Dietary Formic Acid and Vitamin D3 as Growth Effective Supplement for Grass Carp Fingerlings
Dietary Formic Acid and Vitamin D3 as Growth Effective Supplement for Grass Carp Fingerlings
Laiba Shafique1,*, Muhammad Afzal2, Syed Zakir Hussain Shah3, Mahroze Fatima4, Huma Naz5, Saif ur Rehman1, Youchuan Wei1 and Qingyou Liu1,*
1State Key Laboratory of Tropical Biological Resources Protection and Utilization, Guangxi University, Nanning, 530004, China
2Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad
3Department of Zoology, University of Gujrat, Gujrat, Pakistan
4Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore, Pakistan
5Dep Bahawalpur, Pakistan
ABSTRACT
The present research was carried out to evaluate the effectiveness of dietary formic acid and vitamin D3 on growth performance and muscle proximate analyses of grass carp (Ctenopharyngodon idella). Four experimental diets viz. FD1, FD2, FD3 and FD4 having formic acid (%) and vitamin D3 (IU/Kg) 0, 0; 2, 0; 0, 5000 and 2, 5000, respectively were prepared. Fish were fed with experimental diets for 90 days. Growth performance of fish was observed on fortnightly basis throughout the trail. At the end of feeding trail, fish were dissected to obtain the samples of muscles for further analysis. Two-way analysis of variance under RCBD were applied on obtained data. Results demonstrated that growth performance (weight gain, specific growth rate and absolute weight gain) of C. idella fingerlings showed significant improvement by feeding formic acid and vitamin D3. Result showed that C. idella fed with vitamin D3 increased the crude protein, ash and decreased the fat contents in muscles while same result was observed by supplementation of formic acid. In conclusion, dietary formic acid and vitamin D3 improves the growth performance and muscle proximate analysis for the C. idella fingerlings.
Article Information
Received 16 October 2018
Revised 05 November 2018
Accepted 12 November 2018
Available online 26 August 2019
Authors’ Contribution
LS executed the research. MF helped in compiling the data. SZHS did statistical analysis. MA supervised the study. HN and SR helped in writing the article. YW and QL reviewed the manuscript.
Key words
Ctenopharyngodon idella, Growth, Muscles, Formic acid, Vitamin D3.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.sc5
* Corresponding authors: [email protected];
0030-9923/2019/0006-2385 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Aquaculture is the world’s fastest-growing field of agriculture (FAO, 2010). The main objective of aquaculture is to improve the health and growth performance of animals. Feed additives are the best choice (NRC, 1993) to enhance feed properties and growth performance. Antibiotics have long been used in aqua feeds, however, owning to side effects of antibiotics, European Union banned their use in animal production (FAO, 2002; Heuer et al., 2009). Alternatively, antibiotics such as natural immune stimulants, pathogen inhibitors and growth promoters which include plant extracts, probiotics, prebiotics and acidifiers (Devasree et al., 2014; Reda and Selim, 2015; Romano et al., 2015; Selim and Reda, 2015) were used.
Fish meal is an important component of fish diet due to high protein and presence of essential nutrients (Zhou et al., 2004). In the global market the price of fish meal is so high because of its rising demand and limited stock; therefore, it is necessary to search for alternative plant protein sources (Pereira and Oliva-Teles, 2003).
In aquaculture, the replacement of high quality fish meal with plant meal in animal feed is the most cost-effective approach because of reasonable price, high protein content, comparatively balanced amino acid profile and stable supply of plant meals (Storebakken et al., 2000). Plant protein sources contain phytate which cannot be digested (Oliva-Teles et al., 1998), and decreases the digestibility of protein (Spinelli et al., 1983) and utilization of supplementary mineral (Gatlin and Phillips, 1989; Sugiura et al., 2001). Phytate is hydrolysed with the addition of organic acid in the diet (Hossain et al., 2007).
Organic acids (propionic, butyric, formic, citric, malic, sorbic, acetic and lactic acids) and their salts can be used as acidifier in the livestock feed industry and aquaculture, (Defoirdt et al., 2009). The addition of sufficient quantity of organic acids and their salts in the diet can modulate disease resistance and feed quality and also improve the health and growth (Ng and Koh, 2011, 2016).
Acidifiers lower the pH of gastrointestinal tract, enhance the hydrolysis of phytate, kill harmful pathogens, reduce emptying rate of the gastrointestinal tract and improve pepsin activity, nutrient absorption, nitrogen retention and mineral absorption as well as transportation (Ravindran and Kornegay, 1993; Overland et al., 2000; Luckstcadt, 2008).
Cholecalciferol is fat-soluble vitamin (vitamin D3) and essential nutrient for the growth of aquatic organisms. It performs many physiological functions in organisms, due to its vital role in bone formation (Li, 1994). Fish cannot synthesize vitamin D3directly from sunlight like other vertebrates, hence obtains it from diet only (Darias et al., 2011). So, vitamin D3 added in fish diet for proper growth. If excess amount of vitamin D3 is supplemented in the diet, it causes metabolic disorders and calcinosis (Hollis and Wagner, 2004; Horst et al., 2003), while less amount of vitamin D3 can cause tetany (Cho and Slinger, 1979; Barnett et al., 1982; George et al., 1981).
The present study was undertaken to analyze the effect of dietary formic acid and vitamin D3 supplementation on proximate composition in grass carp, Ctenopharyngodon idella.
Materials and methods
The study was carried out at Fisheries Research Farm, University of Agriculture, Faisalabad. Four earthen ponds, each having dimensions 19m×6m×1.5 m were selected and fixed with fine mesh to avoid any unwanted animals to enter the ponds. The ponds were filled with tube well water. Ctenopharyngodon idella (grass carp) (n=25) were stocked after measuring their weight and length in each experimental pond on 1st May 2016. Mixture of inorganic fertilizers that is urea and single superphosphate (1:1), was added in each pond on weekly basis for one month prior to fish stocking. The fish were fed with the fish feed 2% body weight of fishes twice a day. The water level of ponds was maintained throughout the study period. The sampling was done on fortnightly basis and feed ratio was adjusted accordingly. The fish were finally harvested and measured after 90 days feeding period of 1st August 2016.
For feed formulation four experimental diets (FD1, FD2, FD3 and FD4) were prepared that contained formic acid (%) and vitamin D3 (IU/Kg) at following proportion 0, 0; 2, 0; 0, 5000 and 2, 5000, respectively. The duration of experiment was three months. For experimental diet formulations, cereal grinding machine (FFC-45, JIMO, China) was used for grinding and sieving of (0.05 mm) the diet ingredients. The minerals mixed fish oil and vitamins were added after grinding of ingredients. Approperiate amount of water was used for pellet formation. Experimental extruder (Model SYSLG30-IV) was used for the prepration of pellets (3.5 mm), which were dried upto 10% moisture and packed in plastic bags and stored at 20°C during the entire feeding experiment.
Growth performance was assessed on fortnightly basis from each pond in terms of weight gain by the fish. At the end of feeding trial eight fish were starved for a day and dipped in clove oil (3000 mg/L) for 40-60sec to anesthetise (Khajepour et al., 2012). Fish was sacrificed by a sharp blow on head. For proximate analysis standard method of AOAC (1995) were followed to determined moisture at 105oC for 12 h in micro Kjeldahl apparatus used for the determination of crude protein (N x 6.25), Crude fat was estimated by petroleum ether extraction method (Soxtec system HT2 1045) and ash content were measured in electric furnace (Eyela-TMF 3100) by ignition at 650oC for 12 h to constant weight.
The data was analyzed by Two-way ANOVA under RCBD was applied on resulted data (Steel et al., 1996). For the comparison of means Student-Newman-Keuls test was used, when significant differences occurred (Snedecor and Cochran, 1991). For analyzing statistical data CoStat computer package (Version 6.303, PMB320 Monterey, CA 93940, USA) was used.
Weight gain and specific growth rate (SGR) were calculated as:
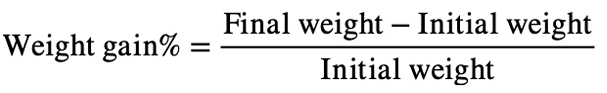

Result and discussion
Table I shows growth performance of fish. All growth parameters were significantly improved by feeding formic acid and vitamin D3 supplemented diet. Muscle proximate analysis is given in Table II. Result showed that C. idella fed with vitamin D3 increased the crude protein (18.30±0.01), ash (2.43±0.02) and decreased the fat (3.84±0.02) contents while same results were observed by supplementation of formic acid for crude protein (18.20±0.02) and ash (2.18±0.02) contents.
The growth rate increased with acidification, because acids increase the mineral availability and their utilization in body. The Organic acids reduce the gastic pH, which improves the activity of many metabolic enzymes leading to improve growth rate (DeWet, 2005; Park et al., 2009). Similarly, Hossain et al. (2007) reported the higher growth rate of pagrus major with the supplementation of citric acid which results in the higher mineral dissociation and forms chelated mineral complexes that are easily absorbed in body. Moreover, organic acid lowers the gastric emptying rate. Present research work showed that supplementation vitamin D3 significantly increased growth rate which may owe to increase utilization of phosphorous. Because of phosphorous utilization Ling-Hong et al. (2015). Similar results were also reported by Ling-Hong et al. (2015) that supplementation of vitamin D increased the growth rate in Wuchang bream. A similar increase in weight gain % of rain bow trout was observed by feeding vitamin D (Coloso et al., 2001).
Table I.- Growth performance of Ctenopharyngodon idella.
|
Diet |
FD1 |
FD2 |
FD3 |
FD4 |
|
FA (%)+ VD3 (IU/kg) |
(0+0) Control |
(2+0) |
(0+500) |
(500+2) |
|
Initial weight (g) |
19.48± 1.05 |
19.13± 0.63 |
19.74± 0.15 |
19.50± 0.75 |
|
Final WG (g) |
181.52± 8.18d |
203.38± 5.31b |
195.36± 2.89c |
220.4± 4.56a |
|
Absolute WG (g) |
162.04± 7.14d |
184.26± 4.68b |
175.62± 2.74c |
200.89± 3.81a |
|
WG (%) |
832.04± 8.03d |
963.57± 7.23b |
889.63± 6.88c |
1030.36± 0.30a |
|
SGR |
3.72± 0.01d |
3.94± 0.01b |
3.82± 0.01c |
4.04± 0.03a |
Values sharing similar letters in a column are non-significant at P<0.05. FA, formic acid; VD3, Vitamin D3, FD, FA+VD3; WG, weight gain; SGR, specific growth rate.
Table II.- Muscle proximate analysis of C. idella.
|
Diet |
FD1 |
FD2 |
FD3 |
FD4 |
|
FA (%)+ VD3 (IU/kg) |
(0+0) Control |
(2+0) |
(0+500) |
(500+2) |
|
Moisture (%) |
75.3±0.04a |
74.8±0.03b |
74.5±0.04b |
75.1±0.04a |
|
Protein (%) |
17.74±0.04d |
18.20±0.02a |
18.30±0.01a |
17.91±0.02c |
|
Crude fat (%) |
3.63±0.05d |
4.13±0.03a |
3.84±0.02c |
3.90±0.04b |
|
Ash (%) |
2.01±0.03b |
2.18±0.02ab |
2.43±0.02a |
1.81±0.03c |
Values sharing similar letters in a column are non-significant at P<0.05. For aabbreviations, see Table 1.
In present study crude protein, crude fat and ash content of muscle were affected by supplementation of vitamin D3 and formic acid. A similar decrease in the lipid content in the muscle of fish were observed by citric acid supplementation, however the yet action is unknown Khajepour et al. (2012). In present research work the effect of vitamin D3 supplementation showed not significant effect on moisture content in muscle of Ctenopharyngodon idella. Similar results were observed by Ling-Hong et al. (2015) who said that addition of vitamin D3 did not affect the moisture content in the muscle of fish while lipid contents, crude protein and ash contents were significantly affected. Moisture is not sensitive indices of dietary vitamin D3. Supplementation of vitamin D in fish diet had non-significant effects on crude lipid in grass carp. This indicated that deficiency of vitamin D in fish body and also decreases the bioavailability of lipids (Jiang et al., 2009).
Conclusion
In conclusion, dietary formic acid and vitamin D3 improves the growth performance and muscle proximate analysis in the Ctenopharyngodon idella fingerlings.
Statement of conflict of interest
The authors declare no conflict of interest.
References
Barnett, B.J., Jones, G., Cho, C.Y. and Slinger, S.J., 1982. J. Nutr., 112: 202-206.
Cho, B.J. and Slinger, C.Y., 1979. Comp. Biochem. Physiol., 63: 291-297. https://doi.org/10.1016/0300-9629(79)90162-2
Coloso, R.M., Basantes, S.P., King, K., Hendrix, M.A., Fletche, J.W., Weis, P. and Ferraris, R.P., 2001. Aquaculture, 202: 145-161. https://doi.org/10.1016/S0044-8486(01)00572-5
Darias, M.J., Mazurais, D. and Koumoundouros, G., 2011. Aquaculture, 315: 49-60. https://doi.org/10.1016/j.aquaculture.2010.12.030
Defoirdt, T., Boon, N., Sorgeloos, P., Verstraete, W. and Bossier, P., 2009. Biotech. Adv., 27: 680-685. https://doi.org/10.1016/j.biotechadv.2009.04.026
Devasree, L.D., Binuramesh, C. and Michael, R.D., 2014. Aquacult. Res., 45: 1581-1590. https://doi.org/10.1111/are.12104
De-Wet, L., 2005. Aqua feeds: Formulation and beyond, 2: 12-14.
Divakaran, S., Leonard, G.O. and Ian, P.F., 2002. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
FAO, 2002. Antibiotic residues in aquaculture products. In: The state of world fisheries and aquaculture, Part 2: Selected issues facing fishers and aquaculturists. Food and Agriculture Organization of the United Nations, pp. 74-83.
FAO, 2010. The state of world fisheries and aquaculture 2010. Rome, pp .197
Gatlin III, D.M. and Phillips, H.F., 1989. Aquaculture, 79: 259-266. https://doi.org/10.1016/0044-8486(89)90466-3
George, J.C., Barnett, B.J., Cho, C.Y. and Slinger, S.J., 1981. Cytobiosis, 31: 7-18.
Heuer, O.E., Kruse, H., Grave, K., Collignon, P., Karunasagar, I. and Angulo, F.J., 2009. Clin. Infect. Dis., 49: 1248-1253. https://doi.org/10.1086/605667
Hollis, B.W and Wagner, C.L., 2004. Anim. J. Clin. Nutr., 79: 717-726.
Horst, R.L., Goff, J.P. and Reinhardt, T.A., 2003. Acta Vet. Scand., 97: 35-50.
Hossain, M.A., Pandey, A. and Satoh, S., 2007. Fish. Sci., 73: 1309-1317.
Jiang, M., Wu, F., Wen, H., Leng, X.J. and Liu, W., 2009. Freshw. Fish., 39: 38-42.
Khajepour, F. and Hosseini, S.A., 2010. World appl. Sci. J., 11: 682-686.
Khajepour, F. and Hosseini, S.A., 2012. J. Anim. Feed Sci., 171: 68-73. https://doi.org/10.1016/j.anifeedsci.2011.10.001
Koh, C.B., Romano, N., Zahrah, A.S. and Ng, W.K., 2016. Aquacult. Res., 47: 357-369. https://doi.org/10.1111/are.12492
Kunitz, M., 1947. J. Gen. Physiol., 30: 291-310. https://doi.org/10.1085/jgp.30.4.291
Li, A.J., 1994. Aquaculture nutrition and feed. China Agriculture Press, Beijing.
Li, J.S., Li, J.L. and Wu, T.T., 2009. Aquacult. Nutr., 15: 415-420. https://doi.org/10.1111/j.1365-2095.2008.00606.x
Ling-Hong, M., Xian-Ping, G., Jun, X., Bo, L., Ke-Bao, W., Jian, Z., Ming-Chun, R., Qun-Lan, Z., Liang-Kun, P. and Ru-Li, C., 2015. Aquaculture, 436: 104-109. https://doi.org/10.1016/j.aquaculture.2014.10.049
Luckstcadt, C. (ed.), 2008. Effect of organic acid containing additives in worldwide aquaculture-sustainable production the non-antibiotic way. In: Acidifiers in animal nutrition: A Guide for feed preservation and acidification to promote animal performance. Nottingham University Press, Nottingham, UK, pp. 71-78.
Ng, W.K. and Koh, C.B., 2011. Application of organic acids in aqua feeds: Impacts on fish growth, nutrient utilisation and disease resistance. In: Standards for acidifiers, principles for the use of organic acids in animal nutrition (ed. C. Luckstcadt). Nottingham University press, Nottingham, UK, pp. 49-58.
Ng, W.K. and Koh, C.B., 2016. Rev Aquacult. https://doi.org/10.1111/raq.1241
NRC, 1993. Nutrient requirements of fish National Academy of Science. National Research Council, Washington, DC, pp. 141.
Oliva-Teles, A., Pereira, J.P., Gouveia, A. and Gomes, E., 1998. Aquat. Living Resour., 11: 255-259. https://doi.org/10.1016/S0990-7440(98)80008-9
Overland, M., Granli, T., Kjos, N., Fjetland, O., Steien, S. and Stokstad, M., 2000. J. Anim. Sci., 78: 1875-1884. https://doi.org/10.2527/2000.7871875x
Park, K.W., Rhee, A.R., Um, J.S. and Paik, I.K., 2009. J appl. Poult. Res., 18: 598-604.
Pereira, T.G. and Oliva-Teles, A., 2003. Aquacult. Res., 34: 1111-1117. https://doi.org/10.1046/j.1365-2109.2003.00909.x
Romano, N., Koh, C.B. and Ng, W.K., 2015. Aquaculture, 435: 228–236.
Ravindran, V. and Kornegay, E., 1993. J. Sci. Fd. Agric., 62: 313-322. https://doi.org/10.1002/jsfa.2740620402
Reda, R.M. and Selim, K.M., 2015. Aquacult. Int., 23: 203-217. https://doi.org/10.1007/s10499-014-9809-z
Selim, K.M. and Reda, R.M., 2015. Fish Shellf. Immun., 44: 496–503.
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods. 8th ed. Ames: Iowa State University Press.
Spinelli, J., Houle, C.R. and Wekell, J.C., 1983. Aquaculture, 30: 71-83.
Steel, R.G.D., Torrie, J.H. and Dinkkey, D.A., 1996.Principles and procedures of statistics. 3rd ed. McGraw Hill Book Co., Singapore, pp. 627.
Storebakken, T., Refstie, S. and Ruyter, B., 2000. Soy products as fat and protein sources in fish feeds for intensive aquaculture. In: Soy in animal nutrition (ed. J.K. Drackley). Federation of Animal Science Societies, Savoy, pp. 127-170.
Sugiura, S.H., Gabaudan, J., Dong, F.M. and Hardy, R.W., 2001. Aquacult. Res., 32: 583-592.
Zhou, Q.C., Tan, B.P., Mai, K.S. and Liu, Y.J., 2004. Aquaculture, 241: 441-451. https://doi.org/10.1016/j.aquaculture.2004.08.044
To share on other social networks, click on any share button. What are these?










