Evaluation of the Fusion Type CpG Adjuvant for the Enhancement of Somatostatin DNA Vaccine in Ram Lambs
Evaluation of the Fusion Type CpG Adjuvant for the Enhancement of Somatostatin DNA Vaccine in Ram Lambs
Yan-Guo Han1, Xiao-Li Peng1, Kai Li1, Yu-He-Tian Zhao1, Xun-Ping Jiang2, Guang-Xin E1, Yong-Ju Zhao1, Jun-Hua Ye3, Li Xu3, Qin-Tao Zhao3 and Yong-Fu Huang1,*
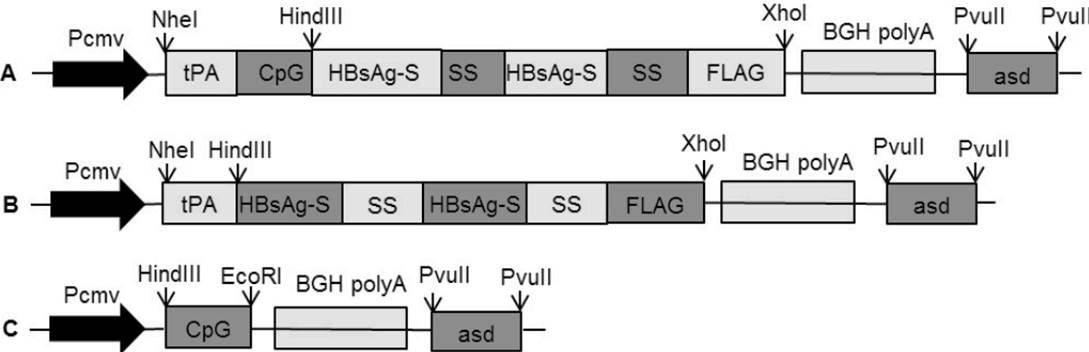
Map of construction of somatostatin (SS) DNA vaccine fused CpG motifs or simple co-injection with CpG: A, map of construction of SS DNA vaccine named ptCS/2SS-asd; B, map of construction of SS DNA vaccine named ptS/2SS-asd; C, map of construction of CpG plasmid adjuvant named pCpG-asd.
Identification of recombinant plasmids ptCS/2SS-asd, ptS/2SS-asd and pCpG-asd by PCR. Lanes 1, 2 and 3, ptCS/2SS-asd, ptS/2SS-asd and pCpG-asd recombinant plasmids were amplified with T7 and BGH primers; Lane M, DL5000 DNA marker. Three bands shown in Lanes 1, 2 and 3 are tPA-CpG-HBsAg-S-2SS-FLAG (1002 bp), tPA-HBsAg-S-2SS-FLAG (975 bp) and CpG (178 bp) fragment, respectively.
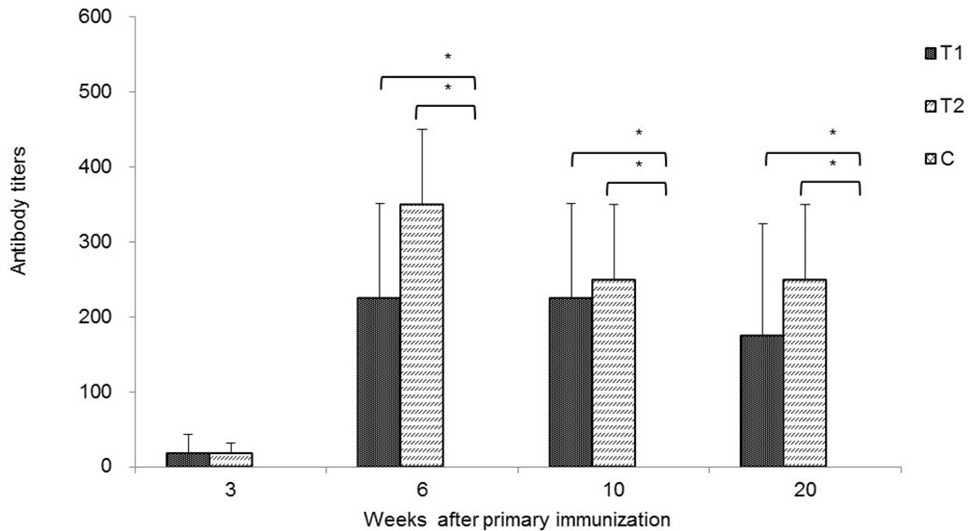
Anti-SS antibody titre in ram lambs. Lamb IgG antibody concentrations against SS were detected at weeks 3, 6, 10 and 20 after primary immunisation in treatment (Groups T1 and T2) and control (Group C) groups. Data are shown as means ± SD; *P < 0.05.
Serum GH concentrations (ng/mL) in Group T1, T2 and C immunised with ptCS/2SS-asd, ptS/2SS-asd + pCpG-asd and naked pVAX-asd, respectively, on weeks 0, 3, 6, 10 and 20 after primary immunisation. Data are shown as means ± SD. *P < 0.05.
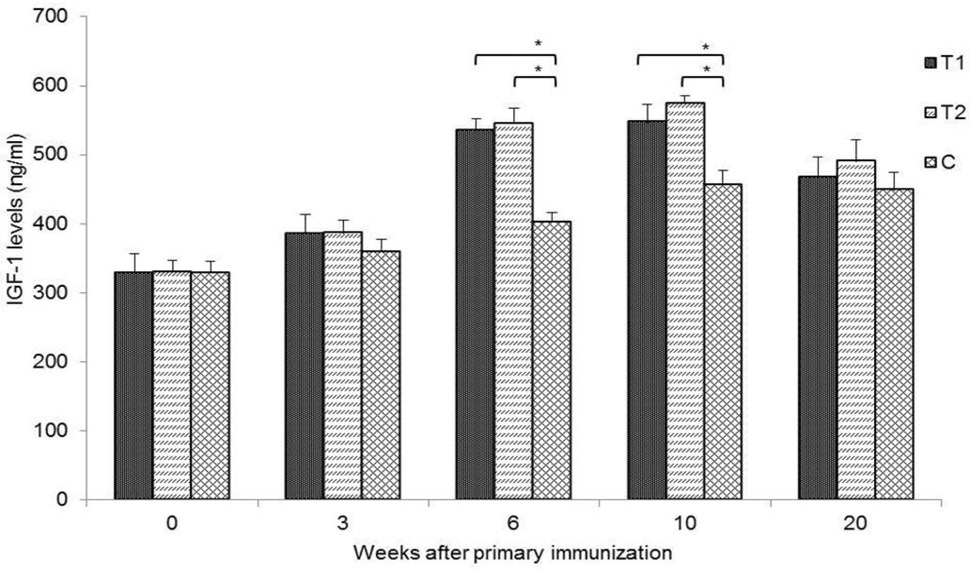
Serum IGF-1 concentrations (ng/mL) in Group T1, T2 and C immunised with ptCS/2SS-asd, ptS/2SS-asd + pCpG-asd and naked pVAX-asd, respectively, on weeks 0, 3, 6, 10 and 20 after primary immunisation. Data are shown as means ± SD; *P < 0.05.
Average body weight (kg) in Group T1, T2 and C immunised with ptCS/2SS-asd, ptS/2SS-asd + pCpG-asd and naked pVAX-asd, respectively, on weeks 0, 3, 6, 10 and 20 after primary immunisation. Data are shown as means ± SD; *P < 0.05.