Effect of Sphingomonas sp., as a Probiotic on Survival, Growth and Biochemical Constituents of Vibrio anguillarum Challenged Labeo rohita Fingerlings
Effect of Sphingomonas sp., as a Probiotic on Survival, Growth and Biochemical Constituents of Vibrio anguillarum Challenged Labeo rohita Fingerlings
Asma Chaudhary1, Qurat-ul-Ain Ahmad1, Afia Muhammad Akram1, Mehwish Iqtedar2 and Javed Iqbal Qazi3,*
1Department of Zoology, Division of Science and Technology, University of Education, Lahore 54770
2Department of Biotechnology, Lahore College for Women University, Lahore
3Department of Zoology, University of the Punjab, Lahore 54590
ABSTRACT
This study was attempted to investigate the probiotic effect of a novel locally isolated bacterium from home-made yogurt, Sphingomonas sp. AsCh-P3 on growth, survival and biochemical constituents of Labeo rohita fingerlings challenged with well-renowned fish pathogenic bacterium Vibrio anguillarum. Positive control-C2 as well as experimental groups was challenged with V. anguillarum intraperitoneally. Probiotic treated feed with antibacterial potential against V. anguillarum was administered to experimental fishes in different groups; G1 (soon after challenge for 30 days), G2 (for 15 days prior and 30 days after challenge) and G3 (for 15 days prior to challenge). The survival rate of fishes was 60% in C2, 66% in G1, 83% in G2 and 76% in G3. Fishes subjected to V. anguillarum (C2) showed significant decrease in growth and biochemical constituents as compared to C1 and experimental groups. The incorporation of probiotic isolate enhanced total proteins, total lipids, DNA contents in G2, G3, average weight, weight gain in G1, G3 than both control groups and specific growth rate in G2. On the contrary carbohydrate contents were found to be decreased significantly in all experimental groups. Henceforth, it has been concluded that on the basis of survival data and body profile, Sphingomonas sp. AsCh--P3 could be used successfully as probiotic bacterium against the fish pathogen prophylactically in Labeo rohita fingerlings.
Article Information
Received 09 October 2018
Revised 22 June 2019
Accepted 12 September 2020
Available online 09 September 2021
Authors’ Contribution
AC designed and performed the experiments. QA and AMA helped in data compilation of analysis. MI wrote manuscript. JIQ guided the trial design and manuscript writing.
Key words
Probiotics, Body composition parameters, Fish biochemical constituents, Fish growth performance, Sphingomonas sp., Aquaculture.
DOI: https://dx.doi.org/10.17582/journal.pjz/20181009061048
* Corresponding author: qazi.zool@pu.edu.pk
0030-9923/2021/0006-2071 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
Introduction
Prevailing higher outbreaks of fish diseases pose a negative impact to the success of aquaculture especially in developing countries. Pathogens are one of the main causative agents for fish diseases. The prevention of fish diseases by application of live pathogen antagonistic bacteria has received widespread interest (Hoseinifar et al., 2018).
Probiotics have achieved considerable acceptance in aquaculture to control disease causing agents. Probiotics are live microbial cultures when added to feed or rearing water, result in improved health status and growth of host animals (Moriarty, 1999; Ghosh et al., 2002). Probiotics have been used to improve feed digestibility by producing fatty acids, vitamin, digestive enzymes that help to break down indigestible components of food and stimulate appetite of fish (Seenivasan et al., 2012; Lara-Flores and Olvera-Novoa, 2013; Abdelhamid et al., 2014). They have been employed as feed additive/supplement, water quality enhancers and available commercially consisting of Gram positive bacteria (Bacillus, Carnobacterium, Lactobacillus acidophilus, Streptococcus, Lactococcus, Micrococcus), Gram negative bacteria (Pseudomonas fluorescens, Pseudomonas chlororaphis, Aeromonas hydrophyla, Enterobacter, Enterococcus), yeast (Saccharomyces cerevisiae, Debaryomyces and Phaffia), microalgae and bacteriophages (Nayak, 2010, 2013; Pandiyan et al., 2013; De et al., 2014; Llewellyn et al., 2014; Hoseinifar et al., 2018; Opiyo et al., 2019). The microbes are involved in resisting pathogenic diseases by secreting antiobiotics, bacteriocin, siderophore lysozyme, etc and treat inflammation of gut, modulate immunity, defend the gut by sticking with mucosal epithelia, produce fatty acids, organic acids, and vitamin B12 (Yan et al., 2002; Vine et al., 2006; Luis-Villaseñor et al., 2011; Mahdhi et al., 2012; Sorroza et al., 2012; Azimirad et al., 2016; Modanloo et al., 2017).
Few putative probiotic candidates known are Bacillus subtilis subsp. spizizenii CC1HG5 and CC2HG7 in Catla (Mukherjee and Ghosh, 2016), B. tequilensis in white leg shrimp, Litopenaeus vannamei (Luis-Villaseñor et al., 2015), Lactobacillus in tilapia (Yu et al., 2019), and B. subtilis in Labeo rohita (Chaudhary et al., 2020). Outbreaks of bacterial diseases contribute to decline in fish production and economic loss that should be controlled properly. In this regard, probiotics appeared as promising candidate to combat pathogens (Hoseinifer et al., 2018). The antipathogenic effects of dietary probiotic supplementation were evident in hybrid bass and striped bass against mycobacterium (Gauthier et al., 2004), to resist vibrio by Arthrobacter-Enterococcus consortium in Atlantic cod (Gadus morhua) (Lauzon et al., 2010) and to increase colonization by Lactobacillus in Atlantic salmon (Salmo salar) (Gupta et al., 2019). Probiotic load or dosage is one of the limiting factors in order to achieve desired results with respect to host. The evaluation of optimum concentration of probiotic is therefore suggested to proliferate microflora of gut to modulate immunity (Donnet-Hughes et al., 1999; Bhatnagar and Raparia, 2014). According to Bhatnagar et al. (2012), 2 × 105 cells of B. coagulans have been proved as effective dose to maximum survival and growth in Catla catla and high mortality was reported in Oncorhynchus mykiss with high dose of L. rhamnosus (1012 CFU/g feed) than lower dose (109 CFU/g feed) (Nikoskelainen et al., 2001). Same results were observed by Bacillus cereus in Cirrhinus mrigala (Bhatnagar and Lamba, 2017). There has been found no specific period for feeding the pre/pro-biotics. It depends on feeding either to fingerlings or grows out. But the optimum dose can be species specific.
Vibriosis is hemorrhagic disease caused by Vibrio anguillarum in fresh as well as brackish water and marine fish. The disease being responsible for drastic economic loss were successfully controlled by probiotics in different fishes such as salmon, rainbow trout, turbot, sea bass, cod, eel, carps, crustaceans and bivalves (Frans et al., 2011; Chatterjee and Haldar, 2012; Chaudhary and Qazi, 2014).
Among fresh water carps, Labeo rohita (rohu) is the most important fish due to good quality meat, rapid growth, consumer’s preference and annual production reaches up to 1.7 million tons in Asia (Anonymous, 2018). The appropriate probiotics when administered are considered as putative candidates to combat pathogens, improve growth for sustained production of fresh water carps to cope with consumers demand. Present study dealt with potential of Sphingomonas sp. to confront V. anguillarum to improve growth performance and body composition of L. rohita fingerlings. According to Ali et al. (2001) body composition parameters are good indicators of the physiological condition and growth of a fish. Proximate body composition includes fat, protein, water and ash contents of fish (Gupakumar, 1998; Hui, 2001) and can be affected by physical, abiotic and biotic factors. Body composition is considered as important component on which consumer preference and selection is based (Konca et al., 2009) and will affect the acceptability of food. L. rohita is an important member of fresh water fishes and it is commercially important due to its food value. The study aimed to improve growth profile and disease resistance by Sphingomonas sp. AsCh-P3 supplemented feed prophylactically with least effect on fish muscle (meat quality). Present investigation is the extension of in vivo assay against vibrosis in L. rohita fingerlings to analyze probiotic effect on muscle composition and growth profile when administered by mixing in feed (Chaudhary and Qazi, 2014). In view of growth potential and popularity among fish consumers, present study was undertaken in L. rohita fingerlings of Pakistan.
Materials and methods
Formulation of fish feed (25% protein)
Fish feed was formulated by mixing locally available ingredients following feed formula with some modification (Ghosh and Mandal, 2015). The ingredients include fish meal (Faisal Fish Feeds, Pakistan) 5.0%, rice polishing (Faisal Fish Feeds, Pakistan) 34.3%, ground nut oil cake (Faisal Fish Feeds, Pakistan) 53.7%, molasses (Aquatic Shipping Trading Co., Pakistan) 4.0%, di calcium phosphate (Polifar Group Limited) 1.0%, table salt (BDH, Middle East FZ-LLC)1.0% and vitamin premix (The Bayer, Pakistan) 1.0%. The feed formula fermented with Bacillus subtilis AsCh-A4 (MF543124) showed 19.36±0.45% relative weight gain and 1.68 FCR than control group. Sphingomonas sp. AsCh-P3 (MF543123) culture was revived overnight in nutrient broth and then centrifuged. The pellet was suspended in PBS up to cell density of 0.5 ± 0.05 at 600 nm. The probiotic suspension (10% v/w) containing 93×106 C.F.U/g was sprayed on autoclaved formulated fish feed. Fish feed was dried in a laminar flow cabinet at room temperature (~27-30°C) up to three days then stored in refrigerator up to 7 days. Fish feed without probiotic isolate served as control feed (Chaudhary and Qazi, 2014).
Source of fish pathogen and probiotic isolate
Fish pathogen Vibrio anguillarum (90-11-287; Sero type 01) was obtained from Royal Veterinary and Agricultural University, Copenhagen, Denmark. The literature about the isolated pathogen was also provided with the courtesy of Skov et al. (1995). V. anguillarum is the most studied etiological agent of vibriosis (Larsen et al., 1994). The O1 and O2 serotypes of V. anguillarum are the virulent strains frequently isolated from diseased fish (Toranzo and Bajra, 1990).
Pathogen caused 40% mortalities by injecting 0.1 ml (56×105 C.F.U/ml) with symptoms such as body reddening, belly swelling and reddening and erythma in eyes in pathogenicity study. While Sphingomonas sp. AsCh-P3 was isolated from home-made yogurt and selected on the basis of antibacterial potential against V. anguillarum in in vitro assay (Chaudhary and Qazi, 2014). As yogurt contains certain bacteria that exert immunostimulatory effect on enteric microflora in human being (Meydani and Ha, 2000). On the basis of this hypothesis, bacteria were isolated from yogurt and used as immunomodulant in L. rohita fingerlings. Moreover, Sphingomonas contain GSL (glyco-sphingolipids) in their cell envelope and are completely devoid of Lipopolysaccharides that are capable of stimulating NKT (Natural killer and T) cells which is a part of innate immune response in L. rohita (Sriram et al., 2005).
Experimental set up
L. rohita fingerlings were collected from Department of Fisheries, Government of Punjab, Pakistan. Initially fish were acclimated for 10 days. Then experiment was designed for 45 days. For 15 days the experimental fishes were fed with respective feed, then pathogen was injected and feed trial expanded for more 30 days. L. rohita fingerlings were identified on basis of phenotypic traits such as body shape, mouth, eye, fin color, lateral line, head size/shape, scale shape and body color. The fingerling has stream-lined body, mouth small and inferior; lips thick and fringed, eyes dorsolateral in position, head without scale, body with cycloid scales, distinct lateral line, slightly greenish on dorsal surface, reddish tinge on ventral and caudal fins (Sarder et al., 2011). The health status of the fish was examined grossly throughout the acclimatization and experimental period for visible lesions or symptoms of vibriosis i.e., reddening of body, swelling and reddening of belly and hemorrhages. Fishes were fed with known amount of feed according to 3% of fish body weight once a day during the period. Temperature was maintained at 24 to 27oC. The fish were reared in glass aquaria (2’ × 1’ × 1’) in University animal house following closed or re-circulating system. Glass aquaria were partially cleaned and two-third water was changed daily. Water was aerated by applying aerators (Soltan, 2016; Xiao et al., 2018).
Each control as well as experimental group comprised of triplicates with 10 fish per aquarium. Each experimental and positive control fish was injected intraperitoneally (i.p.) with 0.1 ml of V. anguillarum suspension (56×105 C.F.U. /ml). Negative control was injected i.p. with comparable amounts of Phosphate buffer saline (PBS).
The negative (C1) and positive control (C2) groups were fed with formulated sterilized feed without probiotic (control feed) for first 15 days and 30 days after i.p. pathogenic injections. In experimental group G1, control feed was administered for 15 days prior pathogenic dose followed by Sphingomonas sp. AsCh-P3 treated feed for 30 days whereas G3 was provided with the Sphingomonas sp. AsCh-P3 supplemented feed prior (for 15 days) and control feed after (for 30 days) pathogenic dose. Experimental group G2 received Sphinogomonas sp. AsCh-P3 enriched feed prior (for 15 days) and after (for 30 days) pathogenic dose.
Growth parameters
After pathogen injection and probiotic supplementation, dead fishes were removed out from aquaria and survival data was recorded. Live fishes were weighed to determine weight gain, survival and specific growth rate at start of experiment (initial reading), at time of injection (R0), at 15 (R1) and 30 (R2) days post pathogen injection. Survival percentage was calculated at termination of experiment by following formula described by Opasola et al. (2013).
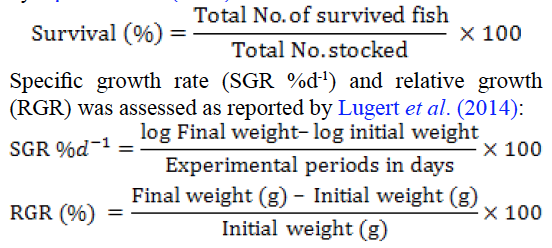
Biochemical analysis of muscle tissue
Muscles of three fishes per replicate were homogenized to extract proteins, lipids, carbohydrates, RNA and DNA (Bregman, 2002). The extraction was carried out for estimation of protein by Lowry et al. (1951) and Sapan et al. (1999), carbohydrates by Dubois et al. (1956), Lichtenthaler and Buschmann (2001) and Jain et al. (2017), lipids by Zollner and Kirsch (1962) and Anschau et al. (2017), and nucleic acids i.e. DNA, RNA by Schnieder (1957) and Jain et al. (2020) following Lowry et al. (1951), phenol sulfuric acid, sulfo-phosphovanillin, diphenyl amine and orcinol methods, respectively.
The ash contents were determined after heating the samples in muffle furnace at 550°C for 3-4 h while moisture contents were determined by drying the sample in oven at 105°C until consistent weight (AOAC, 2016).
Statistical analysis
Values were expressed as mean ± SEM (Standard error mean) for three fish per replicate. All the experimental data were analyzed using one way analysis of variance (ANOVA) at p≤0.05 followed by Dunkun’s multiple range test (SPSS ver.16.0 software, SPSS, Chicago, IL, USA).
Results
Growth performance of L. rohita fingerlings
The dietary supplementation of formulated fish feed with Sphingomonas sp. AsCh-P3 enhanced percent survival rate of V. anguillarum challenged L. rohita fingerlings significantly in G2 (83.3±3.3) and G3 (76.7±3.3) than both control and G1 groups. The percent fish survival of fingerlings infected with V. anguillarum (act as positive control) was 60.0±5.8 than negative control groups as reported in the previous study (Chaudhary and Qazi, 2014), dead fishes were removed daily. The results were tabulated in Table I.
Concerning growth parameters, L. rohita fingerlings fed with probiotic supplemented feed in experimental groups showed significant enhancement in average weight, weight gain and specific growth rate than both control. Highest increase in average weight (g) i.e., 3.8±0.9, 4.6±1.0 in G1 and 3.8±0.8, 4.4±0.9 in G3 were observed after 15 and 30 days post-injection, respectively. Same trend was noted in percent RGR in G1 and G3 groups while highest SGR (1.4±0.1 %d-1) was recorded in G2 group after 30 days (Table I).
Table I.- Growth parameters and percent survival rate of V. anguillarum challenged L. rohita fingerlings fed with probiotic treated diet in different experimental treatments.
|
Parameters |
Negative control (C1) |
Positive control (C2) |
Experimental groups |
|||
|
G1 |
G2 |
G3 |
||||
|
Morphometry |
||||||
|
Initial average weight (g) |
At start of exp. |
2.6±0.3 a |
2.5±0.4 a |
3.3±0.7 b |
3.1±0.6 b |
3.2±0.6 b |
|
Final average weight (g) |
R0 |
2.7±0.3 a |
2.8±0.4 a |
3.4±0.8 b |
3.3±0.6 b |
3.4±0.7 b |
|
R1 |
3.0±0.4 |
3.2±0.4 |
3.8±0.9 |
3.5±0.7 |
3.8±0.8 |
|
|
R2 |
3.5±0.3 a |
3.7±0.5 a |
4.6±1.0 b |
4.1±0.9 b |
4.4±0.9 b |
|
|
Initial average total length (cm) |
At start of exp. |
6.77±0.1 a |
6.7±0.1 a |
6.67±0.2 b |
6.5±0.1 b |
6.5±0.1 b |
|
Final average total length (cm) |
R0 |
7.0±0.1 a |
7.0±0.1 a |
7.1±0.2 a |
6.8±0.1 b |
6.8±0.1 b |
|
R1 |
7.4±0.1 a |
7.3±0.2 a |
7.5±0.2 a |
7.1±0.1 b |
7.1±0.1 b |
|
|
R2 |
7.89±0.2 a |
7.9±0.3 a |
8.5±0.3 b |
7.5±0.1 c |
7.4±0.1 c |
|
|
Initial average width (cm) |
At start of exp. |
1.4±0.02 a |
1.37±0.03 b |
1.4±0.03 a |
1.35±1.3 b |
1.36±1.3 b |
|
Final average width (cm) |
R0 |
1.5±0.03 |
1.5±0.04 |
1.54±0.04 |
1.4±1.4 |
1.5±1.4 |
|
R1 |
1.7±0.04 a |
1.69±0.03 a |
1.73±0.1 a |
1.5±1.4 b |
1.6±1.5 b |
|
|
R2 |
1.9±0.1 a |
1.87±0.1 a |
1.94±0.1 a |
1.65±1.5 b |
1.74±1.5 b |
|
|
Nutritional indices |
||||||
|
Relative growth rate (%) (RGR) |
R0 |
6.04±0.1 a |
9.03±0.1 b |
5.4±0.3 a |
4.0±0.2 c |
7.8±0.2 ab |
|
R1 |
12.5±0.1 a |
16.68±0.1 b |
11.3±0.1 a |
7.3±0.2 c |
10.9±0.2 a |
|
|
R2 |
16.2±0.2 a |
16.6±0.3 a |
19.4±0.6 b |
11.8±0.5 c |
17.1±0.4 a |
|
|
Specific growth rate (% d-1) (SGR) |
R0 |
0.3±0.1 a |
0.6±0.1 b |
0.5±0.1 ab |
0.2±0.1 c |
0.5±0.1 ab |
|
R1 |
0.7±0.2 a |
1.3±0.3 b |
0.6±0.2 a |
0.5±0.1 c |
0.7±0.1 a |
|
|
R2 |
1.1±0.4 a |
1.1±0.1 a |
1.3±0.4 b |
1.4±0.2 c |
1.1±0.3 a |
|
|
% Survival rate (SR) |
At termination of exp. |
100±0.0 a |
60±5.8 b |
66.7±3.3 b |
83.3±3.3 c |
76.7±3.3 bc |
All values represent means of three replicates ±S.E.M. Two values within respective row not sharing a common alphabet differ significantly from each other at p≤ 0.05 at single factor analysis of variance. C1, received sterile PBS 0.1 ml/fish and fed control feed; C2, fish pathogen was injected 0.1ml/fish and fed control feed; G1, the fish were fed probiotic containing feed soon after challenge with fish pathogen for 30 days; G2, the fish were fed probiotic containing feed for 15 days prior and 30 days after challenge with fish pathogen; G3, the fish were fed on the probiotic containing feed for 15 days prior challenge with fish pathogen, thereafter, control feed was given for 30 days ; R0, measurement at time of injection; R1, measurement at 15 days post injection, R1, measurement at 30 days post injection.
Table II.- Effect of Sphingomonas sp. AsCh-P3 on biochemical constituents of L. rohita fingerlings challenged with V. anguillarum.
|
Biochemical constituents |
Negative control (C1) |
Positive control (C2) |
Experimental groups |
|||
|
G1 |
G2 |
G3 |
||||
|
Total protein (mg/g) |
R1 |
26.29±0.40 a |
22.88±0.99 b |
19.75±1.16 b |
22.75±0.29 b |
24.99.±0.09 a |
|
R2 |
25.08±0.52 a |
18.56±0.16 b |
32.27±2.08 c |
30.42±2.22 cd |
26.76±0.58 d |
|
|
Total carbohydrates (mg/g) |
R1 |
23.88±2.00 a |
16.13±0.95 b |
29.71±2.88 c |
20.63±1.45 ab |
17.00±2.00 b |
|
R2 |
26.37±1.04 a |
23.14±1.09 a |
24.46±0.56 a |
16.69±1.10 b |
24.85±2.30 a |
|
|
Total lipids (mg/g) |
R1 |
244.98±11.77 a |
141.06±5.21 b |
209.13±12.94 c |
158.68±4.57 b |
270.45±7.35 a |
|
R2 |
210.78±4.39 a |
155.99±1.37 b |
271.18±17.47 c |
255.59±18.68 c |
281.94±8.14 c |
|
|
DNA (µg/g) |
R1 |
0.71±0.09 a |
1.22±0.04 b |
1.16±0.06 b |
1.14±0.05 b |
1.10±0.04 b |
|
R2 |
1.03±0.07 ab |
0.81±0.06 a |
1.13±0.03 b |
1.23±0.07 b |
1.06±0.05 ab |
|
|
RNA (µg/g) |
R1 |
4.40±0.03 a |
5.16±0.02 b |
5.32±0.23 b |
3.90±0.06 c |
3.85±0.13 c |
|
R2 |
4.21±0.05 a |
4.86±0.08 b |
5.79±0.04 c |
3.57±0.05 d |
3.59±0.04 d |
|
|
Moisture contents (%) |
R1 |
68.02±2.53 a |
74.95±2.21 b |
74.74±1.96 b |
76.46±4.43 b |
82.03±4.00 c |
|
R2 |
69.53±2.02 a |
107.38±2.13 b |
84.69±4.03 c |
74.24±1.55 a |
81.43±3.88 c |
|
|
Ash contents (%) |
R1 |
13.15±0.20 a |
11.44±0.49 a |
9.87±0.58 b |
11.37±0.15 a |
12.45±0.04 a |
|
R2 |
12.54±0.26 a |
9.28±0.08 b |
16.14±1.04 a |
15.21±1.11 a |
13.38±0.29 a |
|
All values represent means of three replicates ±S.E.M. Two values within respective row not sharing a common alphabet differ significantly from each other at p≤ 0.05 at single factor analysis of variance. C1, received sterile PBS 0.1 ml/fish and fed control feed; C2, fish pathogen was injected 0.1ml/fish and fed control feed; G1, the fish were fed probiotic containing feed soon after challenge with fish pathogen for 30 days; G2, the fish were fed probiotic containing feed for 15 days prior and 30 days after challenge with fish pathogen; G3, the fish were fed on the probiotic containing feed for 15 days prior challenge with fish pathogen, thereafter, control feed was given for 30 days; R1, measurement at 15 days post injection, R1, measurement at 30 days post injection.
Biochemical analysis of muscle tissue
The muscle tissue of sampled fish was processed for biochemical characterization of protein, carbohydrates, lipids, RNA, DNA, ash and moisture contents of negative as well as positive control and experimental groups. Healthy fishes with no disease symptoms were sampled after 15 days (R1) and 30 days (R2) for biochemical analysis (Table II). Experimental groups showed increasing trend in total protein, lipid, DNA, moisture and ash contents. The varying degree of increase or decrease contents in experimental fishes after 15 and 30 days than both controls may be due to stress posed by competitive role of probiotics against pathogen. At termination of experiment, experimental fishes showed increasing trend as G1 ˃ G2 ˃ G3 in protein (significant) and ash (non-significant) whereas significant improvement as G3 ˃ G1 ˃ G2 in lipid contents when compared with control groups. Carbohydrate contents were significantly lower in G2 group. Moisture contents were higher significantly in C2, G1 and G3 than C1 as well as G2 groups. The values for RNA contents were higher in G1 and C2 groups after 15 and 30 days post pathogen challenge. The higher DNA contents in C2 after 15 days led to significant lower value at end of experiment as compared to C1 and experimental groups (Table II).
Discussion
L. rohita is regarded as an imperative member of fresh water fishes and is commercially important due to its food value. In view of growth potential and popularity among fish consumers, present study was undertaken to monitor and compare the effects of pathogen and probiotic supplementation on growth performance and body composition of L. rohita fingerlings. Body composition parameters are considered as excellent indicators of the physiological condition and growth of a fish (Ali et al., 2001). These parameters help to assess the level of stress and iterations in metabolic cycles (Wang et al., 2005). The biochemical constituents such as proteins, carbohydrates, lipids, moisture, ash, RNA and DNA contents were determined in muscle of L. rohita fingerlings.
The expansion of aquaculture industry increases the variety of fishes. These fishes are prone to different pathogenic bacteria which causes a great loss. During years, efforts have been made on the reduction of diseases caused by vibrio. Vibriosis is caused by Vibrio anguillarum is reported in fresh water and marine fishes (Frans et al., 2011; Cui et al., 2014). The percent survival rate of L. rohita fingerlings varied from 66.7±5.8 to 83.3±3.3 in experimental fish than positive control 60.0±5.8 in present study as Gram et al. (1999) observed decreased mortality in vibriosis in rainbow trout by probiotic Pseudomonas fluorescens (AH2). According to Welker and Lim (2011) and Hai (2015), Saccharomyces cerevisiae and Bacillus subtilis improve immunity by producing bacitracin and subtilin that contribute towards better survival rate.
This investigation revealed the effect of viable microorganism supplementation into diet, improvement of disease resistance and body composition. Probiotics are microbes to enhance the nutritional values of food. Isolate Shingomonas sp. AsCh-P3 was evaluated for its probiotic potential L. rohita fingerlings. Sriram et al. (2005) described the presence of glycosphingolipid on the cell envelope of Sphingomonas that stimulate natural killer cells and T lymphocytes of L. rohita to respond as innate immunity. Experimental groups were supplied with Sphingomonas sp. AsCh-P3 fortified feed in different feed patterns and effect was observed in biochemical constituents and growth performance of fingerlings. Probiotics improve protein, lipids and ash contents in experimental groups than both controls.
The administration of V. anguillarum in positive control C2 resulted in decrease in average weight, and specific growth rate than experimental groups G1, G2. The decreased growth parameters may be due to pathogenic stress that leads to decreased growth rate because stresses are one of the factors of decreased growth performance in fish (Arthi-Manju et al., 2011). Similarly, V. anguillarum in C2 caused the decrease in protein, lipids, DNA, ash except moisture and RNA contents at termination of experiment.
By keeping in view the growth performance of L. rohita fingerlings, higher average body weight and SGR (%d-1) were recorded in G1 (provided probiotic after infection) and G2 (probiotics prior and after infection), respectively. The results were corroborated with finding of Bairagi et al. (2004) who reported significantly increased final body weight and SGR of common carp than control group by B. subtilis and B. circulans supplemented feed. Similar results were observed by Bagheri et al. (2008), Boonthai et al. (2011) and Parthasarathy and Ravi (2011). Higher relative growth rate (%) was observed in G1 (19.4±0.6) and G3 (17.1±0.4) which were administered probiotics after pathogen injection and prior infection correspondingly. Group G2 received probiotics for long period that is before and after infection showed lower (11.8±0.5) percent RGR. Beghari et al. (2008) reported the decreased growth rate in rainbow trout fry fed with higher levels of B. subtilis and B. licheniformis in combination. Higher probiotic load in feed or dosage attributed reduced growth which may indicate decreased nutrient utilization due to experimental condition variation and experiment duration of probiotic intake. Opiyo et al. (2019) suggested that higher probiotic intake may not be essential in improvement of fish growth and intermediate probiotic dosage may contribute to better health and survival rate instead of enhanced growth.
Protein and lipid contents were improved in experimental groups than positive controls while lower carbohydrates were recorded in C2 as well as experimental groups as compared to C1. In C2, higher moisture and lower lipid as well as ash contents were noted. According to Love (1980), the fish at first consumes lipids from liver and starts to mobilize muscle protein only when fat derived energy has been nearly used up. After that, as protein is utilized, water moves into take its place. Such a shift resulted in the increased water contents that were inversely correlated with protein and fat reserves of their meats (Shimma and Sato, 1985; Mahboob and Sheri, 1997). Carbohydrates being organic nutrients were considered to degrade first due to imposed stress on animal (Gobinath and Ramanibai, 2013). The results of present study were on contrary to findings of Bagheri et al. (2008) who described increase in protein and reduced lipid contents in probiotic enriched diet in Oreochromic mykiss and O. niloticus. According to El-Haroun et al. (2006), increased protein contents can be due to deposition of nutrients. The improvement in protein contents could be resulted by secretion of more proteins by probiotics in Nile tilapia (Opiyo et al., 2019) and conversion of ingested diet effectively into structural protein to build muscles (Rosovitz et al., 1998; Mehrabi et al., 2012; Lara-Flores and Olvera-Novoa, 2013).
At 15 days post pathogen injection, RNA and DNA increased than negative control and experimental groups. At termination of experiment, RNA values stabilized near to negative control and G2, G3 while values of DNA decreased than C1 and experimental groups. In another study, higher DNA contents were observed in L. rohita fingerlings administered with B. subtilis AsCh-A4 fermented fish feed (Chaudhary et al., 2007). Nucleic acid analysis is considered a valuable indictor to assess physiological condition of fish and growth (Smith and Buckley, 2003; Mukherjee and Jana, 2007; Ravikiran and Kulkarni, 2017). In C2, highest values DNA and RNA to some extent are agreed with Khan and Jafri (1991) and Krishnaveni et al. (2013).
Conclusion
By evaluating body composition and growth profile, Sphingomonas sp. AsCh-P3 showed highest protein, carbohydrates and lipid contents followed by average weight and relative weight gain in G1 group when provided after infection in L. rohita fingerlings. Survival and SGR were observed in G2 group fed probiotics prior and after pathogen challenge. The data generated from present study provide information about probiotic incorporation in commercial aquaculture and fish feed supplement for better growth and immunity.
Acknowledgements
This study is a part of first author’s Ph. D work and is grateful to University of the Punjab for financial support for research and publication and Dr. Javed Iqbal Qazi for his guidance.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Ali, M., Salam, A. and Iqbal, F., 2001. Effect of environmental variables on body composition parameters of Channa punctata. BZU J. Res. (Sci.), 12: 200–206.
Abdelhamid, M.A., Refaey, A.M.M., Seden, A.M.E. and Zenhom, O.A., 2014. Effect of different sources and levels of some dietary biological additives on: IV –immunity and haematology of Nile tilapia, Oreochromis niloticus. Egypt J. aquat. Biol. Fish, 18: 49-60. https://doi.org/10.12816/0011096
Anonymous, 2018. FAO yearbook. Fishery and Aquaculture Statistics 2016/FAO annuaire. Statistiques des pêches et de l’aquaculture 2016/FAO anuario. Estadísticas de pesca y acuicultura. FAO, Rome.
Anschau, A., Caruso, C.S., Kuhn, R.C. and Franco, T.T., 2017. Validation of the sulfo-phosphovanillin (SPV) method for the determination of lipid content in oleaginous microorganisms. Braz. J. chem. Eng., 34: 19-27. https://doi.org/10.1590/0104-6632.20170341s20140222
AOAC, 2016. Official methods of analysis (ed. G.W. Latimer Jr.), 20th edition. Association of Official Analytical Chemists, Washington, DC, USA.
Arthi-Manju, R., Felicitta, J., Sakthivel, M., Haniffa, M.A., Vallimmal, S. and Chelladural, G., 2011. Effect of water probiotics on growth performance of Channa punctatus. Int. J. appl. Biores., 1: 25-28.
Azimirad, M., Meshkini, S., Ahmadifard, N. and Hoseinifar, S.H., 2016. The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellf. Immunol., 54: 516–522. https://doi.org/10.1016/j.fsi.2016.05.001
Bagheri, T., Hedayati, S.A., Yavari, V., Alizade, M. and Farzanfar, A., 2008. Growth, survival and gut microbial load of rainbow trout (Onchorhynchus mykiss) fry given diet supplemented with probiotic during the two months of first feeding. Turk. J Fish. aquat. Sci., 8: 43-48.
Bairagi, A., Ghosh, K.S., Sen, S.K. and Ray, A.K., 2004. Evaluation of the nutritive value of Leucaena leucocephala leafmeal, inoculated with fish intestinal bacteria Bacillus subtilis and Bacillus circulans in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings. Aquacult. Res., 35: 436-446. https://doi.org/10.1111/j.1365-2109.2004.01028.x
Bhatnagar, A. and Lamba, R., 2017. Molecular characterization and dosage application of autochthonous potential probiotic bacteria in Cirrhinus mrigala. J. Fish. Sci., 11: 46-56. https://doi.org/10.21767/1307-234X.1000117
Bhatnagar, A. and Raparia, S., 2014. Optimum dietary inclusion level of Bacillus coagulans for growth and digestibility improvement for Catla catla (Hamilton). Int. J. Curr. Res. Rev., 6: 1-10.
Bhatnagar, A., Raparia, S. and Kumari, S., 2012. Influence of isolated Bacillus coagulans on growth performance and digestive enzyme activities of Catla catla. J. Nat. Sci. Sustainable Technol., 6: 225-235.
Boonthai, T., Vuthhiphandchai, V. and Nimrat, S., 2011. Probiotic bacteria effects on growth and bacterial composition of black tiger shrimp (Penaus monodon). Aquacult. Nutr., 17: 634–644. https://doi.org/10.1111/j.1365-2095.2011.00865.x
Bregman, A.A., 2002. Laboratory investigations in cell and molecular biology. John Wiley and Sons, Inc., New York. ISBN: 978-0-471-20133-5
Chatterjee, S. and Haldar, S., 2012. Vibrio related diseases in aquaculture and development of rapid and accurate identification methods. J. mar. Sci. Res. Dev., S1: 002. https://doi.org/10.4172/2155-9910.S1-002
Chaudhary, A., 2007. Growth promoting and bacterial infections controlling roles of probiotics in rohu (Labeo rohita). PhD thesis, University of the Punjab, Lahore, Pakistan.
Chaudhary, A. and Qazi, J.I., 2014. Probiotic antagonism of Sphingomonas sp. against Vibrio anguillarum exposed Labeo rohita fingerlings. Adv. Life Sci., 4: 156-165.
Cui, J., Fan, X.T., Liu, W.Z., Li, H.Y., Zhou, Y.C., Wang, S.F. and Xie, Z.Y., 2014. Isolation and identification of vibriosis pathogens of marine cultured fishes in southern China. Nat. Sci. J. HaiNan Univ., 32: 244–251.
De, B.C., Meena, D.K., Behera, B.K., Das, P., Das Mohapatra, P.K. and Sharma, A.P., 2014. Probiotics in fish and shellfish culture: immunomodulatory and eco physiological responses. Fish Physiol. Biochem., 40: 921–971.
Donnet-Hughes, A., Rochat, F. and Serrant, P., 1999. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: effective dose. J. Dairy Sci., 82: 863-869. https://doi.org/10.3168/jds.S0022-0302(99)75304-X
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F., 1956. Colorimetirc method for determination of sugars and related substances. Anal. Chem., 28: 250-356. https://doi.org/10.1021/ac60111a017
El-Haroun, E.R., Goda, A.M.A.S. and Chowdhury, M.A.K., 2006. Effect of dietary probiotic biogen®supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia, Oreochromis niloticus (L.). Aquacult. Res., 37: 1473–1480. https://doi.org/10.1111/j.1365-2109.2006.01584.x
Frans, I., Michiels, C.W., Bossier, P., Willems, K.A., Lievens, B. and Rediers, H., 2011. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J. Fish Dis., 34: 643–661. https://doi.org/10.1111/j.1365-2761.2011.01279.x
Gauthier, D.T., Rhodes, M.W., Vogelbein, W.K., Kator, H. and Ottinger, C.A., 2003. Experimental mycobacteriosis in striped bass Morone saxatillis. Dis. Aquat. Org., 54: 105-117. https://doi.org/10.3354/dao054105
Ghosh, K. and Mandal, S., 2015. Nutritional evaluation of groundnut oil cake in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after solid state fermentation with a tannase producing yeast, Pichia kudriavzevii (GU939629) isolated from fish gut. Aquacult. Rep., 2: 82–90. https://doi.org/10.1016/j.aqrep.2015.08.006
Ghosh, K., Sen, S.K. and Ray, A.K., 2002. Growth and survival of rohu (Labeo rohita) spawn fed diets supplemented with fish intestinal microflora. J. Acta Ichthol., 32: 83–92. https://doi.org/10.3750/AIP2002.32.1.07
Gobinath, J. and Ramanibai, R. 2013. Studies on growth and survival performance of probiotic supplemented diet on rohu (Labeo rohita) fresh water fish fingerlings. Int. J. Pharm. Res.Bio-Sci., 2: 247-259.
Gram, L., Melchiorsen, J., Spanggaard, B., Huber, I. and Nielsen, T.F., 1999. Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl. environ. Microbiol., 65: 969–973. https://doi.org/10.1128/AEM.65.3.969-973.1999
Gupakumar, K., 1998. Utilization of by catches and low value Fish in India. In: Proceeding of the APFIC Symposium on Fish Utilization in Asia and the Pacific. RAP Publication, Beijing, People’ Republic of China, pp. 29-47.
Gupta, S., Feckaninová, A., Lokesh, J., Košcová, J., Sørensen, M., Fernandes, J. and Kiron, V., 2019. Lactobacillus dominate in the intestine of Atlantic salmon fed dietary probiotics. Front. Microbiol., 9: 3247. https://doi.org/10.3389/fmicb.2018.03247
Hai, N.V., 2015. The use of probiotics in aquaculture. J. appl. Microbiol., 119: 917-935. https://doi.org/10.1111/jam.12886
Hoseinifar, S.H., Sun, Y.Z., Wang, A. and Zhou, Z., 2018. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol., 9: 2429. https://doi.org/10.3389/fmicb.2018.02429
Hui, Y.H., 2001. Meat science and application. CRC Press, pp. 704. https://doi.org/10.1201/9780203908082
Jain, A., Jain, R. and Jain, S., 2020. Estimation of DNA by diphenylamine reaction. In: Basic techniques in biochemistry, microbiology and molecular biology. Springer Protocols Handbooks, Humana, New York, NY, pp. 69-71. https://doi.org/10.1007/978-1-4939-9861-6_22
Jain, A., Jain, R. and Jain, S., 2020. Estimation of RNA using Orcinol method. In: Basic techniques in biochemistry, microbiology and molecular biology. Springer Protocols Handbooks, Humana, New York, NY, pp. 73-75. https://doi.org/10.1007/978-1-4939-9861-6_23
Jain, V.M., Karibasappa, G.N., Arun Suresh Dodamani, A.S. and Mali, G.V., 2017. Estimating the carbohydrate content of various forms of tobacco by phenol-sulfuric acid method. J. Edu. Hlth. Promot., 6: 90. https://doi.org/10.4103/jehp.jehp_41_17
Khan, M.A. and Jafri, A.K., 1991. Protein and nucleic acid concentration in the muscle of catfish Clarias batrachus. Asian Fish Sci., 4: 75-84.
Konca, Y., Kirkpinar, F. and Mert, S., 2009. Effects of Mannan-oligosaccharides and live yeast in diets on the carcass, cut yields, meat composition and colour of finishing turkeys. Asian-Australas. J. Anim. Sci., 22: 550-556. https://doi.org/10.5713/ajas.2009.80350
Krishnaveni, K., Palanivelu, K. and Velavan, S., 2013. Spiritualizing effect of probiotic and spirulina on growth and biochemical performance in common carp (Catla catla). Int. J. Res. Zool., 3: 27–31.
Lara-Flores, M. and Olvera-Novoa, M.A., 2013. The use of lactic acid bacteria isolated from intestinal tract of Nile tilapia (Oreochromis niloticus), as growth promoters in fish fed low protein diets, Lat. Am. J. aquat. Res., 41: 490–497.
Larsen, J.L., Pedersen, K. and Dalsgaard, I., 1994. Vibrio anguillarum serovars associated with vibriosis in fish. J. Fish Dis., 17: 259–267. https://doi.org/10.1111/j.1365-2761.1994.tb00221.x
Lauzon, H.L., Gudmundsdottir, S., Steinarsson, A., Oddgeirsson, M., Petursdottir, S.K., Reynisson, E., Bjornsdottir, R. and Gudmundsdottir, B.K., 2010. Effects of bacterial treatment at early stages of Atlantic cod (Gadus morhua L.) on larval survival and development. J. appl. Microbiol., 108: 624-632. https://doi.org/10.1111/j.1365-2672.2009.04454.x
Lichtenthaler, H. and Buschmann, C., 2001. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Fd. Analyt. Chem., 1: F4.2.1-F4.2.6. https://doi.org/10.1002/0471142913.faf0402s01
Llewellyn, M.S., Boutin, S., Hoseinifar, S.H. and Derome, N., 2014. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol., 5: 207. https://doi.org/10.3389/fmicb.2014.00207
Love, R.M., 1980. The chemical biology of fishes, 3rd edn. Academic Press, Inc., London, UK, pp. 547.
Lowry, O.H., Rosebrough, N.J., Farr, A.J. and Randall, R.J., 1951. Protein measurements with the folin phenol reagent. J. biol. Chem., 193: 265–275.
Lugert, V., Thaller, G., Tetens, J., Schulz, C. and Krieter, J., 2014. A review on fish growth calculation: multiple functions in fish production and their specific application. Rev. Aquacult., 6: 1–13. https://doi.org/10.1111/raq.12071
Luis-Villaseñor, I.E., Voltolina, D., Gomez-Gil, B., Ascencio, F., Campa-Córdova, Á.I., Audelo-Naranjo, J.M. and Zamudio-Armenta, O.O., 2015. Probiotic modulation of the gut bacterial community of juvenile Litopenaeus vannamei challenged with Vibrio parahaemolyticus CAIM 170. Lat. Am. J. aquat. Res., 43: 766–775.
Luis-Villaseñor, I.E., Macías-Rodríguez, M.E., Gómez-Gil, B., Ascencio-Valle, F. and Campa-Córdova, Á.I., 2011. Beneficial effects of four Bacillus strains on the larval cultivation of Litopenaeus vannamei. Aquaculture, 321: 136–144. https://doi.org/10.1016/j.aquaculture.2011.08.036
Mahboob, S. and Sheri, A.N., 1997. Relationship among ovary weight, liver weight and body weight in the grass carp Ctenopharyngodon idella. J. Aquacult. Trop., 12: 255-259.
Mahdhi, A., Kamoun, F., Messina, C., Santulli, A. and Bakhrouf, A., 2012. Probiotic properties of Brevibacillus brevis and its influence on sea bass (Dicentrarchus labrax) larval rearing. Afr. J. microbiol. Res., 6: 6487–6495. https://doi.org/10.5897/AJMR12.1201
Mehrabi, Z., Firouzbakhsh, F. and Jafarpour, A., 2012. Effects of dietary supplementation of synbiotic on growth performance, serum biochemical parameters and carcass composition in rainbow trout (Oncorhynchus mykiss) fingerlings. J. Anim. Physiol. Anim. Nutr., 96: 474-481. https://doi.org/10.1111/j.1439-0396.2011.01167.x
Meydani, A.N. and Ha, W.K., 2000. Immunologic effects of yogurt. Am. J. clin. Nutr., 71: 861–872. https://doi.org/10.1093/ajcn/71.4.861
Modanloo, M., Soltanian, S., Akhlaghi, M. and Hoseinifar, S.H., 2017. The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellf. Immunol., 70: 391–397. https://doi.org/10.1016/j.fsi.2017.09.032
Moriarty, D.J.W., 1999. Disease control in shrimp aquaculture with probiotic bacteria. In: Proceedings of 8th International Symposium on Microbial Ecolology, pp. 237–243.
Mukherjee, A. and Ghosh, K., 2016. Antagonism against fish pathogens by cellular components and verification of probiotic properties in autochthonous bacteria isolated from the gut of an Indian major carp, Catla catla (Hamilton). Aquacult. Res., 47: 2243–2255. https://doi.org/10.1111/are.12676
Mukherjee, S. and Jana, B.B., 2007. Water quality affects SDH activity, protein content and RNA:DNA ratios in fish (Catla catla, Labeo rohita and Oreochromis mossambicus) raised in ponds of sewage-fed fish farm. Aquaculture, 262: 105-119. https://doi.org/10.1016/j.aquaculture.2006.11.013
Nayak, S.K., 2010. Probiotics and immunity: A fish perspective. Fish Shellf. Immunol., 29: 2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Nayak, S.K., 2013, Use of lactic bacteria in fish farming. Aquacult. Asia, 18: 25–27.
Nikoskelainen, S., Ouwehand, A., Salminen, S. and Bylund, G., 2001. Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture, 198: 229-236. https://doi.org/10.1016/S0044-8486(01)00593-2
Opasola, O.A., Adewoye, S.O. and Fawole, O.O., 2013. Growth performance and survival rate of Clarias gariepinus fed Lactobacillus acidophilus supplemented diets. IOSR J. Agric. Vet. Sci., 3: 45-50. https://doi.org/10.9790/2380-0364550
Opiyo, M.A., Jumbe, J., Charles, C., Ngugi, C.C. and Charo-Karisa, H., 2019. Different levels of probiotics affect growth, survival and body composition of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Scient. Afr., 4: e00103. https://doi.org/10.1016/j.sciaf.2019.e00103
Pandiyan, P., Balaraman, D., Thirunavukkarasu, R. George, E.G.J., Subaramaniyan, K., Manikkam, S. and Sadayappan, B., 2013. Probiotics in aquaculture. Drug Invent. Today, 5: 55–59. https://doi.org/10.1016/j.dit.2013.03.003
Parthasarathy, R. and Ravi, D., 2011. Probiotic bacteria as growth promoter and biocontrol agent against Aeromonas hydrophila in Catla catla (Hamilton, 1822). Indian J. Fish., 58: 87–93.
Ravikiran, K. and Kulkarni, R.S., 2015. Nucleic acid content in different tissues of the fish N. notopterus in relation to Se. Int. Lett. Nat. Sci., 34: 1-6. https://doi.org/10.18052/www.scipress.com/ILNS.34.1
Rosovitz, M.J., Voskuil, M.I. and Chambliss, G.H., 1998. Bacillus. In: Systematic bacteriology (eds. A. Balows and B.I. Duerden). Arnold Press, London, pp. 709–720.
Sapan, C.V., Lundblad, R.L. and Price, N.C., 1999. Colorimetric protein assay techniques. Biotechnol. appl. Biochem., 29: 99-108.
Sarder, M.R.I., Yeasin, M., Jewel, M.Z.H., Khan, M.M.R. and Simosen, V., 2011. Identification of Indian major carps (Catla catla, Labeo rohita and Cirrhinus cirrhosus) and their hybrids by phenotypic traits, Allozymes and food habits. Asian Fish. Sci., 24: 49-61.
Schneider, W.C., 1957. Determination of nucleic acids in tissues by pentose analysis. In: Methods in enzymology, Volume III. Academic Press, New York, USA, pp. 680–684. https://doi.org/10.1016/S0076-6879(57)03442-4
Seenivasan, C., Bhavan, P.S., Radhakrishnan, S. and Shanthi, R., 2012. Enrichment of Artemia nauplii with Lactobacillus sporogenes for enhancing the survival, growth and levels of biochemical constituents in the post-larvae of the freshwater prawn Macrobrachium rosenbergii. Turk. J. Fish. aquat. Sci., 12: 23–31.
Shimma, H. and Sato, R., 1985. Composition of proximate composition among the five races of common carp, Cyprinus carpio. Bull. Nat. Res. Inst. Aquacult., 7: 37–43.
Skov, M.N., Pedersen, K. and Larsen, J.L., 1995. Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar 01. Appl. environ. Microbiol., 61: 1540–1545. https://doi.org/10.1128/AEM.61.4.1540-1545.1995
Smith, T.R. and Buckley, L.J., 2003. RNA- DNA ratio in scales from juvenile cod provides a nonlethal measure of feeding condition. Transac. Am. Fish. Soc., 132: 9-17. https://doi.org/10.1577/1548-8659(2003)132<0009:RDRISF>2.0.CO;2
Soltan, M.A.H., 2016. Aquaculture systems. Department of Agriculture, University of Banha, Banha, Egypt.
Sorroza, L., Padilla, D., Acosta, F., Román, L., Grasso, V., Vega, J. and Real, F., 2012. Characterization of the probiotic strain Vagococcus fluvialis in the protection of European sea bass (Dicentrarchus labrax) against vibriosis by Vibrio anguillarum. Vet. Microbiol., 155: 369–373. https://doi.org/10.1016/j.vetmic.2011.09.013
Sriram, V., Du, W., Gervay-Hague, J., Randy R. and Brutkiewicz, R.R., 2005. Cell wall glycosphingolipids of Sphingomonas paucimobilisare CD1d-specific ligands for NKT cells. Eur. J. Immunol., 35: 1692–1701. https://doi.org/10.1002/eji.200526157
Toranzo, A.E. and Barja, J.L., 1990. A review of the taxonomy and sero epizootiology of Vibrio anguillarum, with special reference to aquaculture in the NorthWest Spain. Dis. Aquat. Org., 9: 73–82. https://doi.org/10.3354/dao009073
Vine, N.G., Leukes, W.D. and Kaiser, H., 2006. Probiotics in marine larviculture. FEMS Microbiol. Rev., 30: 404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
Wang, Y.B., Xu, Z.R. and Xia, M.S., 2005. The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish. Sci., 71: 1036–1044. https://doi.org/10.1111/j.1444-2906.2005.01061.x
Welker, T.L. and Lim, C., 2011. Use of probiotics in diets of tilapia. J. Aquacult. Res. Dev., S1: 014. https://doi.org/10.4172/2155-9546.S1-014
Xiao, R., Wei, Y., An, D., Li, D., Ta, X., Wu, Y. and Ren, Q., 2018. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquacult., 11: 863-895. https://doi.org/10.1111/raq.12270
Yan, L., Boyd, K.G. and Burgess, J.G., 2002. Surface attachment induced production of antimicrobial compounds by marine epiphytic bacteria using modified roller bottle cultivation. Mar. Biotechnol., 4: 356–366. https://doi.org/10.1007/s10126-002-0041-x
Yu, L., Qiao, N., Li, T., Yu, R., Zhai, Q., Tian, F., Zhao, J., Zhang, H. and Chen, W., 2019. Dietary supplementation with probiotics regulates gut microbiota structure and function in Nile tilapia exposed to aluminum. Peer J., 7: e6963. https://doi.org/10.7717/peerj.6963
Zollner, N. and Kirsch, K., 1962. Micro determination of lipids by the sulphophosphoanillin reaction. Z. Gesamte exp. Med., 135: 545–561. https://doi.org/10.1007/BF02045455
To share on other social networks, click on any share button. What are these?










