Effect of Formulated Feed as a Subsitute for Live Feed on Growth Performances, Biochemical Composition and Digestive Enzyme Activities of Asian Stinging Catfish, Heteropneustes fossilis (Bloch, 1794) Larvae
Research Article
Effect of Formulated Feed as a Subsitute for Live Feed on Growth Performances, Biochemical Composition and Digestive Enzyme Activities of Asian Stinging Catfish, Heteropneustes fossilis (Bloch, 1794) Larvae
Momotaz Khanom*, Sheikh Mustafizur Rahman, Muhammad Yousuf Ali, Md. Shahin Parvez, Al-Hasan Antu, Md. Nazmul Ahsan
Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna-9208, Bangladesh
Abstract | Substitute of live feed in mass production of fish larvae has recently drawn a great attention due to inadequate supply and inconsistent price. The present study evaluated the effects of early introduction of formulated feed as a substitute for live feed on growth performances of Asian stinging catfish, Heteropneustes fossilis larvae reared in laboratory conditions. Three-day-old larvae hatched through induced breeding (2.0±0.00016 mg; 0.57±0.09cm) were subjected to four feeding treatments in triplicates (100 larvae/aquarium). The catfish larvae were fed with live Tubifex (LT), dry Tubifex (DT), formulated feed (FF), and commercial feed (CF) two times a day for 60 days. Results showed that larvae fed with formulated feed (FF) grew significantly faster than the other treatments (p<0.05). The weight gains were observed as 2.6 g in FF; 2.0 g in CF and 1.9g in LT and 1.54g in DT, respectively. Similarly, FF showed the highest specific growth rate (SGR) of 11.85±0.71%, but significantly differed only from that of DT (SGR=11.1; P< 0.5). Length-weight analysis revealed that the growth of larvae in all treatments showed negative allometric growth (regression slope b ˂3). The highest b value (2.64) was observed in larvae fed with FF. Proximate analysis showed that larvae fed with FF had significantly higher protein content than the other treatments (p<0.05). Water quality parameters remained optimum throughout the experimental period. The feeds used in treatments did not show any deleterious effect on survival. Physiologically, FF led to significantly higher amylase activity in comparison to other feeds, while protease activity was comparable among the feeds except for DT. The results suggest that formulated feed can be used as a potential substitute for live Tubifex for the early stage of larvae of stinging catfish.
Keywords | Enzyme activity, Formulated feed, Growth performance, Stinging catfish, Tubifex
Received | February 22, 2022; Accepted | March 31, 2022; Published | May 15, 2022
*Correspondence | Momotaz Khanom, Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna-9208, Bangladesh; Email: [email protected]
Citation | Khanom M, Rahman SM, Ali MY, Parvez MS, Antu A-H, Ahsan MN (2022). Effect of formulated feed as a subsitute for live feed on growth performances, biochemical composition and digestive enzyme activities of asian stinging catfish, Heteropneustes fossilis (Bloch, 1794) larvae. Adv. Anim. Vet. Sci. 10(6): 1264-1271.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.6.1264.1271
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The Asian stinging catfish (Heteropneustes fossilis), locally known as Shing, is an omnivorous catfish endemic to Bangladesh. It is a high-valued native fish species in Bangladesh, with a high demand in local markets due to its delicious taste, nutritional values (Alok et al., 1993) and medicinal benefits (Froese and Pauly, 2012). Once widely distributed in the natural water bodies of the country, the population of stinging catfish has been declining over the years due to overfishing, habitat destruction and environmental disturbance caused by natural and anthropogenic activities (Dahanukar et al., 2004). However, a large-scale commercial culture of this species is yet to be established in Bangladesh because of a scarcity of good quality seed supply, which, till now, comes from natural sources. Although in recent years, some homestead H. fossilis hatcheries have been established in Mymensingh and Jessore districts of Bangladesh to mitigate the seed supply problems through artificial breeding, large-scale production of this species is yet to get its due momentum. Apart from inferior brood quality and suboptimal water conditions in catfish hatcheries, lack of suitable larval and nursery feeds are the most crucial challenges impeding the widespread adoption of this important aquaculture species in Bangladesh.
The success of aquaculture depends mainly on the availability of supplementary diets rich in essential nutrients that will result in high survivability and fast growth (Giri et al., 2002). Compared to adults, however, the development of a diet for fish larvae is difficult as fish larvae are usually very small and fragile with an undeveloped digestive system (Lavens and Sorgeloos, 1996). Therefore, live feeds are conveniently used for larval feeding (Holt, 1993; People Le Ruyet et al., 1993) as it is believed that live feeds stimulate enzyme secretion resulting in good growth and survival. Tubifex is well known to increase the growth performances of different finfish species such as Chitala chitala (Sarkar et al., 2007), Clarius macrocephalus (Santiago et al., 2003) and catfish (Evangelista et al., 2005; Arslan et al., 2009). However, the regular application of live Tubifex to fish larvae is impractical for developing countries because of inconsistent supply and high price (Evangelista et al., 2005). Besides, the use of live feed from unknown commercial sources can be a potential source of pathogens or parasites that may cause numerous water-borne diseases (Tao et al., 2007). Therefore, the development of artificial larval feeds as the best alternative to costly live feed has always been a subject of intensive research (Gatesoupe and Luquet, 1982).
As mentioned, larval feeding stands as one of the key challenges that hitherto withhold adequate supply of quality seeds for commercial aquaculture of Asian stinging catfish in Bangladesh. Therefore, the development of an artificial feed for catfish larvae is crucial, as it reduces the dependency on live feeds and holds a huge potential in meeting the requirements of food size and nutrition for the larvae (Lavens and Sorgeloos, 2000). While larval feeds are available in the local markets, these are arbitrarily formulated and do not meet the specific nutritional demand of stinging catfish contributing to poor growth and survival of larvae. Previous reports suggest that live and dry Tubifex can be a suitable diet for stinging catfish (Ahmmed et al., 2016; Hossain et al., 2016). However, these studies used fry and fingerlings instead of larvae and did not include any formulated feed for comparison. Therefore, the present study was conducted to observe the efficacy of formulated feed in comparison with a live feed on the early stages of larvae of stinging catfish.
Materials and Methods
Ethics statement
The experimental fish were reared up and maintained according to the guidelines of bioethics and animal care as developed by the Animal Ethics Committee of Khulna University, Bangladesh (KUAEC-2019/11/11).
Experimental design and larval rearing
A 60-days feeding trial was carried out with three days old Asian stinging catfish (H. fossilis) larvae produced through hypophysation technique in the breeding facility of Fisheries and Marine Resource Technology Discipline in Khulna University of Bangladesh. The experimental system consisted of 12 glass aquaria (50 ×36 × 30 cm3) with a total system volume of 50 L each containing a net cage (32cm × 20cm × 29 cm) suspended in the water column. An overhead water shower was installed over each aquarium/net cage to ensure continuous aeration and reduction of accumulated ammonia load. The larvae of 2±0.00016 mg in weight and 0.57±0.09 cm in length were stocked at the rate of 100 in each suspended net cage. Larvae were fed at satiation level with cleaned and fine chopped live Tubifex for three days before commencing the feeding trial. The experimental design consisted of four feed treatments, each with three replicates randomized across the twelve tanks. The experimental feeds consisting of live Tubifex (LT), dry Tubifex (DT), formulated feed (FF), and commercial feed (CF) were used for the feeding trial. Initially, the feed was given ad libitum twice daily for the first 15 days and then gradually adjusted to 10% of the total biomass. To adjust the feed ration, morphometric measurements were taken fortnightly after a 24h feed deprivation period and the calculated amount of daily requirement was given in equal rations at 09 h, 12 h, and 15 h. The experimental tanks were cleaned twice a week after two hours of the last feeding to remove dirt and dead larvae if any, while water quality parameters (temperature, 28±1.5°C; dissolved oxygen, 6.3±0.5mg/L; pH, 7.2±0.6 and ammonia, 0.065±0.03 mg/L) were maintained throughout the experimental period.
Experimental feeds
Live Tubifex (LT) was collected from a local aquarium shop while dry Tubifex (DT) (Royal Feast, R & R Aquarium products) and commercial feed (CF) specially prepared for fish larvae were purchased from the local markets (Agatanursery powder; Agata Feed Mill Ltd, Comilla, Bangladesh). Collected LT were kept in a tray with continuous mild water flow to keep them alive and fresh. Each time, the required amount of LT was picked from the tray, washed thoroughly under tap water, and chopped to fine pieces before feeding to the larvae. FF was prepared at the laboratory by using locally available ingredients, wheat bran; soybean meal; wheat flour; fishmeal; meat and bone meal and soybean oil (Table 1). The protein content and gross energy of the FF were maintained as 40% and 4.16 kcal/kg, respectively as suggested by Siddiqui and Khan (2009) for the rearing of H. fossilis larvae. The price of different experimental feeds and the proximate composition (protein, lipid, ash, and moisture) of all experimental feeds are shown in Table 2. The proximate composition of all experimental feeds and fish obtained at the end of the study was carried out following the methods of AOAC (2012).
Table 1: Composition of ingredients of formulated feed (g 100g diet-1).
| Ingredients | % Inclusions (dry matter basis) |
| Wheat bran | 16.14 |
| Soybean meal | 35.34 |
| Wheat flour | 15.82 |
| Fishmeal | 24.19 |
| Meat and bone meal | 16.59 |
| Soybean oil | 4.50 |
| Vitamin and mineral premix | 0.10 |
Table 2: Proximate composition (%) and cost of experimental feeds.
| Parameter (%) | LT | DT | FF | CF |
| Protein | 49.78±0.01 | 48.50±0.02 | 39.77±0.02 | 30.72±0.01 |
| Lipid | 5.48±0.04 | 8.27±0.03 | 7.98±0.03 | 5.70±0.03 |
| Ash | 7.79±0.01 | 7.26±0.01 | 11.29±0.04 | 8.35±0.02 |
| Moisture | 70.31±0.03 | 11.08±0.02 | 8.26±0.02 | 10.11±0.05 |
| Price (Taka/kg) | 110 | 9000 | 60 | 65 |
Growth parameters
The average body weight (BW) and body length (BL) of 15 larvae randomly collected from each replicate were recorded in the beginning and at every 15 days interval during the experimental period using an electronic balance (Model: FSH; A and D Company, Korea; nearest scale: 0.001g) and ImageJ software, respectively. Survival rates were monitored every day and presented as weekly for 30 days and biweekly for the remaining days. Weight gain of the larvae was calculated according to the formula developed by Sandifer and Smith (1974) while average daily weight gain (ADG) and specific growth rate (SGR) were determined following Chakraborty and Mirza (2008).
Length and weight relationship
The length-weight relationship (LWR) of larvae was determined using the following formula (Le Cren, 1951).
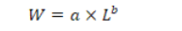
Where; W is the weight of the fish (g), L is the length of the fish (cm), a is the intercept and b is the slope of the linear regression. The equation was linearized by a logarithmic transformation into:
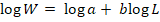
If b corresponds to a value equal to 3 it indicates isometric growth, while more or less than that indicates positive and negative allometric growth, respectively (Ricker, 1975).
Digestive enzyme (amylase and protease) activity assay
Enzyme activity was assayed using crude extracts prepared from a pooled sample of fish at the end of the feeding trial. At first, four fish samples from each replication were roughly macerated from where a pooled sample of 0.5 g was homogenized with 50ml of 0.1 M Tris EDTA (pH 8.0) at4˚C. The tissue samples thus obtained were centrifuged at 12000 rpm for 10 min at 4˚C (Lovett and Felder, 1990) and the supernatants were stored at −80°C until they were used for the enzymatic assays. The amylase activity (E.C. 3.2.1.3) was assayed according to the dinitrosalicylic acid method (Bernfeld, 1955) using 1% soluble starch as the substrate. Briefly, 200µl-crude extracts from each sample in 200µl Tris- EDTA buffer solution was incubated with 100µl 3, 5-dinitrosalicylic (DNS) acid for 30min at 35˚C followed by heating at 70˚C to stop the reaction. The protease activity (E.C. 3.4.21.112) was assayed according to the casein method (Walter, 1984) using 1% casein as the substrate. To assay, this activity, the crude extracts (200µl) from each sample and soluble casein (200µl) were incubated for 30min at 35˚C in which 110mM TCA (200µl) was added to stop the reaction. Absorbance was read at 540 nm for amylase and 280 nm for protease activity using a double beam spectrophotometer (HITACHI, U-2910, Japan). One unit of enzyme activity was defined as the amount of enzyme required to produce 1µg maltose for amylase and 1µg tyrosine for protease in 1 min at 35˚C.
Statistical analysis
One-way ANOVA was used to analyze the data and differences among the treatments followed by Tukey’s comparison test. HSD post hoc was used for multiple comparisons. All statistical analyses were performed using the statistical software package IBM SPSS Statistics V22.0.
Results
Growth performances
In this experiment, all diets were found to be readily accepted by the H. fossilis larvae. The body weight gain (BWG), average daily weight gain (ADG), length gain, % specific growth rate (SGR), and survival rate (%) of larvae after 60 days of feeding trial are presented in Table 3. Analysis of the growth performances showed that at the end of the experiment, larvae fed with FF showed significantly higher growth (weight gain 2.6 g; P<0.05) than those fed with other feeds gaining weight ranging from 1.6 g to 2 g). On the other hand, the larvae showed almost similar growth in length (6.8 to 7.2 cm) among the treatments except those fed with DT (5.1cm) which differed significantly (P<0.05). The SGR of larvae fed with FF was significantly higher (P<0.05) than that of DT-fed larvae, while it remained almost the same in other treatments. Overall, the growth increment was slow upto 30 days of rearing followed by a sharp rise, which was evident till the end of the experiment, regardless of feeding treatments (Figure 1a and 1b). Similarly, the weight and length data were comparable for all feeds up to 30 days, but differed significantly afterwards. The average daily weight gain (ADG) of fish in different treatments ranged between 0.03 to 0.04 g with a significant high gain for larvae fed with FF (P<0.05).
Length and weight relationship
The analysis of LWR of H. fossilis larvae is illustrated in Figure 2. The LWR of larvae fed with different feeds was elucidated through an equation as; W = 0.016* L2.442, r2 = 0.752 for LT; W = 0.269*L1.048, r2 = 0.164 for DT; W = 0.014*L2.638; r2 = 0.619 for FF; and W = 0.013*L2.588, r2 = 0.787 for CF. The growth of all treatments were negative allometric with the growth co-efficient (b) ranging from 1.05 to 2.64. The highest growth co-efficient was observed in larvae fed with FF followed by CF, LT and DT (Figure 2).
The study experienced about 50% mortality of larvae immediately after stocking, and then stabilized upon a time in all feeding trials. This resulted in a survival rate about 55%, 50%, and 45% at the first, second, and third week of the feeding trial, respectively, and remained almost constant onwards throughout the experiment. No mortality was observed after 45 days (Figure 3). FF showed the highest survivability followed by CF, LT, and DT.
Table 3: Growth performance of H. fossilis larvae fed with four different feeds for a 60-day feeding trial.
| Growth parameters | LT | DT | FF | CF |
| Initial body weight (g) |
0.002a |
0.002a |
0.002a |
0.002a |
| Final body weight (g) |
1.98±0.69a |
1.55±0.54a |
2.57±1.19b |
2.00±0.87ab |
| Body weight gain (g) |
1.98±0.69a |
1.54±0.54a |
2.57±1.19b |
2.00±0.87ab |
| Avg. daily weight gain (g) |
0.033a |
0.026a |
0.043b |
0.033a |
| Initial body length (cm) |
0.57±0.09 a |
0.57±0.09 a |
0.57±0.09 a |
0.57±0.09 a |
| Final body length (cm) |
7.16±0.84a |
5.10±0.59b |
7.13±1.05a |
6.80±0.97a |
| Length gain (cm) |
6.59±0.75a |
4.53±0.50b |
6.56±0.96a |
6.23±0.88a |
| Specific growth rate (%) |
11.47±0.53a |
11.07±0.49ab |
11.85±0.71a |
11.43±0.69a |
| Survival rate (%) |
46.17±5.46a |
44.08±8.32a |
51.00±6.19a |
45.83±4.02a |
|
Growth co-efficient (b) |
2.44 | 1.04 | 2.64 | 2.59 |
*Values in the same row with different superscripts are significantly (P<0.05) different.
Proximate composition
Proximate compositions in terms of protein, lipid, ash, and moisture of fish larvae fed four experimental feeds are shown in Table 4. The larvae fed with LT (17.5%) and FF (17.6%) had significantly (P<0.05) higher protein content than those fed with DT (12.3%) and CF (15%). Lipid contents were found quite similar in larvae fed with LT (3.19±0.21%), DT (3.08±0.36%), and FF (3.52±0.50%), while it was found significantly higher in those fed with CF (4.22±0.27%). The ash contents in muscle varied non-significantly among the treatments (2.8 to 3.52%). The significantly higher moisture was observed in larvae fed with DT (81.81±0.93%, P<0.05) while in other treatments it remained almost the same (75.5 to 77.3%).
Digestive enzymes
Spectrophotometry analysis revealed that the dietary treatments showed about 30-fold high protease activity than amylase (Figure 4a and b). The highest mean activity of amylase of 0.19µg/min/mg intestine was detected in fish fed with FF, (Figure 4a) which was significantly higher compared to both DT and CF fed fish (P<0.05). The protease activity was comparable among the feeding trails except for fish fed with DT (Figure 4b). The highest protease activity was found in fish fed with LT (6.5 µg/min/mg intestine), followed by FF (6.3 µg/min/mg intestine), CF (5.3µg/min/mg intestine) and DT (4.5µg/min/mg intestine).
Table 4: Proximate composition of experimental fishes after 60 days of rearing.
| Parameter (%) | LT | DT | FF | CF |
| Protein |
17.54±0.16a |
12.29±1.00c |
17.56±0.18a |
14.95±0.63b |
| Lipid |
3.19±0.21b |
3.08±0.36b |
3.52±0.50ab |
4.22±0.27a |
| Ash |
3.14±0.29a |
2.83±0.24a |
3.35±0.37a |
3.52±0.24a |
| Moisture |
76.13±0.53b |
81.81±0.93a |
75.54±0.12b |
77.31±0.31b |
*Values in the row with different superscripts are significantly (P<0.05) different.
Discussion
Despite being a very popular fish, widespread adoption of stinging catfish aquaculture in Bangladesh is constrained by lack of suitable larval feeds. Artificial and live feeds have been tested as a starter food for the larvae of several catfish species including African sharp tooth catfish, Clarius gariepinus (Adewumi, 2014; El-Sebaie et al., 2014), Asian catfish, Clarias macrocephalus (Evangelista et al., 2005) and Green catfish, Mystus nemurus (Srichanun et al., 2012). However, information on H. fossilis is limited. A study was conducted to determine suitable feed for 30-days old larvae by Ahmed et al. (2016) who obtained the best growth in larvae fed on Tubifex with lower SGR (3.6%) among the treatments with other live foods and commercial feed. While Artemia and Tubifex have been proved to be the best diets for the early stages of numerous teleosts (Jayalekshmi et al., 2017), use of these live feeds in catfish hatcheries is not economically feasible. In addition to high cost, the nutritional values of live feeds can also vary depending on the season of collection, environment, and life stages (Bray and Lawrence, 1992). Thus the main objective of our study was to develop a cost-effective artificial diet using locally available ingredients that is comparable to live feed.
In the study, the early stage of H. fossilis fed with formulated feed performed better in terms of SGR, BWG, and LWR than those reared with Tubifex (live and dried) and commercial feed. This is in agreement with others who report that larvae of other catfish such as C. gariepinus (Appelbaum and McGeer, 1998), C. batrachus (Giri et al., 2002), and C. microcephalus (Fermin and Bolivar, 1991) accepted the dry diet as the first food leading to increased survival and growth. Citing results from other species Wang et al. (2005) suggests that feeding of fish larvae solely on carefully formulated diets or a combination of live and formulated diets results in increased survival and growth in comparison with live food alone. Live Tubifex is usually deficient in some nutrients, particularly some fattyacids,18:3n-3 and 20:3n-3 (Arslan et al., 2009) which are required for larval development. The poor quality Tubifex might be a reason for lowering the growth of larvae fed with LT in our study. Better growth performance of catfish larvae on formulated feed in the present study could be attributed to different ingredients that resulted in better digestibility, bioavailability, and ease of assimilation of nutrition from different ingredients as evidenced by digestive enzyme assays.
The b value in LWRs is an indicator of the food intake and growth pattern of the fish (Nadaf et al., 2013). In the present study, a negative allometric growth (b ˂ 3) was observed in all sorts of feed, indicating an elongated body shape of the fish with a lesser width (Froese, 2006). The b value reported in this study is similar to that reported previously from the same species (2.6 for males and 2.8 for females) (Mia, 1984) as well as from other catfishes, including C. batrachus (1.282) (Sahoo et al., 2019) and C. gariepinus (2.9 for female and 2.74 for male) (Yalcin et al., 2002). Among the four feeds tested in the present study, dry Tubifex produced a b value lower than the limit (2-4) (Tesch, 1971) suggesting inappropriate choice as an alternative to live foods.
Experimental diets had no significant effect on the survival of H. fossilis larvae in the present study, though maximum mortality was observed within a week in all experimental feeds. Survivability in all treatments was stabilized after one month of rearing and remained unchanged till the end of the experiment. High mortality at the early stage could be associated with the onset of air-breathing and the cannibalism nature of catfish larvae (Hecht and Appelbaum, 1987) that subsided over time as the fish were accustomed to routine feeding. The survival rates obtained in the present study were comparable with those reported earlier for C. gariepinus where 50% and 61% survivability were observed with live feed and formulated feed respectively (Ukwe and Kenoye, 2018). On the other hand, Mahmood et al. (2004) obtained the highest rate of survival (61%) for larvae of A. testudineus fed with Tubifex compared to other live foods.
Digestive enzyme activity is a biological indicator to understand how well fish can digest a particular feed ingredient or a formulated diet of several ingredients (Gawlicka et al., 2000). The stinging catfish fed with artificially prepared feed showed increased amylase and protease activities compared to other feeds, which agreed with the findings of Magouz et al. (2020) and Aguilera et al. (2012) for genetically-improved farmed tilapia and tropical gar, respectively. The higher digestive enzyme corresponding with increased growth in larvae fed with laboratory feed indicates that fish larvae have good protein and carbohydrate utilization capability (Srichanun et al., 2012).
Conclusions and Recommendations
The results of the present study suggest that the formulated feed composed of locally available ingredients performed well in terms of growth performance, SGR, survival, enzyme activities, and nutritional quality of stinging catfish larvae. The use of formulated feed will not only reduce the production cost and dependency on live feed but also ensure the sustainable production of this commercially important catfish in the aquaculture system.
Acknowledgments
This research was funded by BAS-USDA (Bangladesh Academy of Sciences- United States Department of Agriculture) project (Project ID: BAS-USDA PALS KU-F1-44).
Novelty Statement
This study explored the efficacy of formulated feed in contrast with live feed on highly valued stinging catfish. Our laboratory experiment revealed that formulated feed could be a potential substitute for live Tubifex for the early stages of larvae of stinging catfish, Heteropneustes fossilis.
Author’s Contribution
Momotaz Khanom: Conceptualization, designing the experiment, laboratory analysis and writing and editing the manuscript. Sheikh Mustafizur Rahman: Reviewing and editing, project administration and fund acquisition. Muhammad Yousuf Ali: Statistical analysis. Md. Shahin Parvez: Reviewing and editing. Al-Hasan Antu: Assisting in data curation and in writing the draft. Md. Nazmul Ahsan: Conceptualization, supervision, writing-review and editing, project administration and fund acquisition.
Conflict of interest
The authors have declared no conflict of interest.
References
Adewumi A (2014). Growth performance and survival of Clarias gariepinus hatchlings fed different starter diets. Adv. Agric. Sci. Eng. Res. 4(6): 1659-1664.
Aguilera C, Mendoza R, Iracheta I, Marquez G (2012). Digestive enzymatic activity on Tropical gar (Atractosteus tropicus) larvae fed different diets. Fish Physiol. Biochem. 38(3): 679-691. https://doi.org/10.1007/s10695-011-9550-8
Ahmmed M, Ghosh S, Sarker J, Islam M, Ahsan M (2016). Impacts of different diets on growth and survival of stinging catfish, Heteropneustes fossilis. Bangladesh J. Vet. Anim. Sci. 4(1): 22-26.
Alok D, Krishnan T, Talwar G, Garg LC (1993). Induced spawning of catfish, Heteropneustes fossilis (Bloch), using D-Lys6 salmon gonadotropin-releasing hormone analog. Aquaculture. 115(1-2): 159-167. https://doi.org/10.1016/0044-8486(93)90366-7
AOAC (2012). Official methods of analysis. 19th Edn., Association of Official Analytical Chemist, Washington DC, USA.,
Appelbaum S, McGeer J (1998). Effect of diet and light regime on growth and survival of African catfish (Clarias gariepinus) larvae and early juveniles. Aquac. Nutr. 4(3): 157-164. https://doi.org/10.1046/j.1365-2095.1998.00064.x
Arslan M, Dabrowski K, Portella MC (2009). Growth, fat content and fatty acid profile of South American catfish, surubim (Pseudoplatystoma fasciatum) juveniles fed live, commercial and formulated diets. J. Appl. Ichthyol. 25(1): 73-78. https://doi.org/10.1111/j.1439-0426.2008.01154.x
Bernfeld P (1955). Amylases, α and β. Meth. Enzymol. 1: 149-154. https://doi.org/10.1016/0076-6879(55)01021-5
Bray WA,Lawrence AL (1992). Reproduction of Penaeus species in capitivity. In: Marine Shrimp Culture: Principles and Practices, A. W. Fast and L. J. Lester (Eds.). Elsevier Science Publishers, Amsterdam,Netherlands, Volume 23: 93-170. https://doi.org/10.1016/B978-0-444-88606-4.50011-4
Chakraborty B, Mirza M (2008). Growth and yield performance of threatened Singi, Heteropneustes fossilis (Bloch) under semi intensive aquaculture. J. Fish. Soc. Taiwan. 35(2): 117-125.
Dahanukar N, Raut R, Bhat A (2004). Distribution, endemism and threat status of freshwater fishes in the Western Ghats of India. J. Biogeogr. 31(1): 123-136. https://doi.org/10.1046/j.0305-0270.2003.01016.x
El-Sebaie H, Mahmoud NH, Mahmoud HI, Saad YM (2014). Biological performance of Pterophyllum scalare larvae fed on artemia and artificial diet. World J. Fish. Mar. Sci. 6(3): 289-294.
Evangelista A, Fortes N, Santiago C (2005). Comparison of some live organisms and artificial diet as feed for Asian catfish Clarias macrocephalus (Günther) larvae. J. Appl. Ichthyol. 21(5): 437-443. https://doi.org/10.1111/j.1439-0426.2005.00643.x
Fermin AC, Bolivar MEC (1991). Larval rearing of the Philippine freshwater catfish, Clarias macrocephalus (Gunther), fed live zooplankton and artificial diet: a preliminary study. Isr. J. Aquac. 43(3): 87-94.
Froese R (2006). Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol. 22(4): 241-253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
Froese R, Pauly D (2012). FishBase. World Wide Web electronic publication. www.fishbase.org, ( 01/2022 ).
Gatesoupe F-J, Luquet P (1982). Weaning of the sole (Solea solea) before metamorphosis. Aquaculture. 26(3): 359-368. https://doi.org/10.1016/0044-8486(82)90169-7
Gawlicka A, Parent B, Horn MH, Ross N, Opstad I, Torrissen O (2000). Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): Indication of readiness for first feeding. Aquaculture. 184(3): 303-314. https://doi.org/10.1016/S0044-8486(99)00322-1
Giri SS, Sahoo SK, Sahu BB, Sahu AK, Mohanty SN, Mukhopadhyay PK, Ayyappan S (2002). Larval survival and growth in Wallago attu (Bloch and Schneider): effects of light, photoperiod and feeding regimes. Aquaculture. 213(1): 151-161. https://doi.org/10.1016/S0044-8486(02)00012-1
Hecht T, Appelbaum S (1987). Notes on the growth of Israeli sharptooth catfish (Clarias gariepinus) during the primary nursing phase. Aquaculture. 63(1): 195-204. https://doi.org/10.1016/0044-8486(87)90071-8
Holt GJ (1993). Feeding Larval Red Drum on Microparticulate Diets in a Closed Recirculating Water System. J. World Aquac. Soc. 24(2): 225-230. https://doi.org/10.1111/j.1749-7345.1993.tb00011.x
Hossain M, Hossain A, Mandal S, Rahman M (2016). Impact of live tubificid worms on the growth and survival of Heteropneustes fossilis (Bloch, 1794) reared in tanks. Bangladesh J. Sci. Ind. Res. 51(3): 175-182. https://doi.org/10.3329/bjsir.v51i3.29417
Jayalekshmi J, Abraham KM, Sobhanakumar K (2017). Growth performance of angelfish, Pterophyllum scalare fed with different live worm diets. J. Aquat. Biol. Fish. 5: 116-122.
Lavens P, Sorgeloos P (1996). Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper No. 361. FAO, Rome,
Lavens P, Sorgeloos P (2000). The history, present status and prospects of the availability of Artemia cysts for aquaculture. Aquaculture. 181(3-4): 397-403. https://doi.org/10.1016/S0044-8486(99)00233-1
Le Cren ED (1951). The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20(2): 201-219. https://doi.org/10.2307/1540
Lovett DL, Felder DL (1990). Ontogenetic Changes in Enzyme Distribution and Midgut Function in Developmental Stages of Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol. Bull. 178(2): 160-174. https://doi.org/10.2307/1541974
Magouz FI, Dawood MA, Salem MF, Mohamed AA (2020). The effects of fish feed supplemented with meal on the growth performance, digestive enzyme activity, and health condition of genetically-improved farmed tilapia (Oreochromis niloticus). Ann. Anim. Sci. 20(3): 1029-1045. https://doi.org/10.2478/aoas-2020-0016
Mahmood S, Ali MS, Anwar-ul-haque M (2004). Effect of different feed on larval/fry rearing of climbing perch, Anabas testudineus (Bloch), Bangladesh: II. Growth and survival. Pak. J. Zool. 36(1): 13-20.
Mia G (1984). Length-weight relationship and condition factor in the air-breathing catfish, Heteropneustes fossilis (Bloch). Bangladesh J. Zool. 12(1): 49-52.
Nadaf S, Bhilave M, Bhosale S (2013). Length-weight relationship (LWR) in freshwater fishes fed on formulated feed. Bionano Frontier. 6(2): 232-236.
People Le Ruyet J, Alexandre JC, Thébaud L, Mugnier C (1993). Marine Fish Larvae Feeding: Formulated Diets or Live Prey? J. World Aquac. Soc. 24(2): 211-224. https://doi.org/10.1111/j.1749-7345.1993.tb00010.x
Ricker WE (1975). Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada, Ottawa, Bulletin 191: 382.
Sahoo SK, Ferosekhan S, Giri SS, Radhakrishnan K, Panda D, SriHari M, Pillai BR (2019). Length–weight relationship and growth performance of different life stages of hatchery-produced magur, Clarias magur (Hamilton, 1822). Aquac. Res. 50(5): 1431-1437. https://doi.org/10.1111/are.14018
Sandifer PA, Smith TIJ (1974). Development of a crustacean mariculture program at south carolina’s marine resources research institute. Proceedings of the annual meeting. World Maricult.Soc., 5(1-4): 431-439. https://doi.org/10.1111/j.1749-7345.1974.tb00210.x
Santiago C, Gonzal A, Ricci M, Harpaz S (2003). Response of bighead carp Aristichthys nobilis and Asian catfish Clarias macrocephalus larvae to free‐living nematode Panagrellus redivivus as alternative feed. J. Appl. Ichthyol. 19(4): 239-243. https://doi.org/10.1046/j.1439-0426.2003.00454.x
Sarkar UK, Deepak PK, Negi RS, Qureshi TA, Lakra WS (2007). Efficacy of different types of live and non‐conventional diets in endangered clown knife fish Chitala chitala (Hamilton‐Buchanan) during its early life stages. Aquac. Res. 38(13): 1404-1410. https://doi.org/10.1111/j.1365-2109.2007.01803.x
Siddiqui TQ,Khan MA (2009). Effects of dietary protein levels on growth, feed utilization, protein retention efficiency and body composition of young Heteropneustes fossilis (Bloch). Fish Physiol. Biochem. 35(3): 479-488. https://doi.org/10.1007/s10695-008-9273-7
Srichanun M, Tantikitti C, Vatanakul V, Musikarune P (2012). Digestive enzyme activity during ontogenetic development and effect of live feed in green catfish larvae (Mystus nemurus Cuv. & Val). Songklanakarin J. Sci. Technol. 34(3): 247-254.
Tao J-J, Gui J-F, Zhang Q-Y (2007). Isolation and characterization of a rhabdovirus from co-infection of two viruses in mandarin fish. Aquaculture. 262(1): 1-9. https://doi.org/10.1016/j.aquaculture.2006.09.030
Tesch F (1971). Age and Growth. In: Methods for Assessments of Fish Production in Freshwaters, W. E. Ricker (Ed.). International Biological Programme, Oxford, 97-130.
Ukwe, Kenoye OI (2018). Comparative Performance of African Catfish (Clarias gariepinus ) Fed Artificial and Live Feeds. Int. J. Poult. Fish. Sci 2(1): 1-5. https://doi.org/10.15226/2578-1898/2/2/00110
Walter H (1984). Probionases: Methods with haemoglobin casein and azocoll as substrates. In: Methods of enzymatic analysis, B. HU (Ed.). Verlag Chemic, Weinheim, Volume V: 270–277.
Wang C, Xie S, Zheng K, Zhu X, Lei W, Yang Y Liu J (2005). Effects of live food and formulated diets on survival, growth and protein content of first-feeding larvae of Plelteobagrus fulvidraco. J. Appl. Ichthyol. 21(3): 210-214. https://doi.org/10.1111/j.1439-0426.2005.00620.x
Yalcin Ş, Solak K, Akyurt İ (2002). Growth of the catfish Clarias gariepinus (Clariidae) in the river Asi (Orontes), Turkey. Cybium. 26(3): 163-172.
To share on other social networks, click on any share button. What are these?





