikhail et al., 2011). However, the previous studies on the ecological impacts of rodents in Egypt were more generalized and did not focused on specific localities. Therefore, this study aimed to (i) identify the distribution of the commensal rodent species at three studied areas at the North Sinai Governorate, Egypt, (ii) determine the factors that influence the distribution pattern of these commensal rodents.
MATERIALS AND METHODS
Ethics statement
The ethics committee approved this study of Suez Canal University. During the monitoring study, rodents were captured, handled, and euthanized according to the guidelines of the Scientific Research Ethics Committee with no: 2020004.
Study area
This investigation was carried out at three different areas (Bir El-`Abd city, Rabaa and Qatia villages) of North Sinai, Egypt from (31°00’38”N 33°00’25”E) to (30°57’35 “N 32°44’ 54 “E). The current study extended over one year, from December 2016 to November 2017.
Trapping of rodents
Trapping methodology was based on calculating the total number of rodents trapped compared to the total number of traps set (Aplin et al., 2003). Eighty wire-live traps (32 x 14 x 10 cm) were placed in the three locations Bir El-`Abd (no=40 traps), Rabaa (no=20 traps), and Qatia (n=20 traps) (Figure 1). The number of the distributed traps per collection was related to the size of the area of the localities. The differences in the number of the applied traps for each collection site were related to the size of the geographical areas and the number of inhabitants in each collection area.
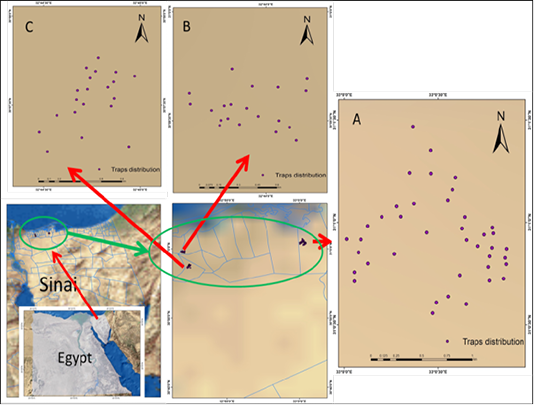
Figure 1: Maps showing the geographical location of the study areas and the pattern of spatial distribution of traps for rodents collection from three localities named Bir El`Abd (A), Rabaa (B) and Qatia (C), located at the North Sinai Governorate, Egypt.
Before setting, traps were thoroughly cleaned with hot water and liquid soap. Then, the traps were pre-baited with fresh food (tomato slices, carrot, fish, cucumber, cheese, and canned salmon). The types of baits were selected randomly to be acceptable by commensal rodents. The baited traps were placed on the floor alongside the wall inside and outside houses, household poultry barns, and livestock animals before the sunset and collected in the early morning before the sunrise once monthly through one year. The trapped rodents were transported with minimum delay inside traps to the laboratory of Zoonoses, Faculty of Veterinary Medicine, Suez Canal University for identification.
Rodent identification
The captured rodents were humanely euthanized for body measurements and identification. The body measurements were recorded in (mm) included the head and body length, tail length, ear length, and hindfoot length, while the body weight was recorded in (gm) according to standards described by Osborn and Helmy (1980). The rodent’s gender was determined by examining the genital region by observing the distance between the anus and the urethral opening and testis in males and the vaginal opening in females. The vagina is a very young female who appeared as a small bald patch immediately to the rear of the urethral opening and was completely covered with a translucent layer of skin.
The rates and indices used were calculated as follows
Rodent index= (number of rodent trapped /total traps distributed). Also, the percentage of every species was estimated as a percent from total rodents captured during the study period as follows:
Prevalence= (number of rodent species/ total rodents captured)*100 to capture seasonal variations in rodents ecology.
Statistical analysis
Statistical analytical package of SPSS 24.0 software was applied for analysis of one-way ANOVA to compare the frequencies of the rodent species in different seasons.
RESULTS AND DISCUSSION
The increase of commensal rodent populations in the environment can cause both economic and public health impacts. Each year, rodents are responsible for financial losses and causing damage to food supplies (Pimentel et al., 2005; Puckett et al., 2016). In the current study, a total of 119 individuals of rodent species were captured from three localities in North Sinai. The collected rodents were identified to three rat species including 82 (68.91%) R. norvegicus, 28 (23.53%) R. r. frugivorus, 6 (5.04 %) R. r. alexandrinus, and one mouse species; M. musculus 3 (2.52%) (Table 1). These species were similar to those identified in other studies in Egypt in Dakahlia governorate (El Kady et al., 2008), in Beheira governorate (Mmetwaly et al., 2009), in Qaliubiya (Yassin, 2009), and in Menofiya (Soliman et al., 2010). Moreover, previous studies that performed in different countries were in harmony with the current finding in terms of such as the studies conducted by Rafique et al. (2009) in Pakistan, Solanki et al. (2013) in India, Cigarroa-Toledo et al. (2017) in Mexico, and Hanafi-Bojd et al. (2007) in Iran. However, another study reported that only R. norvegicus and M. musculus were identified as species distributed in Mashhad, Iran (Moravvej et al., 2015). This finding indicated the widespread of these commensal rodents over varied ecological and geographical areas posing economic impacts in desert and semi-desert areas. Thus, the control of the commensal rodent required global cooperation for combating rodent pests.
The results of this study revealed that the predominant species was R. norvegicus by a prevalence of 68.91% (Table 1). Soliman et al. (2001) reported that R. norvegicus was the dominant species (53.4%) in the Sharkia Governorate, Egypt. Approximately similar findings have been reported in Iran (Hanafi-Bojd et al., 2007) (78%) and (Esfandiari et al., 2017) (67.3%). However, a lower prevalence of 29.5% was reported by (Yassin, 2009) in the Qualubiya governorate, Egypt, and a prevalence of 5.5% in Iran (Dehghani et al., 2012).
The highest trapping success with 76 rodents was exhibited at Bir El-Abd area, followed by Rabaa. Qatia was observed as the lowest rodent trapping with only 20 rodents. R. norvegicus has recorded the dominant species in Bir El-Abd (69.74%) and Rabaa (100%), while R. r. frugivorus in Qatia (70%) was the most frequent. The total rodent index was 0.124 (119 rodents/ 960 trap nights). The rat index of R. norvegicus 0.085 (82/ 960), R.r. frugivorus 0.029 (28/ 960) , R.r.alexandrinus 0.006 (6/ 960) and index of M. musculus 0.003 (3/ 960) (Table 1). The population density of Bir El-`Abd locality is higher than that of Rabaa and Qatia localities. An explanation of this finding could be supported by existing wastes and rubbish that could increase rodent populations. Also, poultry buildings and chicken rooms that are commonly established close to houses provide shelter and increase feed stores that can be attacked by rodents and can increase rodent populations.
The differences in species composition of rodents depend on interspecific compotation, locality, habitat type, neighboring, and preferred food (Desoky et al., 2014). Commensal rodents can adapt to the habitats in different ecological systems (Schweinfurth, 2020). Results showed that R. norvegicus was the only species in Rabaa (100%). This may result from the increasing competition with large rodent species and the suitability of conditions for R. norvegicus living in this area. However, the higher prevalence of R. r. frugivorus in Qatia may be attributed to the ecology of the study area that can provide shelter and feed availability for the commensal rodents more than in the other two study areas.
In general, the overall distribution frequency of females was higher than males with a prevalence of (54.62%) and (45.38%) in females and males, respectively, with a sex ratio male/female of 1:1.2 (Table 1). This finding was consistent with the findings reported by Mmetwaly et al. (2009) in Beheira governorate, Egypt, Asiry and Fetoh (2014) in Saudi Arabia, Allymehr et al. (2012) in Iran, and Omudu and Ati (2010) in Nigeria. Indeed, sexual differences in physiology, behaviour, and evolutionary roles, have been shown to impact both the susceptibility and the exposition to different pathogens (Krasnov et al., 2012). The predominance of females among the collected rodents in this study might be attributed to the more activities of females matched with males. Females repeatedly exit the nests to gain more food during their pregnancy and lactation.
Table 1: Prevalence of rodents’ species captured from three locations in North Sinai, Egypt.
|
Rodent species |
Total number of rodent species / Location site |
|||
| Bir El-`Abd | Rabaa | Qatia | Total | |
| No(%) | No(%) | No(%) | No | |
| R. norvegicus | 53 (69.74) | 23 (100) | 6 (30) | 82 (68.91) |
| R.r. frugivorus | 14 (18.42) | 0 | 14(70) | 28 (23.53) |
| R. r. alexandrinus | 6(7.89) | 0 | 0 | 6 (5.04) |
| M. musculus | 3(3.95) | 0 | 0 | 3( 2.52) |
| Sex | ||||
| Male | 35 (46.05) | 11 ( 47.83) | 8 (40) | 54 (45.38%) |
| Female | 41 (53.95) | 12 (52.17) | 12 (60) | 65 (54.62%) |
| Total | 76 (63.87%) | 23(19.33%) | 20(16.81%) | 119 |
Table 2: Variability in rodent number (Mean ± SE) during different seasons of the study.
| Species |
Season |
||||
| Winter | Spring | Summer | Fall | P value | |
| R.norvegicus |
6.33a±0.52 |
6.00a±0.87 |
5.33a±0.33 |
9.66a±0.18 |
0.200 |
| R.r.frugivorus |
1.00a±0.05 |
3.00a±0.02 |
2.00a±0.06 |
3.33a±0.06 |
0.663 |
| R. r.alexandrinus |
0.33ab±0.01 |
0.00b±0.00 |
0.66ab±0.01 |
1.00a±0.00 |
0.051 |
| M.musculus |
0.33a±0.00 |
0.00a±0.00 |
0.66a±0.01 |
0.00a±0.00 |
0.219 |
| Index | 1.00 | 0.113 | 0.108 | 0.175 | |
a, b, c, d Means carrying different superscripts in the same row are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same row are non-significantly different at (P < 0.05). SE=standard error.
Regarding the effect of season on rodent distribution, there were no significant differences of seasonal effects on rodent occurrence among R. norvegicus, R. r. frugivorus, and M. musculus (P > 0.05) while R. r. alexandrinus showed a significant difference (P ≤ 0.05) among different seasons (Table 2). The highest rodent index was recorded in the fall, followed by spring, summer, and winter with indexes 0.175,0.113,0.108 and 0.100, respectively (Table 2). These findings were consistent with a previous study conducted in the North Sinai governorate (Shoukry et al., 1986), and another study conducted in the Nile Delta (Bakr et al., 1996). However, in the Saint Catherine area, South Sinai, the highest trap index was during May (El-Kady et al., 1998). Although the reproduction is primarily seasonal, although it does take place all year long (Gomez et al., 2008), the increased populations of rodents in the fall season may be related to the suitability of the atmosphere in the fall season for activity and reproduction (Gomez et al., 2008). Identifying the occurrence and pattern of the commensal rats and mice distribution at the North Sinai Governorate could provide valuable data for the rodent combating programs at local and national levels.
CONCLUSIONS AND RECOMMENDATIONS
This study identified the distribution of three rat species (R. norvegicus, R. r. frugivorus, and R. r. alexandrinus), and one mouse species (M. musculus) and captured from three locations in North Sinai, Egypt. The data of this study could be used in planning the rodent control programs and measure the success of combating rodent pests. Further studies are needed for mapping the spread of different rodent species in Egypt and to assess the ecological and zoonotic implications of the commensal rodents.
NOVELTY STATEMENT
This study provided a novel data about the species of the commensal rodent species at the North Sinai Governorate, Egypt, and determined the impacts of locality, gender, and season on the pattern of the distribution. The study provide
valuable data for the rodent combating programs at local and national levels.
AUTHOR’S CONTRIBUTION
D. Farid collected the rodents, performed rodent identification, and drafted the manuscript. A. Youssef edited the manuscript and provided guidance and supervised the laboratory work. A. Elsebae provided support and revised the manuscript. All authors discussed the results and contributed to the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






