Effect of Whole Allium cepa Linn. Bulb Slurry on Haematological and Biochemical Components of Clarias gariepinus Juveniles
Effect of Whole Allium cepa Linn. Bulb Slurry on Haematological and Biochemical Components of Clarias gariepinus Juveniles
Oghenebrorhie M. Oghenochuko1,4*, Olubukola T. Adenubi3, Olusola L. Ajayi3, Fakilahyel M. Mshelbwala3, Johnny O. Olukunle3, Samson A. Rahman3 and Godfrey N.O. Ezeri2
1Animal Science Department, Landmark University, PMB1001, Omu-Aran, Kwara State, Nigeria.
2Department of Aquaculture and Fisheries Management, Federal University of Agriculture, Abeokuta, PMB 2240, Ogun State, Nigeria.
3College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, PMB 2240, Ogun State, Nigeria.
4Landmark University SDG 2, Landmark University Omu-Aran, Kwara State, Nigeria
ABSTRACT
Toxicity of onion (Allium cepa) bulb though documented for man and some livestock but few studies in fish. Onion bulb slurry was administered to Clarias gariepinus juveniles at 200,100,25g/kg and 5, 1.5, 0.4g/l through diets and bath. Cellular immune response, humoral changes, liver and kidney function and histopathology of some visceral organs were examined. Proximate composition of the bulb was determined. Onion bulb revealed presence of carbohydrate (7.82%), protein (4.48%), crude fiber (1.68%), iron (0.5mg/l), magnesium (210mg/l), flavonoids (0.46%), saponins (0.28%), tannins (0.95%). PCV, Hb, RBC and WBC were increased in all treatments but values were higher in bath treatment for RBC (3.0×1012/L), PCV (32.7%), Hb (10.7%). MCV, MCH and MCHC showed similar trend. Similar trends as in RBC and WBC were observed in total proteins. Liver and kidney functionality as expressed by ALT, AST, ALP, creatinine and BUN exhibited no damaging effect on organs. Degenerations were observed in the hepatocytes and epithelia cells in the kidney in some treatments especially in bath treatments. In conclusion, onion bulb showed no toxicity in the blood parameters but dose should be considered to avoid harmful effect on liver and kidneys.
Article Information
Received 28 August 2020
Revised 10 February 2022
Accepted 29 March 2022
Available online 30 January 2023
(early access)
Published 19 December 2023
Authors’ Contribution
OMO data collection, laboratory analysis, prepared manuscript, experimental design, Statistical analysis, interpretation of results. OTA, JOO and GNOE supervision, experimental design, read first and second draft, approved final draft for publication. OLA and FMM histological analysis and result interpretation, read first and second draft, approved final draft for publication. SAR haematology and result interpretation, read first and second draft, approved final draft for publication.
Key words
Cellular immune response, Fish health, Food security, Histopathology, Humoral changes, Onion bulb slurry, Toxicity
DOI: https://dx.doi.org/10.17582/journal.pjz/20200828170831
* Corresponding author: [email protected]
0030-9923/2024/0001-0353 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Allium cepa Linn which is known as onion has been used as folklore medicine for centuries either singly or in combination with other medicinal edible plants by humans against various diseases (Sohail et al., 2011). A lot of studies have been conducted with much attention paid on the pharmacological potential and toxicity of some plants in human alternative medicine (Agber and Anyam, 2016; Ghorani-Azam et al., 2018), however, little has been documented on the toxicity of most plants on farm animals especially fish.
Moreso, fish farming has become the fastest growing, productive and highly harnessed sub-sector of the agricultural sector in Nigeria and the world. This growth is closely followed by the problems of increased challenges of disease outbreak, mortality, increased dependence on antibiotics and the resultant antibiotics resistant issues. This in turn has led to increased research for alternative therapy mostly involving plant medicine. However, challenges such as lack of standardization and incomplete regulation of herbal remedies and incorrect dosing (Grauer, 2003; Obi et al., 2006), posed a major challenge thereby limiting the application of previous research findings on herbal drugs.
Further compounding this issue is the paucity of clinical and toxicological studies on the safety of the onion plant relative to garlic, especially in fish culture. Thus, with the fast-growing demand for herbal drugs as alternative to synthetic antibiotics, this study aimed to determine the quantity of basic active ingredients in the onion bulb and determine its effect in the liver and kidney.
MATERIALS AND METHODS
Ethical statement
The Ethics Committee on the Laboratory Animal Use of the College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria approved all experimental protocols adopted and carried out on the experimental fish during the study which were same with international principles and protocols for use of laboratory animals.
Plant material and proximate analysis
Onion bulbs were bought from the local onion market in Abeokuta, Ogun State, Nigeria. Authentication of the onion bulb was done at the Department of Forestry and Wildlife, FUNAAB and was given the voucher specimen/ID number (UAHA: 018/0001).
The onion bulbs were then peeled, washed and milled using electronic blender (Binatone, BLG-451). The bulb slurry was then screened for some phytocompounds (flavonoids, saponins, tannins, total phenols, etc) using the methods of Harbourne (1998).
Proximate and mineral profile analysis of the onion bulb was done using the methods described by the Association of Analytical Chemists (AOAC, 2005).
Experimental diets
Four experimental rations were compounded for the study with different percentage inclusion of the onion bulb. Three different of the rations used for the treatment were compounded with percentage inclusion of the onion bulb as follows; treatment 1 ration contained 20%, treatment 2 contained 10%, treatment 3 contained 2.5% while the fourth ration did not contain onion bulb and served as control.
Diet ingredients comprising fishmeal [72% crude protein (CP)], soybean meal (44% CP), yellow maize (10% CP), di calcium phosphate (2.5%), vitamin premix (0.5%) and sodium chloride (NaCl) (0.5%) as fixed ingredients were used in formulating four iso-nitrogenous rations of 50% crude protein (CP). Pearson Square method was adopted for formulation of ration. Measurement and mixing of ingredients, pelletizing and drying pelleted feed were carefully done. The onion bulb was peeled, washed, cut and blended using electric blender separately and then incorporated into the mixed ingredients at 20%, 10% and 2.5% for the three treatments respectively, before pelletizing using 3 mesh size locally fabricated pelletizing machine.
Experimental design for sub chronic toxicity studies
A total of 495 juvenile Clarias gariepinus weighing 28.0 ± 1.24g each were divided into 3 groups as A, B and C each comprising of 165 fish. Fish in group A were fed medicated diets containing different concentrations of the onion bulb (200, 100 and 25g/kg of feed) for two weeks. In like manner, fish in group B were exposed to bath treatment with varying concentrations (5, 1.5 and 0.4g/L) of the onion bulb. While Group C were not fed with diets that contains the onion bulb slurry, and so served as control. Fish were distributed into 7 treatments in all and replicated twice (with 15 fish/replicate). Static renewal bioassay system was adopted during the study. The dosage regimen used during the study was selected after a pilot study to test the acute effect of a range of doses on the experimental fish. Mortality and behavioural changes were monitored and recorded.
Experimental fish
Experimental fish (Clarias gariepinus juveniles) were obtained from a fish farm in Abeokuta metropolis and transported in aerated kegs to the Aquaculture and fisheries Management, outdoor fish growing unit of the Department of Aquaculture and Fisheries Management, Federal University of Agriculture, Abeokuta for the study. Acclimation was carried out for 2 weeks, with constant feeding using 3 mm Coppens (Alltech Coppens, Netherlands). Water quality parameters of dissolved oxygen, temperature and pH were monitored using the HANNA water test kit (Model HI98194) in situ all through the study period and were maintained within the optimal range for fish culture; pH = 6.5 - 9, temperature = 28 - 32oC and dissolved oxygen = 4 - 6.5mg/L (Bhatnagar and Devi, 2013).
Blood biochemical analysis
One millilitre of blood was collected from each fish sample between the anal opening and the pelvic fins after anaesthetization. Blood was put into plane bottles and allowed to stand at 26 oC for one hour and for 5 h at 4 °C for blood clotting. After which, clotted blood was centrifuged for 5 min at 1100rpm and serum collected gently with needle and syringe. All serum samples were preserved at -20 °C before analysis.
Haematological analysis
About 0.5ml of blood was collected through the lateral line from each experimental fish at day 0, 7, and 14. Packed cell volume (PCV) and haemoglobin (Hb) were estimated for using the method of Beers et al. (2010) and outlined by the manufacturer of the Cypress diagnostic kit. Dacie and Lewis (1991) was adopted for the determination of total red blood cell count (RBC), total white blood cell count (WBC), granulocytes (neutrophils, eosinophils and basophils) and agranulocytes (lymphocytes and monocytes). Mean corpuscular haemoglobin (MCH), mean corpuscular volume (MCV) and mean corpuscular and haemoglobin concentration (MCHC) were also calculated as follows:
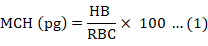
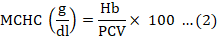
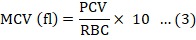
Evaluation of liver damage and function test
Liver enzymes, alanine amino transferase (ALT) and aspartate amino transferase (AST) were measured using enzymatic method (Horder and Sampson, 1991). Total and conjugated bilirubin was as described by Dasgupta et al. (2010). Total protein (TP) concentration was determined by Biuret method described by Zheng et al. (2017), and alkaline phosphatase (ALP) was as described by Ohiri et al. (2013), while albumin concentration was as described by Ueno et al. (2016).
Kidney function evaluation
Urease-Berthelot method as described by Adekunle (2010) was used to estimate for blood urea nitrogen (BUN) while serum creatinine levels were carried out by the Jaffe reaction method according to Perone et al. (1992).
Histopathology
Tissue samples of the liver and kidney were processed and stained according to the method described by Raji and Norouzi (2010). Sections of the liver and kidney were examined with light microscope.
Data analysis
Mean and standard error of means of all data were determined by One-way analysis of variance (ANOVA). Means were separated using the Duncan Multiple Range Test (Duncan, 1955) at significance level of P<0.05.
RESULTS
Proximate composition of onion bulb and experimental diets
Table I shows the proximate composition and Table II shows the mineral profile of onion bulb. Table III shows the percentage composition and proximate composition of the experimental diets. The raw onion bulb on preliminary examination tested strongly positive for tannins and total phenols (Table IV).
Table I. Proximate analysis of the onion bulb.
|
Parameters |
Values (%) |
|
Ash |
0.53 |
|
Fat content |
0.12 |
|
Crude fibre |
1.68 |
|
Crude protein |
4.48 |
|
Moisture content |
85.37 |
|
Carbohydrates |
7.82 |
Table II. Major and minor mineral contents of the onion bulb.
|
Parameters |
Mean ± SE (mg/100g) |
|
Major minerals |
|
|
Calcium |
198.9 ± 0.11 |
|
Sodium |
3.08 ± 0.21 |
|
Potassium |
129.2 ± 0.11 |
|
Phosphorus |
31.6 ± 0.02 |
|
Magnesium |
210.3 ± 0.04 |
|
Minor minerals |
|
|
Iron |
0.5 ± 0.02 |
|
Copper |
0.3± 0.02 |
|
Cobalt |
0.16 ± 0.01 |
|
Selenium |
± 0.02 |
|
Zinc |
0.4± 0.12 |
|
Manganese |
13.6 ± 0.02 |
|
Iodine |
132.9 ± 0.18 |
Showing mean ± SE of the mean.
Haematological analysis
Tables V and VI shows the effect of onion bulb slurry administered via the diets and in the water on haematological parameters of C. gariepinus. There was no significant difference in the levels of PCV, Hb and RBC (P<0.05) among the treated groups considering routes of administration and length of exposure to the onion bulb slurry and control. There was however, significant increase in these parameters at different time of analysis. MCHC, MCH and MCV revealed significant differences (p<0.05), however, there were measurable differences in the treated groups compared to the control group.
Table III. Gross and proximate composition of experimental diets.
|
Components |
Control |
Experimental diets with A. cepa |
||
|
25 g/kg |
100 g/kg |
200 g/kg |
||
|
Ingredients (%) |
||||
|
Fish meal |
26.81 |
26.81 |
26.81 |
26.81 |
|
Soybean meal |
53.61 |
52.36 |
48.61 |
43.61 |
|
Maize |
16.08 |
14.83 |
11.08 |
6.08 |
|
Vitamin premix |
0.5 |
0.5 |
0.5 |
0.5 |
|
Dicalcium phosphate |
2.5 |
2.5 |
2.5 |
2.5 |
|
Toxin binder |
0.1 |
0.1 |
0.1 |
0.1 |
|
Sodium chloride |
0.5 |
0.5 |
0.5 |
0.5 |
|
Onion |
0 |
2.5 |
10 |
20 |
|
Total |
100 |
100 |
100 |
100 |
|
Proximate composition (%) |
||||
|
Moisture content |
8.2±0.02 |
7.4±0.02 |
7.5±0.04 |
8.3±0.06 |
|
Fat |
11.8±0.04 |
12.3±0.01 |
11.3±.06 |
11.5±0.03 |
|
Ash |
9.6±0.02 |
9.1±0.02 |
10.9±0.02 |
10.5±0.03 |
|
Crude fibre |
3.9±0.1 |
4.1±0.03 |
3.9±0.07 |
3.6±0.07 |
|
Crude protein |
50.0±0.27 |
50.6±0.02 |
49.8±0.11 |
49.3±0.06 |
|
Carbohydrates |
16.5±0.07 |
16.5±0.22 |
16.6±0.31 |
16.8±0.67 |
Table IV. Phytocompounds present in the onion bulb.
|
Parameters |
Quantitative (%) |
Qualitative |
|
Alkaloid |
0.75 |
++ |
|
Flavonoid |
0.46 |
+ |
|
Tannin |
0.95 |
++ |
|
Saponin |
0.28 |
+ |
|
Glycosides |
0.54 |
+ |
|
T. Phenol |
0.96 |
++ |
|
Steroid |
0.015 |
+ |
|
Free anthraquinone |
0.28 |
+ |
|
Combined anthraquinone |
1.45 |
+ |
Effect of the onion slurry on biochemical components of fish serum
Humoral immune response was generally affected due to the duration of exposure to treatments in both routes of administration for globulin as well as total protein were lowered in the treated groups than in the control. There was also increased level of albumin in the treated groups. No significant (p>0.05) difference was observed in the levels of ALP and AST between the treated groups and control by day 7 into the experiment. Direct and conjugated bilirubin exhibited no significant variation (P>0.05) in both routes of administration (Tables VII and VIII).
Table V. Effect of Allium cepa diets and bath administered for 7 days on haematological parameters of Clarias gariepinus.
|
Parameters |
Control |
Feed (inclusion levels of onion in g/Kg) |
Bath (onion inclusion levels in g/L) |
||||
|
25 |
100 |
200 |
0.4 |
1.5 |
5 |
||
|
PCV (%) |
25.3± 0.33c |
25.3±0.33bc |
21.0 ± 0.58d |
25.3 ±0.33 bc |
23.0±0.58cd |
24.0±0.58bc |
32.7±0.33a |
|
RBC (×1012/L) |
1.8 ± 0.23c |
2.2±0.00bc |
2.2 ± 0.09b |
2.2 ± 0.12 bc |
1.5 ± 0.06d |
1.8 ± 0.15bcd |
3.0±0.03a |
|
Hb (g/dl) |
7.8 ± 0.49b |
8.3±0.26bcd |
6.9 ± 0.06e |
8.8 ± 0.22 b |
7.6 ± 0.32de |
7.6 ± 0.19de |
10.7±0.12a |
|
MCHC(pg) |
30.8± 1.59b |
32.5±0.68b |
33.2 ± 0.71ab |
34.5 ±0.44 a |
32.7±0.96ab |
32.0±0.09b |
32.3±0.88b |
|
MCV(fl) |
145 ± 17.6a |
116±1.36bc |
118±15.2bc |
116 ± 4.93 bc |
156 ± 4.32a |
132±7.16b |
104±1.86c |
|
MCH(g/dl) |
44.3± 3.04ab |
38.6±0.52cde |
36.8± 2.87cde |
40.0 ±1.18 bcd |
45.1±0.20a |
42.2±2.37bc |
33.7±0.71e |
|
WBC (×109/L) |
12.8 ± 0.34 ab |
10.3 ±0.32c |
12.4 ± 0.38bc |
16.0 ±1.39 a |
11.4±0.15bc |
10.5±0.35bc |
10.4±0.09bc |
|
N (%) |
32.7 ± 0.33 abcd |
27±0.58d |
32.3±0.88abcd |
28.7±2.33 bcd |
27.3±2.03cd |
30.3±0.33abcd |
33±2.89ab |
|
L (%) |
66.3 ± 0.88 abcd |
70.3 ± 0.33a |
64.7 ± 2.03a |
67.3 ±0.88 a |
68.7±2.60a |
65± 0.58a |
66±2.89 a |
|
E (%) |
0.0 ± 0.00 b |
0.0 ± 0.00a |
1.0 ± 0.58a |
1.0 ± 0.58 a |
0.7 ± 0.33a |
0.3± 0.33a |
0.0±0.00 a |
|
B (%) |
0.7 ± 0.33 bc |
1.3 ± 0.33a |
0.3 ± 033a |
1.3 ± 0.89a |
1.3 ± 0.33a |
1.3 ±0.33a |
0.3 ± 0.33a |
|
M (%) |
0.3 ± 0.33 bc |
1.0 ± 0.58a |
1.0 ± 0.58a |
1.0 ± 0.00a |
2.0 ± 0.58a |
1.7 ± 0.67 a |
0.7 ± 0.33a |
Means with different superscripts along the rows indicates significant difference at p<0.05.
WBC, White Blood Cells; M, Monocyte; L, Lymphocyte; E, Eosinophil; N, Neutrophil; B, Basophil; RBC, Red Blood Cells; PCV, Packed cell volume; Hb, haemoglobin; MCHC, Mean corpuscular haemoglobin concentration; MCV, Mean corpuscular volume; MCH, Mean corpuscular haemoglobin.
Table VI. Effect of Allium cepa diets and bath administered for 14 days on haematological parameters of Clarias gariepinus.
|
Parameters |
Control |
Feed (inclusion levels of onion in g/Kg) |
Bath (onion inclusion levels in g/L) |
||||
|
25 |
100 |
200 |
0.4 |
1.5 |
5 |
||
|
PCV (%) |
25.3± 0.33c |
27.3±0.88bc |
31.7±0.33a |
29.0±1.16bc |
27.0±0.58bc |
28.3±0.88bc |
27±1.16bc |
|
RBC (×1012/L) |
1.8 ± 0.23c |
2.2± 0.09b |
2.7 ± 0.03a |
2.3 ± 0.09ab |
2.2± 0.05b |
2.4± 0.06ab |
2.2± 0.15bc |
|
Hb (g/dl) |
7.8 ± 0.49b |
9.3±0.30ab |
10.2±0.09ab |
9.7 ± 0.35ab |
8.7± 0.06ab |
9.3± 0.43ab |
9.0± 3.26a |
|
MCHC(pg) |
30.8± 1.59b |
33.7±0.20ab |
33.1±0.22ab |
33.3±0.10ab |
32.2±0.49ab |
32.6±0.55ab |
36.2±4.16a |
|
MCV(fl) |
145 ± 17.6a |
122.9±1.05b |
119.2±0.98b |
124.3 ±0.99b |
122.± 0.61b |
118.4±0.80b |
131± 3.33a |
|
MCH(g/dl) |
44.3± 3.04ab |
41.4±0.55ab |
39.5±0.10ab |
41.6±0.31ab |
39.6±0.78ab |
38.6±0.87b |
50.9±4.55a |
|
WBC (×109/L) |
12.8 ± 0.34 ab |
12.5±0.43ab |
13± 1.36ab |
13.5±1.44ab |
11.2±0.17ab |
14.9±1.42a |
10.9± 0.22b |
|
N (%) |
32.7 ± 0.33 abcd |
38.0±1.73a |
33.0±1.16abc |
35.3±0.33a |
28.3±2.03bcd |
26.7±3.18 d |
27.7±3.18 cd |
|
L (%) |
66.3 ± 0.88 abcd |
61.7±2.03 d |
65.3±.33abcd |
63.7±0.33bcd |
67.3± 1.45abc |
69.0±2.89 ab |
70.3±2.60 a |
|
E (%) |
0.0 ± 0.00 b |
0.0 ± 0.00b |
0.0 ± 0.00b |
0.7 ± 0.33ab |
1.0 ± 0.58ab |
1.7 ± 0.33a |
0.7 ± 0.33ab |
|
B (%) |
0.7 ± 0.33 bc |
0.7 ± 0.33bc |
1.0 ± 0.58bc |
0.0 ± 0.00c |
1.0 ± 0.00bc |
0.7 ± 0.33bc |
1.3 ± 0.33b |
|
M (%) |
0.3 ± 0.33 bc |
0.0 ± 0.00c |
0.7 ± 0.33bc |
0.7 ± 0.33bc |
2.0 ± 0.00a |
2.3 ± 0.33a |
0.0 ± 0.0c |
Means with different superscripts along the rows indicates significant difference at p<0.05. For abbreviations see Table V.
Table VII. Effect of Allium cepa diets and bath administered for 7 days on serum chemistry parameters of Clarias gariepinus.
|
Parameters |
Control |
Feed (inclusion levels of onion in g/Kg) |
Bath (onion inclusion levels in g/L) |
||||
|
25 |
100 |
200 |
0.4 |
1.5 |
5 |
||
|
AST (U/L) |
64.0 ±3.5a |
67.3±0.33a |
59.0±1.16ab |
57.7±4.33ab |
57.3±0.33ab |
68.3±1.45a |
57.0±1.73ab |
|
ALP (U/L) |
43.7± 0.3a |
44.7 ±0.33 a |
50±4.04a |
46.3 ±2.03 a |
53.0±3.46a |
45.3±3.76a |
48.7±8.37a |
|
ALT (U/L) |
51.7 ±2.4a |
33.0f±1.53 bc |
24.0±1.16 d |
32.7±5.69bc |
33.0±2.31bc |
30.3±1.00cd |
39.0±1.76b |
|
T. Bil (mg/dl) |
0.40 ± 0.01 bc |
0.77±0.11bc |
0.67±0.01bc |
0.66±0.02bc |
0.82±0.05bc |
0.97±0.19ab |
0.91±0.12abc |
|
C. Bil (mg/dl) |
0.28 ± 0.10 ab |
0.41±0.08 a |
0.42± 0.02 a |
0.29± 0.04 a |
0.47±0.09a |
0.29±0.06a |
0.51±0.22a |
|
UREA (mg/dl) |
12.7 ± 0.46 a |
13.7±0.46a |
15.1± 0.55 a |
14.3± 3.73 a |
16.5±1.96a |
13.2±0.92a |
12.9±0.38a |
|
Creatinine (mg/dl) |
1.27 ± 0.15 abc |
1.20±0.17 bc |
0.70±0.00 cd |
0.70± 0.12cd |
1.80±0.21a |
0.93±0.26bcd |
1.30±.06 b |
|
T. protein (g/dl) |
5.00 ± 0.40 ab |
4.40 ± 0.35 abc |
5.47 ± 0.55 a |
4.43 ± 0.78 abc |
3.43 ± 0.26 c |
5.57 ± 0.38 a |
5.13 ± 0.43 ab |
|
Albumin (g/dl) |
2.30 ± 0.29b |
2.20 ± 0.06 a |
2.73 ± 0.15 a |
2.53 ± 0.20 a |
2.50 ± 0.23 a |
2.40 ± 0.12 a |
2.80 ± 0.35 a |
|
Globulin (g/dl) |
2.40±0.12 ab |
2.00±0.29 bc |
2.70 ± 0.40 ab |
1.90 ± 0.58 bc |
0.97 ± 0.03 d |
3.17 ± 0.26 a |
2.33 ± 0.88 bc |
Mean on the same row with different superscripts are significantly different at p<0.05. ALP, Alkaline phosphatase; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; T. Bil, Total bilirubin; C. Bil, Conjugated bilirubin; T. protein, Total protein.
Effect of onion slurry on the liver and kidney of fish
Section of the liver in fish treated via bath showed moderate vacuolar degenerations of hepatocytes while there was moderate necrosis of tubular and glomerular epithelial cells in the kidney. However, in the fish treated orally through feed, section of the liver showed mild vacuolar degeneration of hepatocytes and mild necrosis of tubular and glomerular epithelial cells (Figs. 1 and 2).
DISCUSSION
The onion bulb extract has been presumed by various researchers to be safe for fish (Bello et al., 2014; Saleh et al., 2015; Akrami et al., 2015). However, contrary to these earlier reports, the present study showed that onion bulb extract administered to Clarias gariepinus via dietary inclusion and bath caused varying degree of alterations in the haematological and biochemical parameters as well ashistopathological changes in the liver and kidney According to previous studies on the toxicology of the onion bulb, the plant has the potential to disrupt the haemogram due to its haemolytic effect on the red blood cells, thereby cause haemolytic anaemia in man and other livestock such as water buffalo (Borelli et al., 2009), sheep (Parton, 2000), and cattle (El-Sayed et al., 2015). The reason for this tendency could be as a result of the presence of the organosulfur compounds which is known to damage the red blood cell (Parton, 2000) and the compound was found in high amounts in the onion bulb in the present study.
Table VIII. Effect of Allium cepa diets and bath administered for 14 days on serum chemistry parameters of Clarias gariepinus.
|
Parameters |
Control |
Feed (inclusion levels of onion in g/Kg) |
Bath (onion inclusion levels in g/L) |
|||||
|
25 |
100 |
200 |
0.4 |
1.5 |
5 |
|||
|
AST (U/L) |
64.0 ± 3.5a |
50.0±6.9bc |
40.7±2.6c |
43.0±1.7bc |
49.0±0.6bc |
51.0±0.6b |
50.0±1.2bc |
|
|
ALP (U/L) |
43.7± 0.3a |
24.0±2.3cd |
26.7±3.1bcd |
19.0±1.7d |
28.0±2.6bc |
27.0±0.6bcd |
25.0±2.9 cd |
|
|
ALT (U/L) |
51.7 ± 2.4a |
38.0±2.3b |
27.0±1.7c |
27.0±3.5c |
30.7±2.6bc |
32.0±2.3bc |
26.7±2.0c |
|
|
T. Bil (mg/dl) |
0.40 ± 0.01 bc |
0.48±0.98abc |
0.57±0.74a |
0.34±0.00c |
0.56±0.04a |
0.50±0.03ab |
0.35±0.01c |
|
|
C. Bil (mg/dl) |
0.28 ± 0.10 ab |
0.23±0.01ab |
0.35±0.05ab |
0.21±0.00b |
0.36±0.04 a |
0.27±0.04ab |
0.26±0.00ab |
|
|
Urea (mg/dl) |
12.7 ± 0.46 a |
8.4±0.49c |
9.4±0.27bc |
8.3±0.84c |
7.8±0.38c |
9.6±0.66bc |
10.7±0.33b |
|
|
Creatinine (mg/dl) |
1.27 ± 0.15 abc |
0.57± 0.03 d |
1.03±0.03 c |
1.10±0.17c |
1.60 ± 0.06 a |
0.60 ± 0.06 d |
1.10 ± 0.06 c |
|
|
T. protein (g/dl) |
5.00 ± 0.40 ab |
5.20 ± 0.00 ab |
3.30 ± 0.00 d |
4.63 ± 0.49 abc |
4.20 ± 0.06 c |
4.10 ± 0.23 c |
4.73 ± 0.26 bc |
|
|
Albumin (g/dl) |
2.30 ± 0.29b |
3.00 ± 0.17 ab |
2.27 ± 0.15 b |
2.80 ± 0.17 b |
2.80 ± 0.12 b |
2.80 ± 0.35b |
2.50 ± 0.40 b |
|
|
Globulin (g/dl) |
2.40 ± 0.12 ab |
2.20 ± 0.17 ab |
1.07 ± 0.15 d |
1.90±0.27abcd |
1.30 ± 0.12 cd |
1.30 ± 0.58 cd |
2.07 ± 0.15 abc |
|
Mean on the same row with different superscripts are significantly different at p<0.05. For abbreviations see Table VII.
The high levels in the values of the RBC component (RBC, Hb and PCV) in fish treated through bath than in those treated orally through feed may indicate greater access by the onion bulb compounds to the blood stream thereby increasing the erythropoietic activity of the onion in these treatments. It could also be due to enzymatic breakdown of the compounds in the feed thereby altering the compounds that eventually gets into the blood stream. Also, observed elevation or decrease may not be dose dependent. Bello et al. (2014), Saleh et al. (2015) and Akrami et al. (2015) reported increase in some or all the RBC components.
The PCV level reported in this study differ from that of Coles (1986) and could be attributable to dose variance, handling and time. Also, results obtained in this study were not in agreement with that obtained from other previous studies in which low PCV were reported (Amrevuawho et al., 2016). This could be due to the fact that unlike in our study the fish were challenged before application of the onion treatment. Saleh et al. (2015) also observed a decrease in the RBC of C. gariepinus fed onion powder at varying concentrations. Dose dependent increase observed in the WBC count in treated fish revealed the potential of the onion bulb to stimulate cellular immune response. Findings of this study corroborated earlier study by Akrami et al. (2015) and Bello et al. (2014). The high levels of WBC count in the treated fish indicated that the presence of the onion compounds in circulation was seen as an invasion by xenobiotics, hence, as a defense; an immune response was tailored to counter the invasion. The immune stimulation activity of A. cepa have been well documented Enitan et al. (2012), Bello et al. (2014) and Amrevuawho et al. (2016) and could be related to the high levels of phyto-compounds (alkaloids, flavonoids, saponins and tannins), minerals (iron and copper) and fructo-oligosaccharides (prebiotic activity) present in this plant which enhanced the defence mechanism of the fish (Getahun et al., 2017).
The increase in the values of ALP recorded in the treated groups at 7 days of study could be due to the liver function in the elimination of harmful biochemical waste products and detoxification of certain drugs and environmental toxins which in this case could have been introduced by the feed and onion slurry in the fish environment. However, the levels of this parameter decreased at the 14th day of the study in the treatment groups which could be attributed to poor water quality of the culture environment. This study did not corroborate with the findings of (Akrami et al. 2015; Marzouk et al., 2017). Different parts of the onion plant used, geographical location, and other unknown factors could be responsible for the variation in results. Decrease in the values of AST in the treated did not tally with the liver micrograph and corroborated the findings of Haber et al. (1995) and Davis (2018). However, the study of Bello et al. (2014) was not in agreement with the findings of this study. The variance in the findings could be attributed to the concentration of the onion extract administered in the different studies. Similar trend was also observed in the levels of the enzyme ALT. Bello et al. (2014) and Al-Salahy and Mahmoud (2003) documented similar findings in their studies using A. cepa and A. sativum in fish. Although, low TP levels in this study could be a sign of immunodeficiency, the corresponding low levels of albumin are indicator of liver damage. The level of albumin synthesis reflects the functionality of the hepatocyte mass, thus, the increased levels of albumin observed in both treatments indicated an improvement the synthesis of albumin. This agreed with the report of Mousavi et al. (2016) but was not supported by the study of Enitan et al. (2012) which could be due to the differences of the physiology of the experimental animal. The low levels of globulin in most of the treatments further proved the potency of the onion bulb in preventing diseases that can stimulate immune response, since increase in immunoglobulin is known to be associated with microbial organisms in circulation. This was however not the case in the documentation of Bello et al. (2014).
High levels of creatinine and BUN in the treatments at different concentration in this study indicated that the onion bulb has debilitating effect on the kidney. Result obtained corroborated that of Agbabiaka et al. (2013) and Madibana et al. (2017), but did not support Yilmaz et al. (2012) and could be as a result of differences in the concentration of the onion bulb included in the diet. Reasons for this high BUN levels in the experimental fish could also be credited to high crude protein percentage of the diets as urea, creatinine and uric acid are the by-products of protein digestion.
The histopathological alterations observed in the kidney and liver of experimental fish indicated the toxic potential of the A. cepa bulb in fish. Although this was not visible in the levels of the blood biochemical parameters used for both liver and kidney function tests. Fatty vacuolations in hepatocytes according to Abalaka et al. (2015) could be as a result of pathological response to xenobiotics that are toxic. However, similar degenerations observed in the control could infer that vacuolar changes could be due to other reasons such as feed withdrawal (starvation) prior to commencement of the study which resulted in the increased mobilization of free fatty acids (FFA) from body fat store that resulted to increased synthesis of triglycerides and decreased exportation of same. Necrosis of the tubular and glomerular epithelial cells of the kidney had been documented to be a resultant effect of what is taken by the animal especially plants, drugs and metals (Sancho-Martínez et al., 2015). Moreso, the veterinary pathology blog reported that secondary renal failure may result from plants that cause haemolytic crisis such as red maple leaves and onions. These degenerations were however consistent with bath treatments than in the diet treatments and could be due to the access of the onion slurry into the blood stream as the fish drinks relative to the diets inclusion and the active ingredient may have been acted upon by enzymes in the fish treated orally.
conclusion
In conclusion, feed and bath treatment of fish with onion bulb did not affect heamatological parameters but lowered the levels of ALP, AST and ALT. The kidney functions parameters however were markedly affected by the onion bulb resulting to increase in the values of creatinine and BUN. Although, no significant effect was observed between feed and bath treatment, it is recommended that the plant be administered via feed so as to prevent the problems associated with behavioural changes and marked alterations in the organs. Also, with the high dosage inclusion in the diets, no toxic effect was observed in the blood and some of the serum biochemical parameters of the test fish, although severe degenerative changes were noticed in the liver and kidney, especially in bath treatment.
ACKNOWLEDGEMENT
The authors of this manuscript appreciate the technologist in the Department of Veterinary Physiology, Department of Veterinary Pathology and the staff in the herbarium of the Department of Forestry and Wildlife, and Federal University of Agriculture, Abeokuta, Nigeria. Also, we acknowledge the efforts of the Head of Department and staff of the Biomedicine Department, Forestry Research Institute of Nigeria, Ibadan, Nigeria.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abalaka, S.E., Fatihu, M.Y., Ibrahim, N.D.G. and Ambali, S.F., 2015. Liver histopathological changes in Clarias gariepinus exposed to ethanol extract of Adenium obesum stem bark. J. Morphol. Sci., 32: 22–28. https://doi.org/10.4322/jms.069314
Adekunle, I.M., 2010. Potential nephrotoxicity in African mud catfish (Clarias gariepinus) following exposure to compost derived humic acid. Pak. J. biol. Sci., 13: 835-840. https://doi.org/10.3923/pjbs.2010.835.840
Agbabiaka, L.A., Madubuike, F.N. and Ekenyemi, B.U., 2013. Haematology and serum characteristics of African catfish (Clarias gariepinus Burchell) fed graded levels of Tigernut-based diet. Am. J. exp. Agric., 3: 988-995. https://doi.org/10.9734/AJEA/2013/2979
Agber, W.C. and Anyam, R.W., 2016. Pharmacological activity of some Nigeria plants extracts in the remidiation of alloxan-induced diabetes in rats: A review. Int. J. Pharmacol. Phytochem. Ethnomed., 4: 73-82. https://doi.org/10.18052/www.scipress.com/IJPPE.4.73
Akrami, R., Gharaei, A., Mansour, R.M. and Galeshi, A., 2015. Effects of dietary onion (Allium cepa) powder on growth, innate immune responseand hematobiochemical parameters of beluga (Husohuso Linnaeus, 1754) juvenile. Fish Shellfish Immunol., 45: 828-834. https://doi.org/10.1016/j.fsi.2015.06.005
Al-Salahy, M.B. and Mahmoud, A.B., 2003. Metabolic and histological studies on the effect of garlic administration on the carnivorous fish Chrysichthys auratus. Egypt. J. Biotech., 5: 94-107.
Amrevuawho, M.O., Akinyemi, A.A., Oyewusi, A.J., Bankole, O.M. and Ezeri G.N.O., 2016. Effects of onion (Allium cepa) and chloramphenicol on haematological parameters, histopathology and survival of catfish Clarias gariepinus (Burchell, 1822) sub-adult infected with Pseudomonas aeruginosa. Voms J. Vet. Sci., 11: 1–12
AOAC, 2005. Solids (Total) and moisture in flour, method 925.10. In: Official methods of analysis. 18th Edition, AOAC International, Gaithersburg, Maryland.
Beers, J.M., Borley, K.A. and Sidell, B.D., 2010. Relationship among circulating haemoglobin, nitric oxide synthase activities and angiogenic poise in red- and white-blooded Antarctic notothenioid fishes. Comp. Biochem. Physiol. A. 156: 422-429. https://doi.org/10.1016/j.cbpa.2010.03.027
Bhatnagar, A. and Devi, P., 2013. Water quality guidelines for the management of pond fish culture. Int. J. environ. Sci., 33: 1980–2009. https://doi.org/10.6088/ijes. 2013030600019
Bello, O.S., Olaifa, F.E. and Emikpe, B.O., 2014. Haematological and blood biochemical changes in African catfish, Clarias gariepinus fed walnut (Tetracarpidium conophorum Mull Arg) leaf and onion (Allium cepa Linn) bulb supplemented diets. Am. J. exp. Agric., 4: 1593-1603. https://doi.org/10.9734/ajea/2014/6622
Borelli, V., Lucioli, J., Fernando, H.F., Giovana, H.P., Fleck, R.J., Davi, T.S. and Aldo, G., 2009. Fatal onion (Allium cepa) toxicosis in Water Buffalo (Bubalus bubalis). J. Vet. Diag. Invest., 21: 402–405. https://doi.org/10.1177/104063870902100321
Coles, E.H., 1986. Veterinary clinical pathology. 4th Edition. W.B. Saunders Company, Philadelphia. pp. 17-19.
Dacie, J.V. and Lewis, S.M., 1991. Practical haematology. Church Hill publishers, Livingstone, London. pp. 1-13.
Dasgupta, A., Langman, L.J., Johnson, M. and Chow, L., 2010. Naproxen metabolites interfere with certain bilirubin assays: Elimination of interference by using a Roche bilirubin assay on the Hitachi 917 Analyzer. Am. J. clin. Pathol., 133: 878–883. https://doi.org/10.1309/AJCPN6MWATQ3SZTC
Davis, C.P., 2018. Liver blood test (normal, low and high ranges and results). Retrieved from https://www.medicinenet.com/liver_blood_tests/article.htm 12/06/2019.
Duncan, D.B., 1955. Multiple range and multiple F tests. Biometrics, 11: 1. https://doi.org/10.2307/3001478
El-Sayed, Y.S., El-Okle, O.S.M., Hassan, S.M.H. and Bakir, N.M.A., 2016. Poisoning of cattle feeding on Allium ampeloprasum (Egyptian kurrat). J. Vet. Sci. Med. Diag., 4: 4. https://doi.org/10.4172/2325-9590.1000170
Enitan, S.S., Ajeigbe, K.O., Josiah, S.J. and Ehiaghe, F.A., 2012. Haematological and hepatotoxic potential of onion (Allium cepa) and garlic (Allium sativum) extracts in rats. Eur. J. med. Pl., 2: 290-307. https://doi.org/10.9734/EJMP/2012/1517
Getahun, A., Tesfaye, A. and Muleta, D., 2017. Investigation of the potential benefits and risks of probiotics and prebiotics and their synergy in fermented foods. Singapore J. chem. Biol., 6: 1-16. https://doi.org/10.3923/sjchbio.2017.1.16
Ghorani-Azam, A., Sepahi, S., Riahi-Zanjani, B., Alizadeh-Ghamsari, A., Mohajeri, S.A. and Balali-Mood, M., 2018. Plant toxins and acute medicinal plant poisoning in children: A systematic literature review. J. Res. med. Sci. Off. J. Isfahan Univ. med. Sci., 23: 26-34. https://doi.org/10.4103/jrms.JRMS_629_17
Grauer, R., 2003. Herbal medicine and perioperative care: An Australian perspective. Australas.Anaesth., 58: 105-115.
Haber, M.M., West, A.B., Haber, A.D. and Reuben, A., 1995. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am. J. Gastroenterol., 90: 1250–1257.
Harbourne, J.B., 1998. Phytochemical methods: A guide to modern techniques of plant analysis. 3rd edition. Chapman and Hall Publishers, London, Weinheim. New York. Tokyo. Melbourne Madras. pp. 302.
Horder, M. and Sampson, E.J., 1991. Approved IFCC recommendation on methods for the measurement of catalytic concentration of enzymes Part7: IFCC method for creatinine Kinase (ATP: Creatinine N-phosphotransferase, EC. 2, 7, 3, 2). Eur. J. clin. Chem. clin. Biochem., 29: 435-456.
Madibana, M.J., Mlambo, V., Lewis, B. and Fouché, C., 2017. Effect of graded levels of dietary seaweed (Ulva sp.) on growth, hematological and serum biochemical parameters in dusky kob, Argyrosomus japonicus, Sciaenidae. Egypt. J. aquat. Res., 43: 249-254. https://doi.org/10.1016/j.ejar.2017.09.003
Marzouk, M.S.M., Abdel-Aziz, M.A., Soliman, W.S., Abbas, H.H., Mona, S.Z., Awad, E. and Sahr, B.A., 2017. Effect of some herbal extracts on the health status of cultured Oreochromis niloticus. Res. J. Pharm. Biol. Chem. Sci., 8: 1457-1466.
Mousavi, E., Mohammadiazarm, H., Mousavi, S.E. and Ghatram, E.R., 2016. Effects of inulin, savory and onion powders in diet of juveniles carp Cyprinus carpio (Linnaeus 1758) on gut micro flora, immune response and blood biochemical parameters. Turk. J. Fish. aquat. Sci., 16: 831-838. https://doi.org/10.4194/1303-2712-v16_4_09
Obi, E., Akunyili, D.N., Ekpo, B. and Orisakwe, O.E., 2006. Heavy metal hazards in Nigerian herbal remedies. Sci. Total Environ., 369: 35-41. https://doi.org/10.1016/j.scitotenv.2006.04.024
Ohiri, R.C., Agha, N.C. and Nwachukwu, N., 2013. Variations in phosphatase activity of crude oil and used crankase oil polluted agricultural soil. J. Biol. Agric. Healthc., 3: 143-149.
Parton, K., 2000. Onion toxicity in farmed animals. N. Z. Vet. J., 48: 89. https://doi.org/10.1080/00480169.2000.36168
Perone, R.D., Madias, N.E. and Levey, A.S., 1992. Serum creatinine as an index of renal function: New insight into old concepts. Clin. Chem., 38: 1933-1953. https://doi.org/10.1093/clinchem/38.10.1933
Raji, A. and Norouzi, E., 2010. Histological and histochemical study on the alimentary canal in Walking catfish (Clarias batrachus) and piranha (Serrasalmus nattereri). Iran. J. Vet. Res., 11: 255-261.
Saleh, N.E., Michael, F.R. and Toutou, M.M., 2015. Evaluation of garlic and onion powder as phyto-additives in the diet of sea bass (Dicentrarcus labrax). Egypt. J. aquat. Res., 41: 211-217. https://doi.org/10.1016/j.ejar.2015.03.008
Sancho-Martínez, S.M., López-Novoa, J.M. and López-Hernández, F.J., 2015. Pathophysiological role of different tubular epithelial cell death modes in acute kidney injury. Clin. Kidney J., 8: 548–559. https://doi.org/10.1093/ckj/sfv069
Sohail, M.N., Karim, A., Sarwar, M. and Alhasin, A.M., 2011. Onion (Allium cepa L.): An alternate medicine for Pakistani population. Int. J. Pharm., 7: 736-744. https://doi.org/10.3923/ijp.2011.736.744
Ueno, T., Hirayama, S., Sugihara, M. and Miida, T., 2016. The bromocresol green assay, but not the modified bromocresol purple assay, overestimates the serum albumin concentration in nephrotic syndrome through reaction with α2-macroglobulin. Annls clin. Biochem., 53: 97–105. https://doi.org/10.1177/0004563215574350
Yilmaz, S., Ergun, B. and Celik, M., 2012. Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): Change in body composition and some blood parameters. J. BioSci. Biotech., 1: 217-222.
Zheng, K., Wu, L., He, Z., Yang, B. and Yang, Y., 2017. Measurement of the total protein in serum by biuret method with uncertainty evaluation. Measurement, 112: 16-21. https://doi.org/10.1016/j.measurement.2017.08.013
To share on other social networks, click on any share button. What are these?









