Effect of Exogenous Application of Salicylic Acid, Potassium Nitrate and Methanol on Canola Growth and Phenology under Different Moisture Regimes
Effect of Exogenous Application of Salicylic Acid, Potassium Nitrate and Methanol on Canola Growth and Phenology under Different Moisture Regimes
Abdur Rehman1* and Shad Khan Khalil2
1Agricultural Research Institute Tarnab, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Agronomy, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Canola (Brassica napus L.) is a rich source of vegetable oil. Its production is often limited by shortage of water during reproductive stages. Salicylic acid, potassium nitrate and methanol are considered to induce drought tolerance in plants. Field trials were conducted at Agricultural Research Institute Tarnab, Peshawar-Pakistan to study the effects of moisture stress and foliar application of chemicals at different growth stages on canola growth and phenology during 2015-16 and 2016-17. The experiment comprised of four moisture levels (optimum water, 10%, 20% and 30% reduced irrigation water), three chemicals (salicylic acid 0.5mM, potassium nitrate KNO3 1% and methanol 20% v/v basis) applied at three different stages (bud formation, 20% flowering and 20% pod formation). The experiment was laid out in RCB design with split plot arrangement having three replications. Moisture levels were allotted to main plot while treatments (application of chemicals at different growth stages and water spray) to sub plot. Chemicals were sprayed in solution form on the leaves of the canola plants. Results indicated that reducing irrigation water shortened days to 50% flowering (103 days), days to 50% pod formation (120 days) and days to maturity (161 days). Optimum water supply delayed days to flowering (107 days), days to pod formation (122 days) and days to maturity (166 days). Moisture stress reduced number of branches plant-1 (5.6) and leaves plant-1 (12.8). Application of salicylic acid (SA) at the rate of 0.5mM improved number of branches (6.34), leaves (13.69) and delayed phenological stages of canola compared to KNO3, methanol, water spray and control (no spray). Application of salicylic acid at bud formation stage increased number of branches plant-1 (6.37), leaves plant-1 (13.89) and delayed maturity (163.9 days). Aphids incidence decreased with 30% reduced irrigation water (37 aphids plant-1). Application of salicylic acid also decreased aphids incidence (47.31 aphids plant-1) compared to control. It is concluded that limited water supply affected canola growth and maturity. Salicylic acid was more effective than potassium nitrate, methanol and water spray in improving growth of canola under optimum and limited water supply.
Received | March 01, 2018; Accepted | October 31, 2018; Published | November 16, 2018
*Correspondence | Abdur Rehman, Agricultural Research Institute Tarnab, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Rehman, A. and S.K. Khalil. 2018. Effect of exogenous application of salicylic acid, potassium nitrate and methanol on canola growth and phenology under different moisture regimes. Sarhad Journal of Agriculture, 34(4): 781-789.
DOI | http://dx.doi.org/10.17582/journal.sja/2018/34.4.781.789
Keywords | Phenology, Salicylic acid, Moisture stress, Methanol, Aphids
Introduction
Canola (Brassica napus L.) is the third major source of edible oil in the world after soybean and palm oil (Reyes, 2007). Canola seed contains 40-46% quality oil. Its meal has 38-40% proteins with essential amino acids lysine, methionine and cystine. It is an important oilseed crop of Pakistan cultivated in both irrigated and rain-fed areas of the country (Amjad, 2014). Canola is mostly grown under rain-fed farming systems, its production under irrigated farming is also common in many parts of the world including Australia (McCaffery, 2004).
Abiotic stresses like drought, salinity, high and low temperature adversely affects plant growth and productivity of crops. Abiotic stress cause crop losses worldwide and reduce the average yield of major crops more than 50% (Rodrigez et al., 2005). Among abiotic factors the availability of water is the most important factor which affect crop productivity. Growth and seed oil content of canola increase with the amount of water received (Canola Council of Canada, 2008). Moisture stress in canola reduces yield during many developmental stages. The negative effects of stress are more severe during flowering and silique development (Sinaki et al., 2007). Water stress affects both vegetative and reproductive stages of canola. The adverse effects of water stress in canola are more severe during reproductive stages (Ghobadi et al., 2006). The tolerance of plants to water stress can be increased through breeding and use of plant growth regulators. In recent studies different plant growth regulators have been used to alleviate drought stress injuries in plants (Sayyari et al., 2013). Among these Mepiquat Chloride (Ahmed et al., 2009), Brassinosteroids (Bajguz and Hayat, 2009), Jasmonate (Wang, 1999), 5-aminolevolenic acid (Liu et al., 2011) and Salicylic acid (Senaratna et al., 2000) are common. Salicylic acid, cytokinin, gibberellins and abscisic acid improve the response of plants during drought stress (Farooq et al., 2009).
Salicylic acid is a phenolic compound which regulates physiological processes in plants such as photosynthetic rate, stomatal conductance, enzyme activation and transpiration (Khan et al., 2003). Foliar application of salicylic acid amends the negative effects of water stress and enhance the growth of wheat (Sakhabutdinova et al., 2003). Foliar application of salicylic acid enhance chlorophyll contents in canola (Ghai et al., 2002). Foliar salicylic acid application decreased the transpiration rates in soybean and corn leaves (Khan et al., 2003).
Potash (K) plays a major role in improving plant resistance. Different plant processes like photosynthesis, enzyme activation, turgor pressure and translocation of cations into sink organs are regulated by potash (Mengel and Kirkby, 2001). Plant under drought stress requires more internal K (Cakmak, 2005). During stress conditions, reactive oxygen species (ROS) formation is induced which cause oxidative damage to cells due to which the demand for K increases (Foyer et al., 2002).
Methanol can be an alternative source of carbon for C3 plants which increase its CO2 fixation, growth and yield by inhibiting photorespiration (Safarzade et al., 2005). In plant tissues the increased concentration of methanol has a positive effect on carbon conversion efficiency. It increases leaves expansion by enhancing plant access to Ca+2 which results in increased leaf area (Ramirez et al., 2006). Methanol spray on water deficit plants can increase the chlorophyll content of leaves (Ramberg et al., 2002). Moisture stress has adverse effects on canola growth and development. The present field experiments were designed to evaluate the impact of different moisture levels on canola growth and alleviate moisture stress through foliar application of salicylic acid, potassium nitrate and methanol at different growth stages of canola under agro-climatic conditions of Peshawar valley.
Materials and Methods
Experimental site
To study the effect of moisture stress and foliar chemicals application on canola growth, phenology and aphids incidence, field experiments were conducted at Agricultural Research Institute, Tarnab Peshawar-Pakistan during 2015-16 and repeated in 2016-17. The mean average temperature of the experimental site was 8-24 0C (2015-16) and 8-250C (2016-17) with average monthly rainfall of 13 mm and 19 mm during the growing seasons of canola (Figure 1). Soil of the experimental site was silt loam with low organic matter and nitrogen (Table 1).
Table 1: Soil pre-sowing analysis for physical and chemicals properties (0-30 cm) depth.
| Parameters | Unit | Value |
| Textural class | ----- | Silt loam |
| Clay | % | 24.4 |
| Silt | % | 62.7 |
| Sand | % | 12.8 |
| pH | ----- | 7.95 |
| Electrical conductivity (EC) |
d S m-1 |
0.19 |
| Organic matter | % | 0.65 |
| Total nitrogen | % | 0.031 |
| Phosphorus |
mg kg-1 |
12.6 |
| Potash |
mg kg-1 |
194 |
| Field capacity (FC) | % | 32.04 |
| Permanent wilting point (WP) | % | 18.64 |
| Bulk density |
g cm-3 |
1.37 |
Materials and treatments
Soil samples were taken before sowing and analyzed for bulk density (James, 1993), soil field capacity and permanent wilting point using pressure plate apparatus (Cresswell et al., 2008). Canola hybrid “Hyola 401” was sown on 24th October, 2015 and harvested on 20th April, 2016. The experiment was resown on 21st October, 2016 and harvested on 26th April, 2017. The experiment was laid out in randomized complete block design with split plot arrangement having three replications. The experiment comprised of four moisture levels (optimum water, 10%, 20% and 30% reduced irrigation water), three chemicals (salicylic acid 0.5mM, potassium nitrate (1%) and methanol 20% v/v basis) applied at three different stages i.e. bud formation, 20% flowering and 20% pod formation (Meier, 2001). Moisture levels were assigned to main plots while combination of three chemicals x growth stages were applied to sub-plot. Total 11 treatments were applied to sub-plots i.e. three chemicals at three different stages along with water spray and control (Table 2). All the three chemicals were sprayed in solution form on the leaves of the canola plants. Area of sub plot was 2.70 m2 (1.5m x 1.8m) having six rows with 1.5 m length. Plant to plant distance of 10 cm was maintained through thinning after two weeks of emergence. A basic dose of 100 kg nitrogen ha-1 in two split doses and 60 kg phosphorus ha-1 at sowing time was applied to all the plots.
Moisture stress application
The experiment was carried out under high tunnel structures (rainout shelter) for protection from rainfall during the growing season. A white transparent plastic was used to cover the top of the tunnel. The tunnel was not covered with plastic from sides so that air may circulate freely through it. Plastic cover increased the average temperature of the experimental plots by 1-2 0C. Soil moisture levels were determined through gravimetric method. All the plots received normal water till completion of rosette stage (4-5 leaf stage). After rosette stage moisture stress was imposed. Irrigation was done according to maximum allowable deficit (MAD), when 50% MAD was attained in optimum water plots. The amount of irrigation water was calculated through formula (Panda et al., 2004).
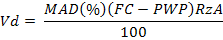
Where; Vd (m3) is volume of water applied; Rz (m) is effective root depth; A (m2) is the area of plot and FC (m3m-3) and PWP (m3m-3) are limits of field capacity and permanent wilting point.
Table 2: Treatment combinations of application at different growth stages (S) and chemicals (C).
| S.No | Application stage (S) | Chemicals (C) |
| 1 | Bud formation | Salicylic acid |
| 2 | Bud formation |
KNO3 |
| 3 | Bud formation | Methanol |
| 4 | Flowering | Salicylic acid |
| 5 | Flowering |
KNO3 |
| 6 | Flowering | Methanol |
| 7 | Pod formation | Salicylic Acid |
| 8 | Pod formation |
KNO3 |
| 9 | Pod formation | Methanol |
| 10 | Water spray | |
| 11 | Control (no spray) |
Observations and statistical analysis
Days to 50% flowering and pod formation were recorded by counting the number of days from sowing till 50% of the plants produced 1-5 flowers and pods respectively. Days to maturity was calculated from date of sowing till 30-40% seeds in the pods turned black/brown on main branches of the plants. The number of branches and number of leaves were counted in five randomly selected plants and averaged to get number of branches and leaves respectively. Aphids incidence was recorded by counting number of aphids on 10 cm terminal main stem of five randomly selected plants during late flowering stage (Mahmoud and Osman, 2015). Number of leaves and branches were recorded during 1st week while aphids incidence was observed during 3rd week of March for both years. Statistical analysis of the data was done through MS-Excel and MSTATC according to the method described by (Gomez and Gomez, 1984). The significance of difference among means was compared through LSD test at P< 0.05 (Steel et al., 1997).
Results and Discussion
Days to 50% flowering
Days to 50% flowering was significantly affected by years, moisture levels and chemicals. Plants in 2016-17 required more days to 50% flowering than 2015-16 (Table 3). Maximum days to flowering (107.2 days) were recorded with plots receiving optimum water supply. Days to flowering deceased with increase in moisture stress. Fewer days to flowering (102.6 days) were observed for 30% moisture level. Application of KNO3 delayed days to flowering (105 days), followed by salicylic acid. The effect of water spray was at par with chemicals application. The untreated plants took less days to 50% flowering compared to treated plots. Application time of chemicals at different growth stages was non significant. Interactions between chemicals (C) x application stages (S) was significant while all other interactions were non significant (Table 3). Water stress might have triggered flowering initiation which resulted in early flowering. Days to flowering decreases with increasing moisture stress (Sinaki et al., 2007). Foliar application of chemicals stimulate the physiological and metabolic processes which enhance vegetative growth and delay days to 50% flowering (Fariduddin et al., 2003).
Days to 50% pod formation
Days to 50% pod formation was significantly affected by moisture levels and chemicals. Maximum days to pod formation (122.4 days) were recorded with optimum water supply (Table 3). Reduction in soil moisture accelerated formation of pods. Fewer days to 50% pod formation (119.9 days) were observed for 30% reduced moisture level. Application of salicylic acid, KNO3 and methanol delayed days to 50% pod formation compared to water spray and control. However, no significant difference was observed among foliar chemicals for days to pod formation. Interaction between moisture levels (M) x chemicals (C) showed that salicylic acid delayed days to pod formation both under optimum and severe stress conditions (Figure 2). The availability of sufficient water might have enhanced the vegetative growth resulting in prolonged duration of pod formation (Kamkar et al., 2012). Application of salicylic acid and KNO3 enhanced the growth of canola (Sibgha et al., 2013) resulting in delay in pod formation.
Table 3: Days to 50% flowering, days to 50% pod formation and days to maturity of canola as affected by moisture levels (M), chemicals (C) and application stages (S) over years.
| Treatments | Days to 50% flowering | Days to 50% pod formation | Days to maturity |
| Year | |||
| 2015-16 | 103.9 b | 119.9 | 159.4 b |
| 2016-17 | 104.9 a | 121.5 | 166.9 a |
| Significance | * | ns | * |
| Moisture levels (M) | |||
| No stress (optimum water) | 107.2 a | 122.4 a | 166.0 a |
| 10% reduced irrigation water | 105.1 b | 121.4 b | 165.2 a |
| 20% reduced irrigation water | 103.5 c | 120.8 bc | 162.5 b |
| 30% reduced irrigation water | 102.6 d | 119.9 c | 160.6 c |
| LSD | 0.31 | 0.94 | 0.92 |
| Chemicals (C) | |||
| Salicylic Acid (SA) 0.5mM | 104.6 b | 121.5 | 164.0 a |
|
Potassium Nitrate (KNO3) 1% |
105.0 a | 121.2 | 163.7 ab |
| Methanol (20% v/v) | 104.4 b | 121.1 | 163.5 b |
| LSD | 0.34 | ns | 0.35 |
| Application Stages (S) | |||
| Bud formation | 104.8 | 121.3 | 163.9 |
| Flowering | 104.5 | 121.3 | 163.6 |
| Pod formation | …… | 121.3 | 163.7 |
| LSD | ns | ns | ns |
| Chemical spray | 104.7 | 121.3 a | 163.7 a |
| Water spray | 104.6 | 120.7 b | 163.3 b |
| Significance | ns | * | * |
| Control | 104.2 b | 120.2 b | 162.6 b |
| Rest | 104.6 a | 121.2 a | 163.7 a |
| Significance | * | * | * |
| Interactions | |||
| MxC | 0.2427 | 0.5794 | 0.000 |
| MxS | 0.4267 | 0.0925 | 0.059 |
| CxS | 0.0172 | 0.0005 | 0.988 |
| MxCxS | 0.2838 | 0.0009 | 0.036 |
Means within each column followed by different letters are significant at P ≤ 0.05 using LSD test.
Days to maturity
Days to maturity was significantly affected by year, moisture levels and chemicals application. Delayed maturity (05 days) was observed in 2016-17 compared to 2015-16 (Table 3). During second year more rainfall occurred in the months of March and April 2017 which lowered the ambient temperature and delayed maturity of canola. Maximum days to maturity (166 days) were taken by plots supplied with optimum water. The increase in moisture stress shortened the maturity period. Among moisture stress plots, 30% reduced moisture level required less days to maturity (160.6 days). The reduction in days to maturity might be a mechanism of plants to escape moisture stress (Sinaki et al., 2007). The availability of sufficient moisture enhanced growth and development of the plants and delayed the maturity process (Kamkar et al., 2012). Application of all the three chemicals delayed maturity compared to water spray and control plots. Among chemicals, salicylic acid took more days to maturity (164 days) compared to KNO3 and methanol. Interaction of moisture and chemicals indicated delay in maturity with application of salicylic acid under optimum water conditions. However, at 30% reduced moisture level, application of KNO3 indicated maximum days to maturity (Figure 3). Application of salicylic acid and KNO3 improved growth and development which delayed maturity. Increase in maturity duration may be due to enhancement of biochemical and physiological processes (Fariduddin et al., 2003) with foliar application of salicylic acid.
Number of branches plant-1
Number of branches were significantly affected by moisture levels (Table 4). Decrease in branches plant-1 occurred with increasing moisture stress. Maximum branches plant-1 (6.93) were recorded for plots supplied with optimum water while 20% and 30% reduced moisture levels decreased number of branches plant-1. Supply of optimum water might have increased growth and more assimilates were translocated to lateral meristems resulting in an increased branches plant-1 (Ahmadi and Bahrani, 2009). Optimum water supply enhanced growth of canola which increased number of branches (Khan et al., 2010). Foliar application of chemicals increased number of branches compared to water spray and untreated plots. Foliar spray of salicylic acid showed maximum number of branches plant-1 (6.34). Application time of chemicals at different growth stages was non significant, however spray at bud formation stage enhanced branches plant-1 (6.37). Interaction between moisture and application stage indicated that chemicals application at bud formation stage increased number of branches at all moisture levels except 30% reduced level (Figure 4). Application of chemicals (salicylic acid and KNO3) enhanced the photosynthetic process which improved overall plant growth (El-Sabagh et al., 2017).
Table 4: Number of branches plant-1, leaves plant-1 and aphids incidence on canola as affected by moisture levels (M), chemicals (C) and application stages (S) over years.
| Treatments |
Branc-hes plant-1 |
Leaves plant-1 |
Aphids incidence plant-1 |
| Year | |||
| 2015-16 | 5.76 | 13.42 | 60.35 a |
| 2016-17 | 6.06 | 13.07 | 53.65 b |
| Significance | ns | ns | * |
| Moisture levels (M) | |||
| No stress (optimum water) | 6.93 a | 14.17 a | 65.64 a |
| 10% reduced irrigation water | 6.29 b | 13.76 a | 57.68 b |
| 20% reduced irrigation water | 5.84 c | 13.24 b | 45.17 c |
| 30% reduced irrigation water | 5.60 c | 12.88 b | 37.00 d |
| LSD | 0.27 | 0.505 | 2.46 |
| Chemicals (C) | |||
| Salicylic Acid (SA) 0.5mM | 6.34 | 13.69 a | 47.31 c |
|
Potassium Nitrate(KNO3) 1% |
6.21 | 13.71 a | 51.76 a |
| Methanol (20% v/v) | 6.25 | 13.46 b | 49.49 b |
| LSD | ns | 0.205 | 1.66 |
| Application Stages (S) | |||
| Bud formation | 6.37 | 13.89 a | 54.07 a |
| Flowering | 6.20 | 13.56 b | 48.44 b |
| Pod formation | 6.23 | 13.42 b | 46.04 c |
| LSD | ns | 0.205 | 1.66 |
| Chemical spray | 6.27 a | 13.62 | 49.52 b |
| Water spray | 5.86 b | 13.13 | 55.54 a |
| Significance | * | ns | * |
| Control | 5.59 b | 12.92 b | 63.88 a |
| Rest | 6.23 a | 13.57 a | 50.12 b |
| Significance | * | * | * |
| Interactions | |||
| MxC | 0.180 | 0.6210 | 0.031 |
| MxS | 0.020 | 0.8018 | 0.000 |
| CxS | 0.400 | 0.0322 | 0.478 |
| MxCxS | 0.020 | 0.3469 | 0.304 |
Means within each column followed by different letters are significant at P ≤ 0.05 using LSD test.
Number of leaves plant-1
Moisture levels, foliar chemicals and their application time significantly affected number of leaves plant-1 (Table 4). Maximum number of leaves (14.1) were observed in plants supplied with optimum water. Number of leaves decreased with decrease in soil moisture and minimum leaves plant-1 (12.8) were recorded for 30% reduced moisture level. Sufficient supply of water to canola increased photosynthetic process and net assimilation rate resulting in increase in leaves plant-1 (Tesfamariam et al., 2010). Presence of sufficient water helped the plant to develop greater leaf area. Norouzi et al. (2008) reported reduced leaf area and less number of leaves with decrease in soil moisture to rapeseed. Foliar application of chemicals increased number of leaves. Maximum leaves plant-1 (13.69) were observed with application of salicylic acid. All the chemicals and water spray increased number of leaves compared to untreated plots. These results are in agreement with (Fayez and Bazaid., 2014) who observed maximum number of leaves and leaf area with application of salicylic acid. Interaction between moisture and chemicals showed that KNO3 increased number of leaves under optimum water conditions while salicylic acid improved number of leaves plant-1 both under optimum and water stress conditions (Figure 5). Data revealed that application of chemicals at bud formation stage increased leaves plant-1 (13.8). Chemicals application at bud formation stage increased photosynthetic efficiency (Leithy et al., 2015) resulting in maximum number of leaves and increased leaf area of canola.
Aphid incidence
Aphids population was significantly affected by moisture levels, foliar chemicals and application time of chemicals. Incidence of aphids on canola plants was higher during 2015-16 compared to 2016-17 (Table 4). Number of aphids on main terminal stem were maximum (65.6 plant-1) on plants supplied with optimum water compared to water stressed plants. Decline was observed in population of aphids (Table 4) with moisture stress. Population of aphids (37) was lower in plots which received 30% reduced moisture. In cowpea water deficit changes the composition and concentration of secondary metabolites, which affects the population of aphids (Agele et al., 2006). Application of chemicals decreased population of aphids compared to control plots. Salicylic acid reduced the aphid incidence (47.3) followed by methanol (49.4). Application of chemicals at pod formation stage was most effective in minimizing population of aphids.
Conclusions and Recommendations
Phenological stages were delayed in 2016-17 compared to 2015-16. This delay in maturity during second year may be due to more rainfall during months of March and April 2017. Days to 50% flowering, 50% pod formation and days to maturity were delayed with optimum water supply. Number of branches and leaves plant-1 were higher under optimum water conditions and decreased with moisture stress. Growth was adversely affected under 30% reduced moisture levels. Delayed phenological stages and maximum number of branches and leaves were recorded with application of salicylic acid. Application of chemicals at bud formation stage were more effective in increasing the number of branches, leaves and delaying maturity of canola. Aphids incidence was minimum under water stress conditions and with foliar spray of salicylic acid. It is concluded that salicylic acid application at the rate of 0.5mM at bud formation stage improved the phenological and agronomic parameters of canola and reduced aphids incidence.
Author’s Contribution
Abdur Rehman: Conducted field experiments, collected the data and prepared draft of the manuscript.
Shad Khan Khalil: Supervised him during analysis, preparation of draft and made corrections.
References
Agele, S.O., T.I. Ofuya and P.O. James. 2006. Effects of watering regimes on aphid infestation and performance of selected varieties of cowpea (Vigna unguiculata L. Walp) in a humid rainforest zone of Nigeria. Crop Prot. 25: 73–78. https://doi.org/10.1016/j.cropro.2005.03.005
Ahmadi, M. and M.J. Bahrani. 2009. Yield and yield components of rapeseed as influenced by water stress at different growth stages and nitrogen levels. American-Eurasian J. Agric. Environ. Sci. 5 (6): 755-761.
Ahmed, M.A., A. Magda, F. Shalaby and M.H. Afifi. 2009. Alleviation of water stress effects on maize by Mepiquat Chloride. Modern Journal of Applied Biological Sciences. (3): 1-8.
Amjad, M. 2014. Oilseed crops of Pakistan. Status paper. Pak. Agric. Res. Counc. Islamabad.
Bajguz, A. and S. Hayat. 2009. Effects of brassinosteroids on the plant responses to environmental stresses. Plant physiol. Biochem. (47): 1-8. https://doi.org/10.1016/j.plaphy.2008.10.002
Cakmak, I. 2005. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nut. Soil Sci. 168: 521– 530.
Canola Council of Canada. 2008. Effects of moisture on canola growth. http://www.canola-council.org/chapter4.aspx#ch4tab2 (verified 13 Jan. 2010). Canola Council of Canada, Winnipeg, MB.
Cresswell, H.P., T.W. Green and N.J. McKenzie. 2008. The adequacy of pressure plate apparatus for determining soil water retention. Soil Sci. Soc. Am. J. 72: 41-49. https://doi.org/10.2136/sssaj2006.0182
El-Sabagh, A., K, A.A. Abdelaal and C. Barutcular. 2017. Impact of antioxidants supplementation on growth, yield and quality traits of canola (Brassica napus L.) under irrigation intervals in north Nile delta of Egypt. J. Exp. Biol. Agric. Sci. 5: (2) 163-172.
Fayez, K.A. and S.A. Bazaid. 2014. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci.13: 45–55. https://doi.org/10.1016/j.jssas.2013.01.001
Fariduddin, Q., S. Hayat and A. Ahmad. 2003. Salicylic acid influences net photosynthetic rate, carboxylation, efficiency of nitrate reductase activity and seed in Brassica juncea L. Photosynthetica, 41: 281-284. https://doi.org/10.1023/B:PHOT.0000011962.05991.6c
Farooq, M., A. Wahid and D.J. Lee. 2009. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta. Physiol. Planta. 31: 937-945. https://doi.org/10.1007/s11738-009-0307-2
Foyer, C.H., H. Vanacker, L.D. Gomez and J. Harbinson. 2002. Regulation of photosynthesis
and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Rev. Plant Physiol. Biochem. 40: 659– 668. https://doi.org/10.1016/S0981-9428(02)01425-0
Ghai, N., R.C. Setia and N. Setia. 2002. Effects of paclobutrazol and salicylic acid on chlorophyll content, hill activity and yield components in Brassica napus L. Phytomorphol. 52: 83–87.
Ghobadi, M., M. Bakhshandeh, G. Fathi, M.H. Gharineh, K. Alamisaeed, A. Naderi and V. Ghobadi. 2006. Short and long periods of water stress during different growth stages of canola (Brassica napus L.). effect on yield, yield components, seed oil and protein contents. J. Agron. 5: 336-341. https://doi.org/10.3923/ja.2006.336.341
Gomez, K.A. and A.A. Gomez. 1984. Statistical procedures for agricultural research. 2nd Ed. Wiley, New York. 680.
James, L.G. 1993. Principles of farm irrigation system design. Krieger Publishing Company, Washington DC, USA.
Kamkar, B., A.R. Daneshmand, F. Ghooshchic, A.H. Shiranirad and A.R.S. Langeroudi. 2011. The effects of irrigation regimes and nitrogen rates on some agronomic traits of canola under a semiarid environment. Agric. water Manage. 98: 1005–1012. https://doi.org/10.1016/j.agwat.2011.01.009
Khan, M.A., M.Y. Ashraf, S.M. Mujtaba, M.U. Shirazi, S. Shereen, S. Mumtaz, M.A. Siddique and G.M. Kaleri. 2010. Evaluation of high yielding canola type Brassica genotypes/ mutants for drought tolerance using physiological indices as screening tool. Pak. J. Bot. 42(6): 3807-3816.
Khan, W., B. Prithiviraj and D.L. Smith. 2003. Photosynthetic responses of corn and soybean to foliar application of salicylates. Plant Physiol. 160: 485-492. https://doi.org/10.1078/0176-1617-00865
Leithy, S.M., B.A. Leila, E.F. Abdallah and M.S. Gaballah. 2015. Response of canola plants to antitranspirant levels and limited Irrigation. American-Eurasian J. Sustainable Agric. 9 (4): 83-87.
Liu, D., Z.F. Pie, M.S. Naeem, D.F. Ming, H.B. Liu, F. Khan and W.J. Zhou. 2011. 5-aminolevolinic acid activates antioxidative defense system and seedling growth in Brassica napus L. under water-deficit stress. J. Agron. Crop Sci. 1-12.
Mahmoud, M.F. and M.A.M. Osman. 2015. Management of cabbage aphid, Brevicoryne brassicae L. on canola crop using neonicotinoids seed treatment and salicylic acid. J. Phytopathol. Pest Manage. 2(3): 9-17.
McCaffery, D. 2004. Irrigated canola—Management for high yields. Grain Res. Dev. Corporation, Griffith, Australia.
Meier, U. 2001. Growth stages of mono and dicotyledonous plants. BBCH monograph 2nd Edi. Federal biological research centre for agriculture and forestry. https://en.wikipedia.org/wiki/BBCH-scale.
Mengel, K. and E.A. Kirkby. 2001. Principles of Plant Nutrition. 5th edition. Kluwer Academic Publishers, Dordrecht. https://doi.org/10.1007/978-94-010-1009-2
Norouzi, M., M. Toorchi, G.H. Salekdeh, S.A. Mohammadi, M.R. Neyshabouri and S. Aharizad. 2008. Effect of water deficit on growth, grain yield and osmotic adjustment in rapeseed. J. Food, Agric. Environ. 6 (2): 3 1 2 - 3 1 8.
Panda, R.K., S.K. Behera and P.S. Kashyap. 2004. Effective management of irrigation water for maize under stressed conditions. Agric. Water Manage. 66: 181–203. https://doi.org/10.1016/j.agwat.2003.12.001
Ramberg, H.A., J.S.C. Bradley, J.S.C. Olson, J.N. Nishio, J. Markwell and J.C. Osterman. 2002. The role of methanol in promoting plant growth: An. update. Rev. Plant Biochem. Biotechnol. 1: 113-126. http://www.springerlink.com/content/e6k1q7547531145u/
Ramirez, I., F. Dorta, V. Espinoza, E. Jimenez, A. Mercado and H. Pen a-Cortes. 2006. Effects of foliar and root applications of methanol on the growth of Arabidopsis, tobacco and tomato plants. J. Plant Growth Regul. 25: 30-44. https://doi.org/10.1007/s00344-005-0027-9
Reyes, S.U. 2007. Canola oil. Available at http://www.ats.agr.gc.ca/asean/4359e.htm (verified 15 Jan. 2010). Agric. Agric. Food Canada.
Rodriguez, M., E. Candales and O. Borras-hidalgo. 2005. Molecular aspects of abiotic stress in plants. Biotecnol. Apl. 22(1): 1-9.
Safarzade, V.M.N., G.H. Normohamadi, E.M. Haravan and B. Rabiei. 2005. Effect of methanol on peanut Growth and yield (Arachis hypogaea L.). Iran. J. Agric. Sci. 1: 88-103.
Sakhabutdinova, A.R., D.R. Fatkhutdinova, M.V. Bezrukova and F.M. Shakirova. 2003. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulgarian. J. Plant Physiol. 21: 314-319.
Saxena, M.C. and L. Singh. 1985. A note on leaf area estimation of intact maize leaf. India. J. Agron. 10: 457 – 459.
Sayyari, M., M. Ghavami, F. Ghanbari and S. Kordi. 2013. Assessment of salicylic acid impacts on growth rate and some physiological parameters of lettuce plants under drought stress conditions. Int. J. Agric. Crop Sci. 5 (17): 1951-1957.
Senaratna, T., D. Touchell, E. Bunn and K. Dixon. 2000. Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plant. Plant Growth Regul. 30: 157-161. https://doi.org/10.1023/A:1006386800974
Sibgha, N., H.R. Attar and M. Ashraf. 2013. Interactive effects of watering regimes and exogenously applied osmoprotectants on earliness indices and leaf area index in cotton (Gossypium hirsutum L.) Crop Pak. J. Bot. 45(6): 1873-1881.
Sinaki, J.M., H.E. Majidi, A.H. Shirani-Rad, G. Noormohammadi and G. Zarei. 2007. The effects of water deficit during growth stages of canola (Brassica napus L.). Am-Eurasian J. Agric. Environ. Sci. 2(4): 417-422.
Steel, R.G.D., J.H. Torrie and D.A. Dicky. 1997. Principles and Procedures of Statistics, A Biometrical Approach. 3rd Edition, McGraw Hill, Inc. Book Co., New York, 352-358.
Tesfamariam, E.H., J.G. Annandale and J.M. Steyn. 2010. Water stress effects on winter canola growth and yield. Agron. J. 102(2): 658-666. https://doi.org/10.2134/agronj2008.0043
Wang, S. 1999. Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. (Abstract). https://doi.org/10.1007/PL00007060
To share on other social networks, click on any share button. What are these?








