Electrochemistry of Vitamin D and Biosensors for its Determination: A Review
Mini-Review
Electrochemistry of Vitamin D and Biosensors for its Determination: A Review
Nargis Sardar1*, Maria Binte Sarfraz2, Sufian Rasheed3 , AKM Rezwan Sardar4 , Fahamida Zaman5, Arsalan Rasheed6,7*
1Department of Material Science and Electrical Engineering, State Research Institute, Center for Physical Sciences and Technology Vilnius, Lithuania; 2Department of Physiology, Army Medical College, National University of Medical Sciences, Rawalpindi, Pakistan; 3HEJ Research Institute of Chemistry, Karachi, Pakistan; 4Department of Textile and Apparel Marketing, Primeasia University, Dhaka, Bangladesh; 5Department of Microbiology, Primeasia University, Dhaka, Bangladesh; 6Department of Zoology, Abdul Wali Khan University Mardan, Pakistan; 7SST (Bio-Chemistry), Elementary and Secondary Education Department (E&SED), Khyber Pakhtunkhwa, Pakistan.
Abstract | Vitamin D play a vital role in health, therefore, it is necessary to find a sensitive, selective as well as quick and easy technique for its determination. This review focuses on the Vitamin D investigations of electrochemical biosensors that have been conducted in recent years. According to the research, the practical use of electrochemical biosensors is attributed to the existence of UV radiation and transactivation of pharmaceutical items, food, or even human blood plasma in the detection of Vitamin D from diverse samples, including Vitamin D production in nature. Among the most commonly used electrochemical biosensors for vitamin D detection are Ab-25OHD/SPE/ FMTAD, CYP27B1/GCE, SiO2/GO/Ni(OH)2/GCE, BSA/Ab-VD2/CD-CH/ITO, BSA/Anti VD/Fe3O4 PANnFs/ITO, BSA/Ab-VD/Asp-Gd2O3NRs/ITO, 25OHD Antibody, 25OHD, 25OHD Antibody, IoT Enabled Enzyme Embossed Biosensor, Au-Pt NPs/APTES/FTO and GCN-β-CD/Au nanocomposite. The proposed electrochemical biosensors utilized in the previous publications studied were based on glassy carbon, carbon dots, or carbon paste, functionalized with the various electrochemical biosensors. Further research should be conducted on existing problems and future opportunities of the present electrochemical sensors for the determination of vitamin D.
Keywords: Vitamin D, Biosensor, Radiation, Carbon dots, Glassy carbon, Carbon paste
Received | December 30, 2021 Accepted | February 05, 2022; Published | March 01, 2022
*Correspondence | Nargis Sardar, Arsalan Rasheed, Department of Material Science and Electrical Engineering, State Research Institute, Center for Physical Sciences and Technology Vilnius, Lithuania; Department of Zoology, Abdul Wali Khan University Mardan, Pakistan; Email: [email protected], [email protected]
Citation Sardar N, Sarfraz MB, Rasheed S, Sardar AKMR, Zaman F, Rasheed A (2022). Electrochemistry of vitamin d and biosensors for its determination: a review. S. Asian J. Life Sci. 10(1): 1-6.
DOI | http://dx.doi.org/10.17582/journal.sajls/2022/10.1.1.6
ISSN | 2311–0589
INTRODUCTION
Vitamin D (also known as “calciferol”) is a class of fat-soluble secosteroids that promotes calcium, magnesium, and phosphate absorption in the intestine, and enhances several other biological effects. Vitamin D is associated with the guideline of calcium homeostasis and bone metabolism by playing out its capacities in target tissues, including the digestion tracts, kidneys, and bones. Developing proof proposes that Vitamin D assumes a significant part in numerous tissues, including skeletal muscle. Early clinical translations of a myopathy related with extreme Vitamin D insufficiency perceived the expected connection between nutrient D and muscle. Toxicity to Vitamin D is much rare (Holick, 2007). It is caused due to the administration of excessive doses of Vitamin D rather than the exposure to sunlight. Toxicity threshold has not yet being established for Vitamin D.; however, according to certain research, for the ages of 9 – 71 the acceptable upper intake level (UL) is 4,000 IU per day. (Ross, 2011), Other study indicates that a continuous consumption of more than 1250 g per day (50,000 IU) in healthy persons might cause overt toxicity after a few months and raise blood 25-hydroxyVitamin D levels to 150 ng/mm or higher. (Holick, 2007). Patients suffering from primary hyperparathyroidism are substantially more susceptible to Vita
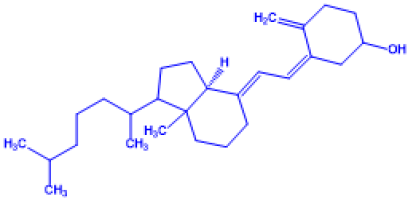
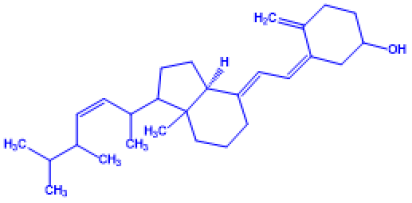
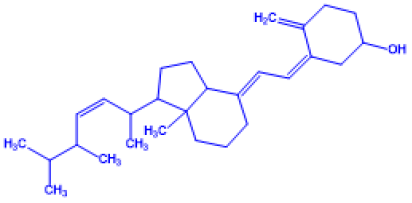
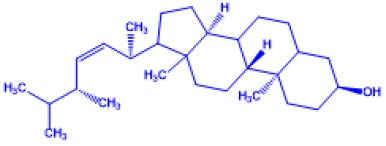
Table 1: Important Chemical structure of Vitamin D
| Name of Vitamin D | Chemical Name | Structure | Chemical Composition |
|
Vitamin D1 |
(3S,9R,10R,14S)-17-((2R,5R,Z)-5,6-dimethylhept-3-en-2-yl)-10,13-dimethyl hexadeca hydro-1H-cyclopenta [a] phenanthren-3-ol |
|
Mixture of molecular compounds of ergocalciferol with lumisterol, 1 :1 |
|
Vitamin D2 |
(Z)-3-((E)-2-(1-((Z)-5,6-dimethylhept-3-en-2-yl)-7a-methyl hexahydro-1H-inden-4(2H)-ylidene)ethyli dene)-4-methylene cyclohexanol |
|
Ergocalciferol (made from ergosterol) |
|
Vitamin D3 |
(Z)-3-((E)-2-(7a-methyl-1-(6-methyl heptan-2-yl)hexahydro-1H-inden-4(2H)-yli dene)ethylidene)-4-methylenecyclohexanol |
|
Cholecalciferol (made from 7- |
|
Vitamin D4 |
(Z)-3-((E)-2-((3aS)-1-((Z)-5,6-dimethylhept-3-en-2-yl)-7a-methyl hexahydro-1H-inden-4(2H)-ylidene)ethyli dene)-4-methylenecy clohexanol |
|
22-dihydroergocalciferol |
|
Vitamin D5 |
(Z)-3-((E)-2-((3aS)-7a-methyl-1-(6-methyl heptan-2-yl)hexahydro-1H-inden-4(2H)-yli dene)ethylidene)-4-methylenecyclohexanol |
|
Sitocalciferol (made from 7- |
min D. All patients with primary hyperparathyroidism will have low levels of Vitamin D. Maternal hypercalcemia may enhance fetal susceptibility to Vitamin D effects and lead to mental retardation syndrome and facial abnormalities. (Vieh, 1999). Idiopathic infantile hypercalcemia is caused by a CYP24A1 gene mutation, which reduces Vitamin D decomposition. Infants with this mutation are more sensitive to Vitamin D and are at risk of hypercalcemia if they consume more of it (EFSA, 2006). Dahlquist et al. (2015) found that deleterious effects were only observed at 25(OH)D serum concentrations over 200 nmol/L. It is important that women who are pregnant or breastfeeding should get doctor’s advice prior to using a vitamin D supplement. The Food and Drug Administration (FDA) has recommended makers of liquid Vitamin D supplements that droppers containing such goods should be clearly and precisely labelled for 400 foreign units (1 IU is the biological equivalent of 25ng cholecalciferol/ergocalciferol); FDA, 2017). The indication of hypercalcemia is increased urination and thirst. Excessive calcium accumulation in soft tissues and organs such as the liver, kidneys, and heart result in discomfort and organ damage if hypercalcemia is not treated. (Holick, 2007; Brown, 2013; Insel, 2015). Anorexia, vomiting, and diarrhoea are the most common symptoms of Vitamin D insufficiency. and they are the same as hypercalcemia. The toxicity of Vitamin D is controlled by the discontinuation of the supplementation of this vitamin and restricting calcium intakes. Sunlight exposure over lengthy periods of time does not generally result in Vitamin D toxicity. The amounts of Vitamin D precursors produced in the skin reach equilibrium, and any more Vitamin D produced is decomposed. (Vieth, 1999).
These facts show that determination of Vitamin D in human organism is important especially if one uses food supplements with this vitamin. Therefore, the fast and effective methods for analysis of this vitamin are needed. One of such methods are electrochemical sensing, this review determination of Vitamin D with electrochemical sensors or methods are overviewed. In order to understand how this vitamin could be determined electrochemically, basics of compounds and transformations within Vitamin D are explained and discussed.
Basic information on Vitamin D and its analogues
Vitamin D (Vitamin D) production in nature is dependent on the presence of UV light and transactivation in the liver and kidneys. Vitamin D comes in a variety of forms in living beings (Horst et al., 1986; Table 1). Vitamin D1 contains a 1:1 ratio of the lumisterol and D2 molecular compounds. Vitamin D2 or trivial name - ergocalciferol & Vitamin D3 or trivial name - cholecalciferol are the two primary form. Without a subscription, Vitamin D is usually D2 or D3, or both. Collectively, these are recognized as calciferol (Karim & Hoor, 2015). In 1931, Vitamin D2 was characterized for the first time (Vaja, 2019). In 1935, the molecular structure of Vitamin D3 was discovered and proved to be the result of UV irradiation of 7-dehydrocholesterol. The various forms of Vitamin D are secosteroids, which are steroids formed by the the breakage one of the bonds in the steroid rings. The structural distinction between Vitamin D2 and D3 is that the side chain of D2 includes a double bond between carbons 22 and 23 and the carbon 24 methyl groups. The structural difference among Vitamin D2 and D3 is that the side chain of D2 has a methyl groups on carbon 24 and a double bond between carbons 22 and 23 (Trashin & Pchelintsev, 2010).
Number of species synthesize the 7-dehydrocholesterol (trivial name - cholecalciferol) Vitamin D3 and several fungi synthesize the ergosterol Vitamin D2 (trivial name - ergocalciferol). Vitamin D2 and Vitamin D mechanism of action (Bikle, 2014). According to the International Union of Pure and Applied Chemistry, metabolites generated by Vitamin D2 are frequently referred to as er- or ergo prefixes to distinguish them from D3 equivalents. (IUPAC, 1982). Vitamin D2 metabolites tend to link the Vitamin D binding protein. Alternatively, Vitamin D3 may be hydroxylated to calcifediol via sterol 27-hydroxylase (CYP27A1), while Vitamin D2 doesnt. From position 24, ergocalciferol can be hydroxylated directly. This hydroxylation also adds to a greater degree of inactivation: whereas calcitriol activity reduces to 60% of its original following 24-hydroxylation, (Holick et al., 1973), the activity of ercalcitriol on conversion to ercalcitetrol decreases 10-fold.
The transition of 7-dehydrocholesterol to Vitamin D3 occurs in two steps. (Fig.1; Holick 1987; Deluca, 2014). First, 7-dehydrocholesterol is photolyzed by UV light in a 6-electron conrotatory ring-opening electro cyclic process, yielding previtamin D3. Second, previtamin D3 spontaneously isomerises to Vitamin D3 in an antara-facial-sigmatropic hydride transfer. In an organic solvent, the transition of previtamin D3 into vitamin D3 takes around 12 days at room temperature. The conversion of previtamin D3 into vitamin D3 in skin is approximately ten times quicker than in an organic solvent. (Holick, 2004). The conversion of ergosterol into Vitamin D2 bears some resemblance, with photolysis yielding previtamin D2, which isomerizes to Vitamin D2 (Eyley, 1975). The rate of previtamin D2 transformation to methanol from Vitamin D2 is similar with that of previtamin D3. (Banerjee & Bhunia, 2009). Photochemically, vitamin D3 is generated from 7-dehydrocholesterol with in skin of majority vertebrate species, which includes humans. (Keegan, 2013).
7-dehydrocholesterol, a precursor of vitamin D3, is produced in quite substantial quantities. (Crissey et al., 2003). At wavelengths of 290–315 nm, 7-dehydrocholesterol interacts with UVB light (Holick, 2018). These wavelengths are found in sunshine and also the light generated by UV lamps in sunbeds (which emit ultraviolet mostly in UVA spectrum but often generate 4 -10% of overall UV emissions as UVB). Because glass nearly totally filters UVB rays, there is insufficient light availability from windows. (Ray, 2005; Bolton, 2013). Adequate quantities of Vitamin D can be obtained by exposing the arms, legs, and face to the sun for 15 to 30 minutes twice per week, or around 25% of the typical sunburn duration. The lower level of sunshine and more exposure time is required for the darker skin.
The toxicity of Vitamin D from UV radiation is impossible since the skin reaches a point where the vitamin is degraded at the same rate as it is produced. (Holick, 2007; Holick, 2009; Holick, 2002). UV radiation is absorbed or reflected by sunscreen, preventing it from reaching the skin (Holick et al., 1987). Sunscreen with an SPF of 8 on the UVB spectrum diminishes Vitamin D’s synthetic potential by 95%, while SPF 15 reduces it by 98 percent. (Ross et al., 2011). The skin consists of two main layers: the inner layer called the dermis while outer layer is known as thinner epidermis, mainly composed of connective tissue (Yousef et al., 2021). The thick epidermis consists of five strata in the soles and palms; from outside to inside they are stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. In keratinocytes, the basal stratum and the spinosum stratum are the two innermost strata that synthesize vitamin D (Holick et al., 1987; Blumberg et al., 2016). Table 2 summarizes various biosensors that are used to determine vitamin D levels.
| Biosensor/Electrode/ Recognition element | Technique | References |
| Ab-25OHD/SPE/ FMTAD | SPR | Carlucci et al., 2013 |
| DPV | Chauhan et al., 2019 | |
| CYP27B1/GCE | CV | Ozbakir et al., 2015 |
|
SiO2/GO/Ni(OH)2/GCE |
DPV | Canevar et al., 2014 |
|
BSA/Ab-VD2/CD-CH/ITO |
DPV |
Sarkar et al., 2018 |
|
BSA/AntiVD/Fe3O4PANnFs/ITO |
DPV | Chauhan et al., 2018 |
|
BSA/Ab-VD/Asp-Gd2O3NRs/ITO |
DPV | Chauhan et al., 2019 |
| 25OHD Antibody | SPR | Carlucci et al., 2013 |
| 25OHD | SPR | |
| 25OHD Antibody | DPV | |
| IoT Enabled Enzyme Embossed Biosensor | DPV | Ghosh et al., 2021 |
| Au-Pt NPs/APTES/FTO | FE-SEM, FT-IR, XRD, XPS, CAM, CV, DPV, EIS. | Kaur et al., 2020 |
|
GCN-β-CD/Au nanocomposite |
EIS, CLIA |
Anusha et al., 2022 |
* DPV:Detection Using Differential Pulse Voltammetry, SPR:Surface Plasmon Resonance, CV:Cyclic Voltammetry, Anti-VD3:anti-VD3 1 A hydroxylase enzyme antibody, Au-Pt NPs/APTES/FTO: gold-platinum nanoparticles supported on 3-(aminopropyl)triethoxysilane modified fluorine tin oxide glass electrode, FE-SEM: field emission scanning electron microscope, FT-IR: Fourier Transform Infrared Spectroscopy/Analysis, XRD: X-ray Powder Diffraction, XPS: X-ray photoelectron spectroscopy, CAM:contact angle measurement, EIS:electrochemical impedance spectroscopy, GCN–β-CD@Au/GCE: graphitic carbon nitride hybridized with β-cyclodextrin, CLIA: Chemiluminescent immunoassays
Conclusions
Considering the significance of Vitamin D in health, it is vital to develop a sensitive, selective, quick, and simple technique for determining it. This review outlines some of the novel electrochemical biosensors used to determine Vitamin D in the presence of UV radiation and subsequent activation of pharmaceutical products, food, or human plasma, since there is a global need to be able to employ a quick approach to evaluate Vitamin D levels from a range of samples. The biosensors used for the electrochemical determination of Vitamin D were functionalized via various electrochemical materials based on carbon dots, glassy carbon, or carbon paste. Also, the option of electrochemical biosensor for Vitamin D determination should be affected by the form of specific samples used in the applications. For practical applications, highly selective and efficient analytical techniques that can span vast concentration ranges and fulfill low concentration limits for Vitamin D are required. The advantages of the electrochemical biosensors presented in this review, such as high sensitivity, selectivity, stability, and repeatability, allow them to be employed as prospective instruments for evaluating Vitamin D in medical, therapeutic, food, or other domains. The carbon-based electrochemical discussed in this study demonstrates a rapid electrochemical biosensor determination of Vitamin D on the functionalized electrochemical surface, as well as a fast kinetic process and high electrochemical biosensor activity. Furthermore, the design and production of the material utilized to detect Vitamin D is focused on lowering the fouling impact of the electrochemical surface, which might lead to biological application.
acknowledgements
The authors wish to thank the peer reviewers and South Asian Journal of Life Sciences EFSA staff for the support provided to improve and publishing this review.
conflict of interest
The authors have declared no conflict of interests.
novelty statement
In this study, some efficient electrochemical biosensors for Vitamin D detection has been reviewed.
authors contribution
Nargis Sardar designed the work and drafted the manuscript; AKM Rezwan Sardar and Fahamida Zaman collected the data; Arsalan Rasheed performed data analyses and co-write the manuscript; Sufian Rasheed and Maria Binte Sarfraz critically revised the manuscript for necessary actions. All the authors approved the final manuscript for publication.
REFERENCES
Anusha T, et al (2022). «Fabrication of electrochemical immunosensor based on GCN-β-CD/Au nanocomposite for the monitoring of vitamin D deficiency.» Bioelectrochemistry. 143: 107935. https://doi.org/10.1016/j.bioelechem.2021.107935
Banerjee P, Bhunia AK (2009). Mammalian cell-based biosensors for pathogens and toxins. Trends Biotechnol. 27: 179–188. https://doi.org/10.1016/j.tibtech.2008.11.006
Bikle DD (2014). Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. Mar 20;21(3):319-29 https://doi.org/10.1016/j.chembiol.2013.12.016.
Bolton J (2013). UV FAQs. International Ultraviolet Association. Archived from the original on 2013.
Blumberg JB, Frei B, Fulgoni III VL, Weaver CM, Zeisel SH (2016). Vitamin and mineral intake is inadequate for most Americans: what should we advise patients about supplements?. J. Family Pract. 65(9): S1-S1. https://doi.org/10.3390/nu9080849
Brown JE (2013). Nutrition through the life cycle. Cengage Learning, 5th Edition. Stanford; 2013. Pages 37: 257-508.
Canevari TC, FH Cincotto, R Landers, SA Machado (2014). Synthesis and characterization of α-nickel (II) hydroxide particles on organic-inorganic matrix and its application in a sensitive electrochemical sensor for Vitamin D determination. Electrochim Acta., 147: 688-695. https://doi.org/10.1016/j.electacta.2014.10.012
Carlucci L, G Favero, C Tortolini, M Di Fusco, E Romagnoli, S Minisola et al (2013). Several approaches for Vitamin D determination by surface plasmon resonance and electrochemical affinity biosensors. Biosens Bioelectron, 40 (1): 350-355. https://doi.org/10.1016/j.bios.2012.07.077
Chauhan D, PK Gupta, P.R. Solanki (2018). Electrochemical immunosensor based on magnetite nanoparticles incorporated electrospun polyacrylonitrile nanofibers for Vitamin D3 detection. Mater. Sci. Eng. C. 93: 145-156. https://doi.org/10.1016/j.msec.2018.07.036
Crissey SD, Ange KD, Jacobsen KL, Slifka KA, Bowen PE, Stacewicz-Sapuntzakis M, et al.. (2003). «Serum concentrations of lipids, Vitamin D metabolites, retinol, retinyl esters, tocopherols and selected carotenoids in twelve captive wild felid species at four zoos». J. Nutrit. 133 (1): 160–6. https://doi.org/10.1093/jn/133.1.160
Dahlquist DT, Dieter BP, Koehle MS (2015). «Plausible ergogenic effects of Vitamin D on athletic performance and recovery». IntSoc. Sports Nutr. 12: 33 (2015). https://doi.org/10.1186/s12970-015-0093-8
Deluca HF (2014). History of the discovery of Vitamin D and its active metabolites. BoneKEy Rep. 3: 479. https://doi.org/10.1038/bonekey.2013.213
EFSA staff (2018). Update of the tolerable upper intake level for vitamin D for infants. EFSA Journal 16(8). https://doi.org/10.2903/j.efsa.2018.5365
Eyley SC, Williams DH (1975). Photolytic production of Vitamin D. The preparative value of a photo-sensitiser. J. Chem. Society, Chem. Commun. (20): 858a. https://doi.org/10.1039/c3975000858a
FDA Cautions on Accurate Vitamin D Supplementation for Infants. Food and Drug Administration (FDA) (2017). https://www.medindia.net/health-press-release/FDA-Cautions-on-Accurate-Vitamin D-Supplementation-for-Infants-73270-1.htm.
Ghosh, M, Chiranjib K (2021). «An IoT Enabled Enzyme Embossed Biosensor for Determination of Vitamin D Level in Human Blood Sample.» Modern Techniques in Biosensors. Springer, Singapore, 95-109. https://doi.org/10.1007/978-981-15-9612-4_4
Holick MF, Kleiner-Bossaller A, Schnoes HK, Kasten PM, Boyle IT, DeLuca HF (1973). 1,24,25-TrihydroxyVitamin D3. A metabolite of Vitamin D3 effective on intestine. J. Biolog. Chem. 248 (19): 6691–6. https://doi.org/10.1016/S0021-9258(19)43408-X
Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL (1986). 24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process. J. Biolog. Chem. 261 (20): 9250–6. https://doi.org/10.1016/S0021-9258(18)67647-1
Holick MF, Smith E, Pincus S (December 1987). «Skin as the site of Vitamin D synthesis and target tissue for 1,25-dihydroxyVitamin D3. Use of calcitriol (1,25-dihydroxyVitamin D3) for treatment of psoriasis». Archiv. Dermatol. 123 (12): 1677–1683a. https://doi.org/10.1001/archderm.123.12.1677
Holick MF (2002). Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health». Current Opinion in Endocrinology, Diabet. Obesit. 9 (1): 87–98. https://doi.org/10.1097/00060793-200202000-00011
Holick MF (2002). Sunlight and Vitamin D: both good for cardiovascular health. J. Gen. Internal Med. 17 (9): 733–5. https://doi.org/10.1046/j.1525-1497.2002.20731.x
Holick MF (2007). Vitamin D deficiency. The New England J. Med. 357 (3): 266–81. https://doi.org/10.1056/NEJMra070553
Holick MF (1987). Photosynthesis of Vitamin D in the skin: effect of environmental and life-style variables. Federat. Proceed. 46 (5): 1876–82.
Holick MF (2004). Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. American J. Clin. Nutrit. 79 (3): 362–71. https://doi.org/10.1093/ajcn/79.3.362
Holick MF (2009). Vitamin D status: measurement, interpretation, and clinical application. Ann. epidemiol. 19(2): 73-78. https://doi.org/10.1016/j.annepidem.2007.12.001
Holick MF (2018). «Chapter 4: Photobiology of Vitamin D». In Feldman D, Wesley Pike J, Bouillon R, Giovannucci E, Goltzman D, Hewison M (eds.). Vitamin D: Volume 1: Biochemistry, Physiology and Diagnostics (4th ed.). London, UK: Academic Press. ISBN 978-0-12-809965-0.
Insel P, Ross D, Bernstein M, McMahon K (2015). Nutrition. Jones & Bartlett Publishers, Burlington, 2015.
IUPAC-IUB (1982). Joint Commission on Biochemical Nomenclature: Nomenclature of Vitamin D. Recommendations. European J. Biochem. 124 (2): 223–7.
Justin L. Kaplan, Robert S. Porter (2011). The Merck Manual of Diagnosis and Therapy, Wiley, 2011.
Karim N, Hoor T (2015). Vitamin D-Not Just a Simple Vitamin. The Journal of Bahria University Medical and Dental College Karachi, Pakistan. 5(3)143-145.
Kaur A, et al (2020). «Goldplatinum bimetallic nanoparticles coated 3-(aminopropyl) triethoxysilane (APTES) based electrochemical immunosensor for vitamin D estimation.» J. Electroanalyt. Chem. 873: 114400. https://doi.org/10.1016/j.jelechem.2020.114400
Keegan RJ, Lu Z, Bogusz JM, Williams JE, Holick MF (2013). Photobiology of Vitamin D in mushrooms and its bioavailability in humans». Dermato-Endocrinol. 5 (1): 165–76. https://doi.org/10.4161/derm.23321
Ozbakir HF, D. Sambade S. Majumdar L. Linday S (2015). Banta. Detection of 25-HydroxyVitamin D3 with an enzyme modified electrode. J Biosens Bioelectron, 7 (193): 2 https://doi.org/10.4172/2155-6210.1000193
Ray CC (2005). & HYPERLINK «https://www.nytimes.com/2005/05/17/science/17qna.html»A Sunshine Vitamin D. The New York Times. Archived from the original on 2005.
Ross AC, Taylor CL, Yaktine AL, Del Valle HB, Institute of Medicine (IoM). 8, Implications and Special Concerns, (2011). Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al (2011). J. Clin. Endocrinol. Metabol. 96 (1): 53–8.
Sarkar T, H. Bohidar, P.R. Solanki (2018). Carbon dots-modified chitosan based electrochemical biosensing platform for detection of Vitamin D. Int. J. Biol. Macromol., 109: 687-697. https://doi.org/10.1016/j.ijbiomac.2017.12.122
Shaima S, Iltaf S, Asma Al M (2019). A Narrative Role of Vitamin D and Its Receptor:With Current Evidence on the Gastric Tissues, Int. J. Mol. Sci. 20: 3832. https://doi.org/10.3390/ijms20153832
Tolerable Upper Intake Limits for Vitamins And Minerals. European Food Safety Authority. 2006.https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf
Trashin S, Pchelintsev N (2010). Electrochemical biosensors. Chem. Soc. Rev. 39: 1747–1763. https://doi.org/10.1039/b714449k
Vaja A, Parikh A, Patel G, Anand V, Chaudhari B, Patel A, Katariya S (2019). Vitamin D Deficiency And Its Effect on Severity of Asthma In Adult. Global Journal For Research Analysis (GJRA). 30;8(11).
Vieth R (1999). «Vitamin D supplementation, 25-hydroxyVitamin D concentrations, and safety». American J. Clin. Nutrit. 1999, 69(5): 842–56. https://doi.org/10.1093/ajcn/69.5.842
Yousef H, Alhajj M, Sharma S (2021). Anatomy, Skin (Integument), Epidermis. [Updated 2021 Jul 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470464/
To share on other social networks, click on any share button. What are these?