Gastric Evacuation in Brook Trout (Salvelinus fontinalis) Fry: Effect of Body Size
Gastric Evacuation in Brook Trout (Salvelinus fontinalis) Fry: Effect of Body Size
Kadir Seyhan1,*, Nimet Selda Başçinar2, Nadir Başçinar3 and Umar Khan3
1Department of Maritime Business Administration, Faculty of Marine Science, Karadeniz Technical University, Trabzon, 61530, Turkey
2Central Fisheries Research Institute, Vali Adil Yazar Cad., No: 14 Şana 61250 Yomra, Trabzon, Turkey
3Department of Fisheries Technology Engineering, Faculty of Marine Science,
Karadeniz Technical University, Trabzon, 61530, Turkey
ABSTRACT
This study was aimed to determine the gastric evacuation (GE) in brook trout (Salvelinus fontinalis) fry and to estimate the effect of body mass on their GE rate (GER). A group of small and large sized S. fontinalis fry (ranging 0.39−0.66 and 0.76−1.46 g) was fed with commercial pellets under similar conditions to avoid the ingress of any other variable such as temperature and dietary energy density. Their stomach contents were sampled at predetermined postprandial times, and were dried at 60° C to constant weight. The course of GE in both sizes of S. fontinalis fry was best described by the square root model. The relationship between GER and body mass was then determined by a power function of fry mass that can be summarized by the equation (g h-1), where St is current stomach mass (g), M is fry mass (g), and t is time (h). The course of GE in S. fontinalis fry is similar to that previously reported for adult S. fontinalis.
Article Information
Received 30 August 2016
Revised 04 July 2018
Accepted 12 March 2019
Available online 13 March 2020
Authors’ Contribution
KS presented and planned the project and statistically analyzed the results. NB performed the experimental work with the help of NSB. UK wrote the manuscript.
Key words
Commercial pellets, Salmonidae, Square root model, Stomach emptying
DOI: https://dx.doi.org/10.17582/journal.pjz/20160830030220
* Corresponding author: [email protected]
0030-9923/2020/0003-1173 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
The relevance and importance of gastric evacuation (GE) experiments to quantify the daily rations of wild fish (Elliott and Persson, 1978; Bromley, 1994; dos Santos and Jobling, 1995; Seyhan and Grove, 1998; Andersen, 2001) as well as cultivated fish (Windell et al., 1972; Talbot and Higgins, 1983; Riche et al., 2004; Khan et al., 2016; Başçınar et al., 2016) are well recognized. Studying GE rates (GER) in fish will help to quantify their daily ration amount to avoid any overfeeding or underfeeding as both of these are dangerous to fish health and the economic feasibility of aquaculture systems: overfeeding causes the degradation of water quality (e.g. ammonia poisoning, low oxygen levels, low pH levels) and increases faecal production as well as the waste of expensive feed (Fateh et al., 2005), whereas underfeeding of fish can lead to poor growth of fish and sometimes even fish death (Jobling et al., 2012).
The GER is influenced by temperature (Jobling, 1981; Bromley, 1994), fish size (Nobel, 1973; Mills et al., 1984; Andersen, 1998), meal size and energy density of the diet (Grove et al., 1978; Jobling, 1987; Andersen, 2001). Most of GE experiments include adult fish and only a few focus young fish (Nobel, 1973; Silva and Owoyemi 1983; Röusch, 1987; Karjalainen et al., 1991; Bernreuther et al., 2009).
GE experiments have been carried out on adult brook trout (Salvelinus fontinalis) under various factors such as at different temperatures (Sweka et al., 2004; Başçınar et al., 2016), body and meal sizes (Khan et al., 2016; Bascinar et al., 2017). According to aforementioned studies the GE of S. fontinalis is best described by the square root model except Sweka et al. (2004) who chose a linear model over the square root though the square root model best fit their data obtain at 12.1 and 17.0°C.
In this study, the GE in S. fontinalis fry with reference to the effect of body size on GER was determined. The fry were fed with commercial pellets and their stomach contents were recovered by the discussed method at predetermined postprandial times.
Materials and methods
Two different sizes fry (ranging 0.39−0.66 and 0.76−1.46 g respectably) were procured from the Surmene Faculty of Marine Sciences, Trabzon (Table I). They were stocked in two separate aquaria of 10 L which were facilitated with recirculating water system where the oxygen saturation was ensured by means of continuous air-bubbling. The fish were fed four times daily with 800µ size commercial pellets (crude protein, 55%; crude fat, 12% obtained from Skretting Aquaculture, www.skretting.com.tr) to apparent satiation for a week prior to experiments. The same feed type was used during the GER experiments.
The fish fry were deprived of food for 72 h before starting the GE experiments. They were fed (group-feeding) to apparent satiation; the feeding period lasted for 15 minutes. The uneaten feed was collected by water-pipe. The stomach contents of fry were then sampled at predetermined postprandial times under Stereo microscope. The recovered stomach contents were dried in an Ecocell Drying Oven at 60°C to constant weight. Fish were killed using an overdose of anaesthesia (20 ppm benzocaine).
The simple regression method used by He and Wurtsbaugh (1993), Pääkkönen and Marjomäki (1997), Sweka et al. (2004) and Bascinar et al. (2016, 2017) was applied to the GE data of S. fontinalis fry. The best fit model was determined on the basis of adjusted r2 and the residual sum of squares (RSS) values.
Linear model ntegrated:
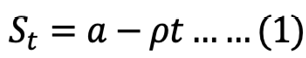
Square root model integrated:
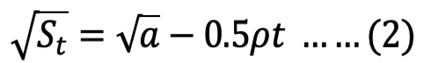
Exponential model integrated:
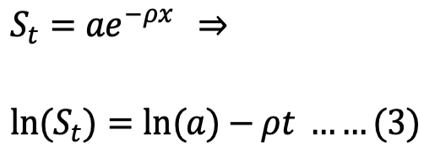
where St is the recovered stomach content mass (g) at time t (h), a is the intersection of the regression line with the y-axis that represents the mean ingested meal size S0, and r is the rate parameter (g h-1).
The parameters of equations 1, 2, and 3 were determined by PROC GLM procedure from SAS (SAS Institute Inc, 2015). After determining the model that best fit the GE data of S. fontinalis fry, the effect of body size on GER was then determined by a simple power function.
Results
The course of GE in each size S. fontinalis fry was best described by the square root model. The square root model consistently provided a higher value of adjusted r2 and the lowest value of RSS (Table II). The differences between square root and linear models were small compared to that of the exponential model.
Fry body mass (g) was used to quantify the effect of body size on GER. The rate parameters obtained by the square root model were plotted (y-axis) against the mass of fry (x-axis) and a curve line was provided using the simple power function. Hence, the relationship between body size and GER can be summarized as:

where St is current stomach mass (g), M is fry mass (g), and t is time (h).
Table I.- Basic experimental data (mean ± S.E.) from gastric evacuation experiments on brook trout Salvelinus fontinalis fry fed on commercial pellets.
|
Exp. no. |
Temperature (°C) |
Mass (g) |
Total length (cm) |
Meal size (g)* |
Obs. (n) |
|
1 |
17.3 |
0.49 ± 0.01 |
4.44 ± 0.03 |
0.003 ± 0.000 |
21 |
|
2 |
17.3 |
1.08 ± 0.03 |
5.39 ± 0.03 |
0.020 ± 0.000 |
24 |
*stomach contents recovered at time 0
Table II.- Estimates (mean ± S.E.) of the intercept and rate parameter ρ in the square root model, linear model and exponential model from gastric evacuation data of brook trout Salvelinus fontinalis fry fed meals of commercial pellets.
|
Square root model |
Linear model |
Exponential model |
||||||||||
|
Exp. no. |
a |
ρ (×10-2) |
RSS |
Adj. r2 |
a |
ρ (×10-2) |
RSS |
Adj. r2 |
a |
ρ (×10-2) |
RSS |
Adj. r2 |
|
1 |
0.53 ± 0.01 |
-1.32 ± 0.04 |
0.00 |
0.923 |
0.03 ± 0.00 |
-46.00 ± 0.30 |
1.27 |
0.924 |
-57.55 ± 1.46 |
-42.39 ± 0.01 |
3.02 |
0.812 |
|
2 |
1.38 ± 0.04 |
-3.30 ± 0.14 |
0.00 |
0.766 |
0.18 ± 0.00 |
-0.31 ± 0.00 |
0.00 |
0.755 |
-38.67 ± 1.02 |
-36.75 ± 0.01 |
4.43 |
0.730 |
This equation, obtained from combined GE data of both fry sizes was used to provide the GE curves in Figure 1.
Discussion
The square root model adequately described the course of GE in both sized S. fontinalis fry which is in accordance with the results obtained from adult S. fontinalis in previous studies (Khan et al., 2016; Başçınar et al., 2016, 2017). Khan et al. (2016) fed adult S. fontinalis with a range of meal sizes (100%, 50% and 25% of satiation meal size) and found the square root model to adequately describe the GE of S. fontinalis independent of meal size. The square root model also described the GE of vendace (Coregonus albula) L. fry fed with live zooplankters: copepod nauplii and copepodids (Karjalainen et al., 1991). Whereas, the course of GE in sprat (Sprattus sprattus) fry was reported to be best described by so called surface-area dependent model (Bernreuther et al., 2009).
In this study, the effect of body size on GER of S. fontinalis was quantified using fry mass instead of length. While for adult S. fontinalis Khan et al. (2016), and Başçınar et al. (2017) used the fish total length (though they also estimated the mass exponent) to quantify the effect of body size on the GER of adult S. fontinalis in accordance with Andersen (2001). However, in the present study using S. fontinalis fry length to quantify the effect of body size on GER gave an unrealistic length exponent value (4.79). In contrast to fry length, using fry mass gives a realistic value for the mass exponent (1.31) and the summarized equation (4) adequately provided a good curve to the data (Fig. 1). Bernreuther et al. (2009) obtained a mass exponent value of 0.503 for sprat fry.
Conclusion
The result of this study, together with the result of Khan et al. (2016), suggested that the GE of S. fontinalis fed commercial pellets can be adequately described by the square root model. The square root model can therefore from a limited number of growth experiments be used to estimate the stomach fullness at return of appetite as well as the fullness that provides optimum food conversion efficiency and maximum growth rate. This information can then be extrapolated to other situations (temperature and energy density of feed), and this way reduce the number of time consuming and expensive growth experiments.
Acknowledgement
This study was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK) under Grant No. 113O362.
Statement of conflict of interest
Authors have declares that there is no conflict.
References
Andersen, N.G., 1998. J. Fish. Biol., 52: 743-755. https://doi.org/10.1006/jfbi.1997.0617
Andersen, N.G., 2001. J. Fish. Biol., 59: 1198-1217. https://doi.org/10.1006/jfbi.2001.1731
Başçınar, N., Başçınar, S.N., Seyhan, K. and Khan, U., 2016. Pakistan J. Zool., 48: 1899-1904.
Başçinar, N.S., Başçinar, N., Khan, U. and Seyhan, K., 2017. Indian J. Fish., 64: 50-54. https://doi.org/10.21077/ijf.2017.64.3.59243-08
Bernreuther, M., Temming, A. and Herrmann, J.P., 2009. J. Fish Biol., 75: 1525-1541. https://doi.org/10.1111/j.1095-8649.2009.02353.x
Bromley, P.J., 1994. Rev. Fish Biol. Fisher., 4: 36-66. https://doi.org/10.1007/BF00043260
dos Santos, J. and Jobling, M., 1995. ICES J. Mar. Sci., 52: 209-219. https://doi.org/10.1016/1054-3139(95)80036-0
Elliott, J. and Persson, L., 1978. J. Anim. Ecol., 977-991. https://doi.org/10.2307/3682
Fateh, D., Minehira, K., Schwarz, J.M., Periasamy, R., Park, S. and Tappy, L., 2005. Diabetes, 54: 1907-1913. https://doi.org/10.2337/diabetes.54.7.1907
Grove, D., Loizides, L. and Nott, J., 1978. J. Fish Biol., 12: 507-516 https://doi.org/10.1111/j.1095-8649.1978.tb04195.x.
He, E. and Wurtsbaugh, W.A., 1993. Trans. Am. Fish Soc., 122: 717-730. https://doi.org/10.1577/1548-8659(1993)122<0717:AEMOGE>2.3.CO;2
Jobling, M., 1981. J. Fish Biol., 19: 29-36. https://doi.org/10.1111/j.1095-8649.1981.tb05808.x
Jobling, M., 1987. J. Fish Biol., 30: 299-314. https://doi.org/10.1111/j.1095-8649.1987.tb05754.x
Jobling, M., Alanärä, A., Noble, C., Sánchez-Vázquez, J., Kadri, S. and Huntingford, F., 2012. Aquacul. Behav., 7: 183-219. https://doi.org/10.1002/9781444354614.ch7
Karjalainen, J., Koho, J. and Viljanen, M., 1991. Aquaculture, 96: 343-351. https://doi.org/10.1016/0044-8486(91)90163-2
Khan, U., Seyhan, K., Başçınar, N. and Başçınar, S,N., 2016. J. Fish Biol. 89: 1227–1238. https://doi.org/10.1111/jfb.13021
Mills, E.L., Ready, R.C., Jahncke, M., Hanger, C.R. and Trowbridge, C., 1984. Can. J. Fish. aquat. Sci., 41: 513-518. https://doi.org/10.1139/f84-061
Nobel, R., 1973. Trans. Am. Fish. Soc., 102: 759-763. https://doi.org/10.1577/1548-8659(1973)102<759:EROYYP>2.0.CO;2
Pääkkönen, J.P. and Marjomäki, T.J., 1997. J. Fish Biol., 50: 555-563. https://doi.org/10.1111/j.1095-8649.1997.tb01949.x
Riche, M., Haley, D., Oetker, M., Garbrecht, S. and Garling, D., 2004. Aquaculture, 234: 657-673. https://doi.org/10.1016/j.aquaculture.2003.12.012
Röusch, R., 1987. J. Fish Biol., 30: 521-531. https://doi.org/10.1111/j.1095-8649.1987.tb05779.x
SAS Institute Inc, 2015. SAS Institute Inc SAS/IML® 14.1 User’s Guide, SAS Institute Inc, Cary, NC.
Seyhan, K. and Grove, D., 1998. Fish Res., 38: 233-245. https://doi.org/10.1016/S0165-7836(98)00165-9
Silva, S. and Owoyemi, A., 1983. J. Fish Biol., 23: 347-355. https://doi.org/10.1111/j.1095-8649.1983.tb02914.x
Sweka, J.A., Keith Cox, M. and Hartman, K.J., 2004. Trans. Am. Fish. Soc., 133: 204-210. https://doi.org/10.1577/T02-064
Talbot, C. and Higgins, P., 1983. J. Fish Biol., 23: 211-220. https://doi.org/10.1111/j.1095-8649.1983.tb02896.x
Windell, J., Hubbard, J. and Horak, D., 1972. Progr. Fish-Cult., 34: 156-159. https://doi.org/10.1577/1548-8640(1972)34[156:ROGEIR]2.0.CO;2
To share on other social networks, click on any share button. What are these?










