Determination of Heavy Metals in Various Tissues of Locally Reared (Baladi) Chicken in Jazan Region of Saudi Arabia: Assessment of Potential Health Risks
Determination of Heavy Metals in Various Tissues of Locally Reared (Baladi) Chicken in Jazan Region of Saudi Arabia: Assessment of Potential Health Risks
Mohammed Al Bratty1, Hassan A. Alhazmi1, Somaya Jadoh Ogdi1, Jana Ahmad Otaif1, Abdul Jabbar Al-Rajab2, Mohammad Firoz Alam3 and Sadique Akhtar Javed1,*
1Department of Pharmaceutical Chemistry, College of Pharmacy, Jazan University, Jazan 45142, Saudi Arabia
2Centre for Environmental Research and Studies, Jazan University, Jazan 45142, Saudi Arabia
3Department of Pharmacology and Toxicology, College of Pharmacy, Jazan University, Jazan 45142, Saudi Arabia
ABSTRACT
Consumption of foodstuffs is considered to be one of the major routes of metal exposure to human population. In the present study, concentration levels of seven heavy metals were measured in different body parts of Baladi chicken from Jazan region, Saudi Arabia using Inductively Coupled-Plasma Optical Emission Spectrometry (ICP-OES). The concentrations of heavy metals in various chicken parts were recorded to be in the range of nd-0.01, 0.07-0.18, 1.08-3.95, 9.61-119.0, nd-0.16, 0.13-1.10, and 8.63-67.58 mg/kg for Cd, Cr, Cu, Fe, Ni, Pb and Zn respectively. Relatively higher metal concentrations were observed in bone, lungs and brain, which are generally not consumed by the local population. Meat, the most consumed part of the chicken has been found to be among the least contaminated tissues. Importantly, the lead (Pb) content was found to be of serious concern in the tested chicken samples, as it was measured to be higher than its maximum permissible limit (0.1 mg/kg) in poultry meat set by WHO and European Union. The estimated daily intakes (EDIs) of the tested metals through the consumption of the Baladi chicken were found to be lower than their respective reference oral doses (RfD) set by USEPA. The non-carcinogenic health risks to the target population due to exposure of the tested metals were assessed by estimating the Hazard Quotient (HQ) and Hazard Index (HI) values. The HQ and HI values observed in this estimation were less than one, suggesting that the exposure of these heavy metals through the consumption of Baladi chicken is unlikely to produce potential health risks to the population of the tested region.
Article Information
Received 12 June 2017
Revised 30 August 2017
Accepted 02 October 2017
Available online 03 July 2018
Authors’ Contribution
MAB conceived, designed and supervised the work and participated in data interpretation. AJAR, SJO and JAO performed the experimental work, collected and interpreted the data. HAA performed data analysis. MFA and SAJ collected samples and prepared and revised the manuscript.
Key words
ICP-OES, Heavy metals, Baladi chicken, Jazan, Hazard quotient.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.4.1509.1517
* Corresponding author: sajaved@jazanu.edu.sa
0030-9923/2018/0004-1509 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
Chicken meat is widely consumed throughout the world and it constitutes one of the major sources of essential nutrients mainly protein, in addition to minerals, vitamins and fats (Schonfeldt and Gibson, 2008). In Saudi Arabia, its consumption is very high, in-fact the highest among all the available meat types. Two types of chickens are consumed in Saudi Arabia: broiler and baladi. Baladi chicken is a native chicken species, grown locally by the individuals in their homes and is characterized by relatively smaller size and varying colors. They lay small brown eggs and are adapted to the local environment (Al-Yousef, 2007). The consumption of Baladi chicken by the local population is considerably high, however, it is lower than broiler chicken because of easy availability and low cost of the later.
Heavy metals intake at the local, regional and global levels is a major issue because it affects the structural and functional integrity of the ecosystem. The contamination of meat by heavy metal pollutants is a serious concern for food safety and a threat to human health because most of these metals are toxic even at very low concentration levels (Abduljaleel et al., 2012). Some investigations on heavy metal contents in meat samples indicated the direct correlation with animal feeds and suggested that the bioaccumulation of these metals in animal feeds are the main source of intake (Kim and Koo, 2007; Sedki et al., 2003). The distribution and bioaccumulation of trace elements varies significantly from one animal species to another and also differ from one tissue type to another tissue of the same animal (John and Jeanne, 1994). Some heavy metals are potentially toxic such as lead, mercury, cadmium and arsenic, whereas some are considered to be essential such as copper, manganese, iron, selenium, cobalt, nickel, zinc etc. The toxic metals are extremely harmful even at trace levels, if exposed to the body over a long period of time and excessive intake of essential metals may also produce toxic effects (Abduljaleel et al., 2012; Celik and Oehlenschlager, 2007; Nighat et al., 2016; Uluozlu et al., 2009). Heavy metals intake through ingestion of food items is among the most common route of exposure of toxic elements to human beings. It accounts for more than 90% exposure in comparison to other pathways such as inhalation and dermal adsorption (Loufty et al., 2006). Since last few decades, heavy metal contamination in foodstuffs such as meat and meat products, vegetables and fish has unprecedentedly increased, hence, exposure to toxic metals becoming one of the major threats to human health (Bortey-Sam et al., 2015).
Long term exposure of toxic metals in animals and human beings above their threshold levels may have serious health consequences and can cause a variety of non-carcinogenic as well carcinogenic hazards (Lai et al., 2010; Zheng et al., 2007). Excessive intake of cadmium can increase the risk of post-menopausal breast cancer and induce genetic alteration in various other cancer-related genes resulting in the increased incidence of cancer formation (Itoh et al., 2014; Zhitkovich et al., 1995). An increased lead content is reported to increase the incidence of cardiovascular diseases in adults and suppress the intellectual and cognitive development and behavior in children (EC, 2006). Lead exposure can also affect the blood production and interfere with certain enzyme system in human body (Sezgin et al., 2003).
The trace element contamination in various chicken tissues have been extensively studied and reported in the literature (Abduljaleel et al., 2012; Alturiqi and Albedair, 2012; Bortey-Sam et al., 2015; Mahmoud and Abdel-Mohsein, 2015; Caldas et al., 2016; Rehman et al., 2012; Uluozlu et al., 2009). However, the baladi (native) chicken species grown in Saudi Arabia is among the ignored foodstuffs in this respect, there is scarcity of the data on heavy metal contents and its possible adverse effects to the consumer population. To the extent of our knowledge, so far no investigation has been reported for the estimation of trace elements in the tissues of Baladi chicken in Jazan region. Therefore, the objectives of this investigation were: to measure the contents of seven heavy metals including Cd, Cr, Cu, Fe, Ni, Pb and Zn in various parts of the locally grown Baladi chicken, to reveal the behavior of these tissues towards the accumulation of metal contaminants and to evaluate the health risk posed by the tested elements to the human population via consumption of these chicken parts. The sample solutions were prepared by microwave digestion technique and the concentrations of heavy metals were measured by using Inductively Coupled-Plasma Optical Emission Spectrometry (ICP-OES).
Materials and methods
Reagents and instruments
The chemicals and reagents used in this study were of analytical grade. Double de-ionized water (resistivity 18.2 MΩ-cm) used for dilutions, was produced in our laboratory using Milli-Q water purifier system (Millipore, USA). HNO3 (70%) and H2O2 (30%) were purchased from Sigma-Aldrich, Germany. Prior to their use, all the glass and plastic wares were soaked in dilute HNO3 (10%) overnight and then rinsed with de-ionized water. Standard solutions for all the tested heavy metals were prepared from their stock solutions (1000 mg/L) procured from Ultra Scientific, USA. The tissue samples were digested in advanced microwave digestive system (Ethos-one, Milestone USA). Maximum temperature (300°C) and pressure (1595 psi) was applied. The Teflon vessels (100 mL capacity), used for all digestions were cleaned with concentrated nitric acid and dried before each run. The content of heavy metals was estimated using ICP-OES (iCAP 6000 Series, Thermo Scientific, USA), equipped with auto-sampler (CETAC ASX-520, USA).
Study area
Jazan province is located in the south-west (16° 53’ N and 42° 33’ E) region of Saudi Arabia, directly attached with Yemen border. The city of Jazan is the capital of the province. Enormous agricultural activities across the Jazan region are considered to be the major contributor in the environmental pollution. A variety of fruits, vegetables and grains are cultivated in the area. Moreover, Jazan is the economic city having a number of industries including copper, aluminum and steel industries, in addition to a huge power station, oil port, desalination station and enormous automobile works.
Sample collection
For the purpose of sample collection the tested region was divided into five sub-regions. A total of 48 live adult Baladi chickens were collected during the months of November-December 2016 from across the region (Jazan, n = 8; Abu Arish, n = 12; Sabya, n = 11; Derb, n = 9 and Samta, n = 8). The collected chickens were then directly transported to Environmental Research Laboratory, Jazan University, where different tissue parts including meat, liver, lungs, gizzard, heart, skin, cartilage, bone and brain were isolated after dissection and stored in clean polythene bags at -20 °C till further processing.
Sample digestion and preparation
The chicken tissue samples were digested by applying microwave digestion procedure described by Uluozlu et al. (2009) with slight modification. Approximately 1.5 g of each tissue (properly mixed) was weighed and transferred to cleaned Teflon digestion vessels followed by the addition of 9.0 mL HNO3 (70%) and 3.0 mL hydrogen peroxide (30%). The digestion vessels were capped and placed into microwave digestion system. The following digestion program was applied: 250 W for 2 min, 0 W for 2 min, 250 W for 6 min, 400 W for 5 min and 550 W for 8 min followed by ventilation time for 8 min. All the digests were clear. The digested samples were transferred into 100 mL plastic tubes and filled up to the mark with de-ionized water. Blank was prepared using the same procedure.
Quantification of heavy metals
The sample solutions were analyzed using an ICP-OES (iCAP 6000 Series, Thermo Scientific) for the quantification of cadmium, chromium, copper, iron, nickel, lead and zinc. The following instrument operating parameters were set for the analysis: carrier gas–argon; pump rate–50 rpm; nebulizer type–concentric; RF forward power–1350 W; nebulizer gas flow–0.6 L/min; auxiliary gas flow–1 L/min; coolant gas flow–12 L/min and integration time–5 to 15 sec. The instrument was calibrated using standard solutions for each selected elements and the calibration standard curve was established at three concentration levels (0.1, 1 and 10 ppm). The analytical wavelengths of investigated elements were 226.502 nm (Cd), 205.559 nm (Cr), 324.754 nm (Cu), 238.204 nm (Fe), 221.647 nm (Ni), 220.353 nm (Pb) and 213.856 nm (Zn). All the samples were injected in triplicate and the data were monitored using iTEVA iCAP Software (Thermo Scientific). The concentrations of each element were calculated in mg/kg on fresh weight basis of the chicken tissue samples.
Potential health risk assessment
The non-carcinogenic risk for adults and children populations in the Jazan region of Saudi Arabia due to heavy metals exposure through the consumption of Baladi chicken parts was assessed as per the method described by US Environmental Protection Agency (USEPA, 1989). The estimated daily intake (EDI) values of the trace elements through the consumption of the edible chicken parts (meat, liver, gizzard, heart, cartilages and skin) were calculated from the observed mean concentrations of heavy metals in the chicken samples and the average daily consumption of Baladi chicken in the region. The EDI (mg/kg/day) has been estimated using the following equation (Eq. 1):
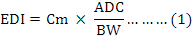
Where, Cm (mg/kg on fresh weight basis) is the average metal concentrations in chicken samples observed in this study, ADC (kg/person/day) is average daily basis consumption of Baladi chicken in the region and BW is body weight of the target population, 70 kg for adult (USEPA, 1989) and 30 kg for children (Bortey-Sam et al., 2015). The average daily consumption of Baladi chicken was assumed to be 0.126 kg and 0.084 kg for adult and children, respectively based on a survey conducted by our team in Jazan region during the sample collection. Furthermore, based on this survey, bone, lungs and brain were not included in the risk assessment because these parts are generally not consumed by the target population.
The non-carcinogenic risks due to trace metals through the consumption of Baladi chicken by the local inhabitants is expressed as HQ (Hazard Quotient), which is the ratio of EDI (estimated in this study) of a pollutant with the reference oral dose (RfD), which is an estimated amount of tolerable daily intake of the contaminants by the humans during a life time. The HQ was determined by using the following mathematical equation (Eq. 2) provided in the USEPA guidelines (USEPA, 1989):
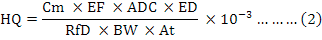
Where, Cm (mg/kg on fresh weight basis) is the average metal concentrations in chicken samples observed in this study, ADC (kg/person/day) is average daily basis consumption of Baladi chicken in the region, BW is body weight of the target population, ED is exposure duration (70 years), EF is exposure frequency (365 days/year), At is average exposure time (ED x 356 days) and RfD is oral reference dose (mg/kg/day). The RfD (ingestion) values used in this assessment were 1.00E-03, 3.00E-03, 4.00E-02, 2.00E-02, 3.50E-03 and 3.00E-01 for the elements Cd, Cr, Cu, Ni, Pb and Zn respectively (De Miguel et al., 2007) and 7.00E-01 for Fe (Harb et al., 2015). If the HQ values is less than unity (<1), the exposure of pollutant to the population is unlikely to cause obvious adverse effects.
It was assumed that the level of adverse effects to the exposed population would be proportional to sum of the multiple contaminants. Subsequently, the Hazard Index (HI) was calculated by the addition of the Hazard Quotients (HQs) of all the metals assessed in this study. HI is considered to be an estimated potential risk of the exposure of multiple heavy metals (USEPA, 1989). The HI was calculated using the following equation (Eq. 3):
HI = ∑HQ = HQCd + HQCr + HQCu + HQFe +
HQNi + HQPb + HQZn ……… (3)
Statistical analysis
The calculated results of heavy metal determination were presented as mean ± SD and one way analysis of variance (ANOVA) was employed to assess the significant variation of metal contents between the different tissue parts. Pearson correlation test was applied to evaluate the relationship between heavy metal concentrations in different tissue samples.
Results and discussion
Table I shows that the trace elements levels in the tested chicken samples recorded to be in the range of nd-0.01 mg/kg for Cd; 0.07-0.18 mg/kg for Cr; 1.08-3.95 mg/kg for Cu; 9.61-119.0 mg/kg for Fe; nd-0.16 mg/kg for Ni; 0.13-1.10 mg/kg for Pb and 8.63-67.58 mg/kg for Zn on fresh weight basis.
Cadmium
Cadmium levels in the tested chicken sample were reported to be well below its permissible limits of 0.05 mg/kg in meat, 0.5 mg/kg in liver and 0.5-1.0 mg/kg in offal for human consumption (EC, 2006). The average Cd concentration in all tissues of Baladi chicken in the tested region was observed to be 0.006 mg/kg. The highest Cd level (0.01mg/kg) was recorded in liver, skin and meat samples, while remained undetected in heart, cartilage, gizzard and brain samples. In comparison to this study, Cd concentration in the chicken samples were observed in the range from 0.25-6.09 μg/kg in Turkey (Uluozlu et al., 2009), 1-2 μg/kg in Canada (Dabeka and Mckenzie, 1995) and 0.05-0.9 mg/kg in Nigeria (Onianwa et al., 2000). Accumulation of cadmium in human body may induce dysfunction of kidneys and reproductive system. The animals could be exposed to Cd through their contacts with cadmium-plated articles, municipal wastes, paints, plastics and electroplating wastes.
Chromium
Chromium is one of the essential elements and its presence in the diet is particularly important because of its role in lipid metabolism and insulin function (Bratakos et al., 2002). In this study, average Cr content was observed to be 0.13 mg/kg in the tested chicken samples and was in the range from 0.07-0.18 mg/kg. The highest Cr level (0.18 mg/kg) was found in liver and brain followed by skin (0.17 mg/kg) samples. The least Cr content (0.07 mg/kg) was detected in meat samples. The mean Cr content observed in present study was found to be higher than its concentration (0.1 mg/kg) present in most food items (Kumpulainen, 1992). The daily dietary intake of Cr recommended by US National Academy of Sciences is in the range of 50-200 μg/day (NAP, 1989). Other studies on chicken samples have reported Cr levels ranging from 0.11-0.21 μg/g in Greek (Bratakos et al., 2002) and 0.01-0.72 μg/g in Turkey (Uluozlu et al., 2009). Chromium is one of the components of corrosion resistant steel and also used in pigments, wood preservatives and leather tanning (Verla et al., 2015).
| Tissues |
Cd |
Cr |
Cu |
Fe |
Ni |
Pb |
Zn |
| Liver |
0.01±0.003* |
0.18±0.05* |
1.28±0.06* |
119.0±8.89* |
nd |
0.14±0.04* |
20.73±2.48 |
| Bone |
0.008±0.006 |
0.11±0.01 |
1.53±0.29 |
35.75±5.43 |
nd |
0.54±0.06 |
67.58±5.23 |
| Heart |
nd |
0.08±0.01 |
3.26±0.34 |
42.80±5.41 |
0.16± 0.05 |
0.13±0.02 |
32.76±4.08 |
| Meat |
0.01±0.002 |
0.07±0.01 |
1.08±0.20 |
9.61±1.44 |
nd |
0.22±0.05 |
9.38±1.24 |
| Cartilage |
nd |
0.16±0.02 |
1.71±0.27 |
28.69±4.48 |
0.01± 0.002 |
0.50±0.08 |
22.25±2.93 |
| Lungs |
0.005±0.002 |
0.16±0.02 |
1.21±0.20 |
55.69±6.02 |
nd |
0.25±0.04 |
22.92±3.96 |
| Gizzard |
nd |
0.09±0.02 |
1.15±0.10 |
28.24±4.11 |
0.03± 0.005 |
0.14±0.04 |
36.98±5.76 |
| Brain |
nd |
0.18±0.03 |
3.95±0.75 |
17.68±2.22 |
0.03± 0.004 |
1.10±0.22 |
15.58±0.91 |
| Skin |
0.01±0.002 |
0.17±0.04 |
1.60±0.48 |
18.35±2.94 |
nd |
0.38±0.09 |
8.63±1.08 |
| Average |
0.005 |
0.13 |
1.86 |
39.53 |
0.02 |
0.38 |
26.31 |
|
Min - Max |
nd-0.01 |
0.07-0.18 |
1.08-3.95 |
9.61-119.0 |
nd-0.16 |
0.13-1.10 |
8.63-67.58 |
*Significant variation between different tissue parts (p<0.05). The metal concentrations are in mg/kg on fresh weight basis. Each sample was injected in triplicate. nd, not detectable.
Copper
The average concentration of Cu in Baladi chicken samples was recorded to be 1.862 mg/kg, which is higher than its maximum permissible limit (1.0 mg/kg) in poultry set by FAO/WHO Codex Alimentarius and European Commission (Zhuang et al., 2014). The highest and lowest Cu concentrations were recorded in brain (3.95 mg/kg) and meat (1.08 mg/kg) samples respectively. In previous studies, the levels of Cu in the chicken samples were reported in the range from 1.00-1.13 μg/g in Nigeria (Onianwa et al., 2001), 0.3-3.5 μg/g in Brazil (Ferreira et al., 2005), 0.10-114 μg/g (Uluozlu et al., 2009) in Turkey and 0.34-3.67 μg/g in Ghana (Bortey-Sam et al., 2015). The level of Cu detected in this work was found to be similar to the reported values in the literature, except that of Turkey, where markedly elevated levels of Cu has been reported in chicken samples. Copper is considered to be vital as well as toxic (at higher levels) for the biological systems. Copper gets access to the foodstuffs through food processing, contaminated environments from certain agricultural inputs, metal-based works and is transferred to the crops from contaminated soils (Onianwa et al., 2001).
Iron
The average iron content in Baladi chicken samples in this study was detected to be 39.53 mg/kg. The maximum and minimum iron concentrations were recorded in liver (119.0 mg/kg) and meat (9.61 mg/kg), respectively. No reported data on the maximum permissible iron concentration in chicken samples could be obtained. Iron is an essential element and its presence in adequate amount in diet is very important to minimize the incidence of anemia. Iron deficiency mainly occurs when there is an inadequate intake of iron, presence of elements that interfere with the iron absorption and bioavailability, during pregnancy and high menstrual loss in women and fast growth in children (Lynch and Baynes, 1996). In the previous studies, iron content in chicken samples were reported to be in the range of 2.91-155 μg/g in Turkey (Uluozlu et al., 2009) and 6.6-542.3 mg/kg in in Ghana (Bortey-Sam et al., 2015).
Nickel
Highest Ni content in Baladi chicken samples was found in heart (0.16 mg/kg), which is lower than the maximum permissible Ni level of 0.5 mg/kg in foods (WHO, 2000). The average Ni content measured in the present study was found to be 0.02 mg/kg. Ni remained undetected in most of the chicken tissues including liver, bone, meat, lungs and skin. Nickel is not considered to be potentially toxic element, however its chronic exposure may be toxic and can produce respiratory complication and even may be carcinogenic (Bortey-Sam et al., 2015). Ni concentrations in chicken samples reported in previous studies were in the range of 0.01-2.08 μg/g in Turkey (Uluozlu et al., 2009) and 0.01-0.55 mg/kg in Ghana (Boetey-Sam et al., 2015) and an average of 1.67 μg/g in Nigeria (Onianwa et al., 2001). Nickel is one of the naturally abundant metals, apart from that it enters the environment through incineration of wastes, combustion of diesel oil and coal. Stainless steel kitchen utensils, tobacco, dental and orthopedic implants and inexpensive jewellery are among the potential sources of nickel (Cempel and Nikel, 2006).
Lead
In this study, maximum lead concentration was found in brain (1.10 mg/kg) followed by bone (0.54 mg/kg) samples of Baladi chicken. Heart was found to possess the lowest lead content (0.13 mg/kg), while the meat sample was reported to be contaminated with Pb at relatively higher level (0.22 mg/kg). Higher levels of Pb in brain and bone in comparison to meat sample may be explained by the fact that, after absorption, Pb is effectively distributed to brain, kidneys and bones and finally get stored in bones, where it accumulates over the time (WHO, 2016). It is worth mentioning that, all the tested tissue types of Baladi chicken were found to contain higher Pb levels than the maximum permitted lead concentration (0.1 mg/kg) in poultry set by FAO/WHO Codex Alimentarius and European Union (Zhuang et al., 2014). Lead is one of the potentially toxic contaminants in the environmental samples and its higher level in foodstuffs is of particular concern because as per WHO, no level of lead exposure is considered to be safe. The maximum permissible Pb intake for adults is 3 mg per week (FAO/WHO, 1976). The binding capacity of lead to sulfhydryl group present in various enzymes is one of the major causes of lead poisoning. The elevated level of lead exposure may increase cardiovascular complications in adults and affect intellectual development in children population (Uluozlu et al., 2009). In the literature, Pb contents in the chicken tissues were reported to be in the range from 0.01-0.40 μg/g in Turkey (Uluozlu et al., 2009), 0.13-0.38 mg/kg in Ghana (Bortey-Sam et al., 2015), which are lower than that observed values in the present work. The major sources of lead are paints, plastic facings, insecticides, ceramics, rubber products glass fittings, vehicle exhaust and batteries etc. (Verla et al., 2015).
Zinc
Zinc is abundant in living organisms because of its important role in the biological systems. It is known to participate in various biochemical pathways in human beings. Immunological abnormalities, growth retardation, loss of appetite and skin abnormalities are some of the complications of zinc deficiency in the body. Zinc is relatively non-toxic element (as per US National Library of Medicine, oral LD50 is ~3 g/kg body weight, which is ten-fold greater than Cd), however, its long-term exposure of elevated amount may produce toxic effects including copper deficiency, respiratory disorders, GIT disturbances and enhanced risk of prostate cancer (Plum et al., 2010). The average Zn level in the tested chicken samples was found to be 26.31 mg/kg. The highest Zn level was reported to be in bone (67.58 mg/kg), which is higher than the Zn permissible limit of 50 mg/kg in meat (EC, 2006). The lowest Zn contents were recorded in skin (8.63 mg/kg) and meat (9.384 mg/kg) samples. In previous studies Zn values in chicken samples were reported to be 1.21-24.3 μg/g in Turkey (Uluozlu et al., 2009) and 2.87 μg/g in Nigeria (Onianwa et al., 2001). The major sources of zinc contamination are agricultural products, antifouling paints, food wastes and particles released from the tires and brake linings of the vehicles. Zinc is also used to galvanize iron and steel to avoid rusting (Verla et al., 2015).
Overall, the results of the current study indicated that the tested chicken samples possessed an unequal distribution of trace elements among the different body parts. Cu, Fe and Zn were observed in relatively higher concentrations than other tested heavy metal, where Fe concentration is the highest followed by Zn and Cu, while Cd was detected at the lowest concentration. Cu, Fe and Zn belong to essential elements and relatively higher levels may be related to their physiological roles in various chicken tissues (Carpene et al., 1995). Kanakaraju et al. (2008) has stated that Fe tend to accumulate at higher levels in human beings to invertebrates because it plays important roles in the body system as an essential element. The measured average heavy metal concentrations were observed in the following descending order: Fe>Zn>Cu>Pb>Cr>Ni>>Cd. The higher heavy metals levels were observed in liver, bone and brain samples. Out of these bone and brain are generally not consumed, while the liver is less favorite among the population in the region. Meat is the most consumed part of the chicken, which has been found to be among the least contaminated tissues by the tested heavy metals. However, the levels of heavy metals especially lead observed in this study are cause of concern, suggesting the need of regular monitoring of these pollutants in the tested region.
The variation in heavy metal contents among different tissue parts was assessed using one-way ANOVA method at 95% confidence level. Significant variation (p<0.05) in the concentrations of Cd, Cr, Cu, Fe and Pb has been recorded in different chicken parts, whereas insignificant variation was observed for Ni and Zn contents. Pearson correlation test was performed to evaluate the relationship between the observed heavy metal concentrations in this study. The values of correlation coefficient (r) are shown in Table II. Good correlations were observed between Ni-Cu and Pb-Cu (r = 0.616 and 0.631, respectively). Positive correlations were observed between heavy metals, Cr-Cd, Fe-Cd, Cu-Cr, Fe-Cr, Pb-Cr, Zn-Fe and Zn-Ni with the corresponding r values of 0.086, 0.302, 0.130, 0.379, 0.440, 0.085 and 0.130, respectively. However, negative correlation was seen between Cu-Cd, Ni-Cd, Pb-Cd, Zn-Cd, Ni-Cr, Zn-Cr, Fe-Cu, Zn-Cu, Ni-Fe, Pb-Fe, Pb-Ni and Zn-Pb with the corresponding r values of -0.558, -0.505, -0.313, -0.206, -0.436, -0.375, -0.216, -0.074, -0.033, -0.393, -0.183 and -0.026, respectively. The positive correlation co-efficient suggests that the heavy metal contents are probably being controlled by similar sources.
Table II.- Correlation between heavy metal concentrations of different tissues of Baladi chicken.
|
Cd |
Cr |
Cu |
Fe |
Ni |
Pb |
Zn |
|
| Cd |
1 |
|
|
|
|
|
|
| Cr |
0.086 |
1 |
|
|
|
|
|
| Cu |
-0.558 |
0.130 |
1 |
|
|
|
|
| Fe |
0.302 |
0.379 |
-0.216 |
1 |
|
|
|
| Ni |
-0.505 |
-0.436 |
0.616 |
-0.033 |
1 |
|
|
| Pb |
-0.313 |
0.440 |
0.631 |
-0.393 |
-0.183 |
1 |
|
| Zn |
-0.206 |
-0.375 |
-0.074 |
0.085 |
0.130 |
-0.026 |
1 |
Effects of consumption of heavy metal contaminated chicken parts
The estimated intake (EDIs) of toxic elements for adults and children through the consumption of Baladi chicken parts in Jazan region of Saudi Arabia were calculated and presented in Table III. The EDIs of all the trace elements were calculated using the average metal concentrations estimated in different edible parts (meat, liver, gizzard, heart, cartilages and skin). The calculated EDIs for all the metals in this assessment were found to be lower than their respective oral reference doses (RfDs) set by United State Environmental Protection Agency. The children are more susceptible to acute and chronic effects of intake of chemical contaminants because they consume more food per unit of their body weight as adult (ENHIS, 2007), this could result in higher intake of toxic elements through food consumption in children than adults. Accordingly, the EDIs estimated in this study for children were higher than their adult counterparts. The non-carcinogenic risks from the tested heavy metals via ingestion of Baladi chicken parts were evaluated on the basis of Hazard Quotient (HQ).
| Metals |
Cma |
EDI |
Hazard quotient (HQ) |
Hazard index (HI) |
|||
|
Adults |
Children |
Adults |
Children |
Adults |
Children |
||
| Cd | 0.006 |
1.08E-05 |
1.68E-05 |
1.08E-02 |
1.68E-02 |
|
|
| Cr | 0.13 |
2.30E-04 |
3.58E-04 |
7.68E-02 |
1.19E-01 |
|
|
| Cu | 1.68 |
3.02E-03 |
4.70E-03 |
7.56E-02 |
1.18E-01 |
|
|
| Fe | 41.12 |
7.40E-02 |
1.15E-01 |
1.06E-01 |
1.64E-01 |
|
|
| Ni | 0.03 |
5.76E-05 |
8.96E-05 |
2.88E-03 |
4.48E-03 |
|
|
| Pb | 0.25 |
4.50E-04 |
7.00E-04 |
1.29E-01 |
2.00E-01 |
|
|
| Zn | 21.79 |
3.92E-02 |
6.10E-02 |
1.31E-01 |
2.03E-01 |
0.53 |
0.83 |
aBone, lungs and brain samples were not included in this risk assessment because these tissues are generally not consumed by the target population.
The estimated HQ values of individual metals were found to be less than 1 (Table III), suggesting that the target population is unlikely to suffer any adverse health effect from the exposure of these toxic elements through the consumption of Baladi chicken. The highest HQ values for adults and children were recorded for Zn (1.31E-01 and 2.03E-01, respectively) followed by Pb (1.29E-01 and 2.00E-01, respectively), while the least values were observed from Ni (2.88E-03 and 4.48E-03, respectively) intake. The HQ values for adult and children populations due to exposure of the tested heavy metals increased in the order of Ni<Cd<Cu<Cr<Fe<Pb<Zn. To assess the cumulative risk due to exposure of multiple toxic elements, Hazard Index (HI) have been calculated, which is considered to be the sum of HQ values for all the individual metals. The HI values for both adults and children (0.53 and 0.83, respectively) were found to be below 1 (Table III), suggesting no significant health risk due to cumulative exposure of the tested metals to the target population through the consumption of different edible parts of the Baladi chicken in Jazan region. The bone, lungs and brain were not included in this risk assessment because these parts are generally not consumed by the local population in the region.
Overall, the risk parameters (EDI, HQ and HI) calculated in this study were found to be in the safe limits, indicating that the exposure of adult and children population to heavy metals through consumption of edible parts of Baladi chicken alone will not pose noticeable adverse health effects. However, the effects of the exposure through other sources such as ingestion of other foodstuffs, intake or dermal adsorption of contaminated water, inhalation of urban aerosol, ingestion of playground dust particles (especially children) etc. were not estimated in this study. These environmental exposures would be addition to the intake through chicken consumption; hence the collective risks to the population cannot be assessed correctly. However, the health risks due to exposure of toxic elements through the consumption of Baladi chicken cannot be ignored because the accumulation of toxic pollutants in the human body over the time can produce serious toxic effects.
Conclusion
The concentrations of seven heavy metals were measured in different tissues of Baladi chicken collected from across the Jazan region, Saudi Arabia. The samples were prepared by microwave digestion in HNO3 and H2O2 (3:1) and the determination was done by ICP-OES. The mean concentrations of heavy metals observed in the following decreasing order: Fe>Zn>Cu>Pb>Cr>Ni>>Cd. The results of this investigation have indicated that the different organs and tissues have varying capabilities towards the accumulation of heavy metals. It was also noticed that the essential elements (Cr, Cu, Fe and Zn) were found to present in higher levels than the toxic elements (Cd, Ni and Pb). However the Pb content in the tested chicken parts is of serious concern because it has been reported to be higher than its maximum permissible limit (0.1 mg/kg) in poultry meat set by FAO/WHO Codex Alimentarius and European Union. Metal contents were found to be higher in non-edible chicken parts such as bone, lungs and brain, while chicken meat, the most consumed part found to be among the least contaminated ones. The estimated daily intakes (EDIs) of the tested heavy metals through the consumption of Baladi chicken were low when compared to their respective oral reference doses (RfDs) set by USEPA. The EDIs for children were more than those of adults. The non-carcinogenic risks on the local inhabitants were assessed based on the estimation of Hazard Quotient (HQ) and Hazard Index (HI) for both adults and children. The observed values of HQs and HI were found to be less than unity (<1), which suggests that, the exposure of trace elements through the consumption of Baladi chicken is unlikely to pose any adverse health effect to the population of the tested region. However, the exposure of these metals through other sources has not been considered in this assessment, which would be addition to the exposure through Baladi chicken consumption.
Acknowledgement
The authors are grateful to Deanship of Scientific Research, Jazan University for financial support under the 4th Future Scientist Program (Project Number: FS4-40) to conduct this study.
Statement of conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
Alturiqi, A.S. and Albedair, L.A., 2012. Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt. J. aquat. Res., 38: 45-49.
Al-Yousef, Y.M., 2007. A survey study on the distribution of Saudi baladi chickens and their characteristics. Int. J. Poult. Sci., 6: 289-292. https://doi.org/10.3923/ijps.2007.289.292
Bortey-Sam, N., Nakayama, S.M.M., Ikenaka, Y., Akoto, O., Baidoo, E., Yohannes, Y.B., Mizukawa, H. and Ishizuka, M., 2015. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxic. environ. Safe., 111: 160-167. https://doi.org/10.1016/j.ecoenv.2014.09.008
Bratakos, M.S., Lazos, E.S. and Bratakos, S.M., 2002. Chromium content of selected Greek foods. Sci. Total Environ., 290: 47-58. https://doi.org/10.1016/S0048-9697(01)01057-9
Caldas, D., Pestana, I.A., Almeida, M.G., Henry, F.C., Salomão, M.S. and de Souza, C.M., 2016. Risk of ingesting As, Cd, and Pb in animal products in north Rio de Janeiro state, Brazil. Chemosphere, 164: 508-515. https://doi.org/10.1016/j.chemosphere.2016.08.130
Carpene, E., Serra, R. and Isani, G., 1995. Heavy metals in some species of water fowl of Northern Italy. J. Wildl. Dis., 31: 49-56. https://doi.org/10.7589/0090-3558-31.1.49
Cempel, M. and Nikel, G., 2006. Nickel: A review of its sources and environmental toxicology. Polish J. environ. Stud., 15: 375-382
Dabeka, R.W. and Mckenzie, D.A., 1995. Survey of Pb, Cd, Fe, Ni, Co in food composites and estimation of dietary intakes of these elements. J. AOAC Int., 78: 897-909.
De Miguel, E., Iribarren, I., Chaco´n, E., Ordon˜ez, A. and Charlesworth, S., 2007. Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere, 66: 505-513. https://doi.org/10.1016/j.chemosphere.2006.05.065
ENHIS, 2007. Fact sheet No. 4.4, CODE: RPG4_Foo- d_EX1. European Environment and Health Information System, World Health Organization.
FAO/WHO, 1976. List of maximum levels recommended for contaminants by the Joint FAO/WHO Codex Alimentarius Commission. Vol. 3, Second Series. CAC/FAL, Rome, pp. 1-8.
Ferreira, K.S., Gomes, J.C. and Chaves, J.B.P., 2005. Copper content of commonly consumed food in Brazil. Fd. Chem., 92: 29-32. https://doi.org/10.1016/j.foodchem.2004.07.004
Harb, M.K., Ebqa’ai, M., Al-rashidi, A., Bakri H. Alaziqi, B.H., Al Rashdi, M.S. and Ibrahim, B., 2015. Investigation of selected heavy metals in street and house dust from Al-Qunfudah, Kingdom of Saudi Arabia. Environ. Earth Sci., 74: 1755-1763. https://doi.org/10.1007/s12665-015-4184-2
John, H.H. and Jeanne, I.R., 1994: Food additives, contaminants and natural toxins. Modern nutrition in health and disease, 8th ed., Part II. Lea & Febiger, pp. 1597-1608.
Kanakaraju, D., Jios, C.A. and Long, S.M., 2008. Heavy metal concentration in the razor clam (Solen spp.) from muara tebas, Sarawak. Malay. J. Analyt. Sci., 12: 53-58.
Kim, J. and Koo, T.H., 2007. Heavy metal concentrations in diet and livers of black-crowned night heron Nycticorax nycticorax and grey heron Ardea cinerea chicks from Pyeongtaek, Korea. Ecotoxicology, 16: 411-416. https://doi.org/10.1007/s10646-007-0143-3
Kumpulainen, J.T., 1992. Chromium content of foods and diets. Biol. Trace Elem. Res., 32: 9-18. https://doi.org/10.1007/BF02784582
Loutfy, N., Fuerhacker, M., Tundo, P., Raccanelli, S., El Dien, A.G. and Ahmed, M.T., 2006. Dietary intake of dioxins and dioxin-like PCBs, due to the consumption of dairy products, fish/seafood and meat from Ismailia city, Egypt. Sci. Total Environ., 370: 1-8. https://doi.org/10.1016/j.scitotenv.2006.05.012
Mahmoud, A.M. and Abdel-Mohsein, H.S., 2015. Health risk assessment of heavy metals for Egyptian population via consumption of poultry edibles. Adv. Anim. Vet. Sci., 3: 58-70. https://doi.org/10.14737/journal.aavs/2015/3.1.58.70
Nighat, S., Nadeem, M.S., Mahmood, T., Kayani, A.R., Mushtaq, M. and Hassan, M.M., 2016. Estimation of heavy metals in Indian flying fox Pteropus giganteus (Brünnich, 1782) from Punjab, Pakistan. Pakistan J. Zool., 48: 1787-1792.
Onianwa, P.C., Adeyemo, A.O., Idowu, O.E. and Ogabiela, E.E., 2001. Copper and zinc contents of Nigerian foods and estimates of the adult dietary intakes. Fd. Chem., 72: 89-95. https://doi.org/10.1016/S0308-8146(00)00214-4
Onianwa, P.C., Lawal, J.A., Ogunkeye, A.A. and Orejimi, B.M., 2000. Cadmium and nickel composition of Nigerian foods. J. Fd. Comp. Anal., 13: 961-969. https://doi.org/10.1006/jfca.2000.0944
Rehman, K.U., Andleeb, S., Mahmood, A., Bukhari, S.M., Naeem, M.M. and Yousaf, K., 2012. Assessment of heavy metals in different tissues of broilers and domestic layers. Glob. Vet., 9: 32-37.
Schonfeldt, H.C. and Gibson, N., 2008. Changes in the nutrient quality of meat in an obesity context. Meat Sci., 80: 20-27. https://doi.org/10.1016/j.meatsci.2008.05.025
Sedki, A., Lekouch, N., Gamon, S. and Pineau, A., 2003. Toxic and essential trace metals in muscle, liver and kidney of bovines from a polluted area of Morocco. Sci. Total Environ., 317: 201-205. https://doi.org/10.1016/S0048-9697(03)00050-0
Sezgin, N., Ozcan, H.K., Demir, G., Nemlioglu, S. and Bayat, C., 2003. Determination of heavy metal concentrations in street dusts in Istanbul E-5 highway. Environ. Int., 29: 979-985. https://doi.org/10.1016/S0160-4120(03)00075-8
Uluozlu, O.D., Tuzena, M., Mendila, D. and Soylak, M., 2009. Assessment of trace element contents of chicken products from Turkey. J. Hazardous Materials, 163: 982-987. https://doi.org/10.1016/j.jhazmat.2008.07.050
USEPA, 1989. Human health evaluation manual, EPA/540/1-89/002, Vol. I. Office of Solid Waste and Emergency Response. US Environmental Protection Agency. Washington, DC. Available from: http://www.epa.gov/superfund/programs/risk/ragsa/index.htm.
WHO, 2000. Report of the Fifty-Third of the joint FAO/WHO Expert Committee on food additives. Technical Report Series No. 896, Geneva.
WHO, 2016. Fact sheet, Reviewed September 2016. World Health Organization. http://www.who.int/mediacentre/factsheets/fs379/en/ (Access date: 5 April 2017).
Zheng, N., Wang, Q.C. and Zheng, D.M., 2007. Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao zinc plant in China via consumption of vegetables. Sci. Total Environ., 383: 81-89. https://doi.org/10.1016/j.scitotenv.2007.05.002
Zhitkovich, A., Voitkun, V. and Costa, M., 1995. Glutathione and free amino acids form stable complexes with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis, 16: 907-913. https://doi.org/10.1093/carcin/16.4.907










