Implications of Saline Water Irrigation for Linseed on Seed Germination, Seedling Survival and Growth Potential
Implications of Saline Water Irrigation for Linseed on Seed Germination, Seedling Survival and Growth Potential
Muhammad Abdul Qayyum1*, Farhat Bashir2, Muhammad Mudassar Maqbool3, Anser Ali3, Saqib Bashir1 and Qaiser Abbas4
1Department of Soil and Environmental Sciences, Faculty of Agricultural Sciences, Ghazi University, Dera Ghazi Khan, Pakistan; 2Soil and water testing laboratory, Dera Ghazi Khan, Pakistan; 3Department of Agronomy, Faculty of Agricultural Sciences, Ghazi University, Dera Ghazi Khan, Pakistan; 4Department of Economics, Ghazi University, Dera Ghazi Khan, Pakistan.
Abstract | Saline water irrigation is considered a severe threat for plants seed germination because it has highest concentration of soluble salts. This study aims to examine the effect of NaCl salt at 100 mM and 200 mM concentrations on linseed (Linum usitatissimum L.) germination, plant growth, nutrients and soluble salts uptake. The results revealed that tested four linseed genotypes showed an overall germination rate of 86-94% at 100 mM NaCl (T2) and 78-84% at 200 mM NaCl (T3) and seedling survival rate of 80-90% at T2 and 40-60% at T3. Seedling survival was related to seedling vigor and tolerance to salinity, as salt tolerant genotypes with healthy seedlings showed better survival and growth rate under salt stress conditions. The minimum relative growth rate was recorded as 68% to respective control in S-907 genotype at 100 mM NaCl while it was 38% of respective control in C-99-3-115 genotype at 200 mM NaCl stress conditions. In linseed growth, K+/Na+ratio proved to be a critical factor in relative growth rate and biomass production while the ability of linseed genotypes to accumulate Na+ at root level and to restrict the entry of this ion to upper parts of the plant seemed to be one of the distinct features of salt tolerant genotypes.Salt tolerant genotypes of linseed expressed a genetic vigour to combat salinity stress by restricting Na+ entry at root level and by using this trait through biotechnology, plant breeders can produce salt tolerant crops.
Received | March 26, 2019; Accepted | October 31, 2019; Published | November 26, 2019
*Correspondence | Muhammad Abdul Qayyum, Department of Soil and Environmental Sciences, Faculty of Agricultural Sciences, Ghazi University, Dera Ghazi Khan, Pakistan; Email: [email protected]
Citation | Qayyum, M.A., F. Bashir, M.M. Maqbool, A. Ali, S. Bashir and Q. Abbas. 2019. Implications of saline water irrigation for linseed on seed germination, seedling survival and growth potential. Sarhad Journal of Agriculture, 35(4): 1289-1297.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.4.1289.1297
Keywords | Relative growth rate, K+:Na+ ratio, Salt tolerance, Genotypes
Introduction
Germination and seedling growth is crucial for initial crop stand under stress conditions. Selection of suitable cultivars showing quick and identical germination under salt stress can add to uniform seedling establishment. Soil salinity and brackish water irrigation imposes severe hazard for crop production especially reducing economic growth in areas of arid and semiarid climate around the globe. Generally, seed germination, seedling growth and seedling vigour are considered very crucial traits for crop productivity in salt stressed soils (Ratnakar and Rai, 2013).
The manifestation of high contents of soluble salts in growth media significantly reduces germination rate and ultimately germination percentage of seeds is declined (Wu et al., 2015; Muhammad and Hussain, 2010b). Several studies indicates that seeds of many species which express maximum germination in salt free water, show high sensitivity for salt stress during germination and seedling growth (Al-Taisan, 2010; Berrichi et al., 2010; Keshavarzi et al., 2011; El-Naim et al., 2012; Kandil et al., 2012; Moosavi et al., 2013; Sikha et al., 2013). The injurious impacts of salts occur on seed germination because of osmotic stress and toxicity of specific ion (Munns et al., 2006; Sayar et al., 2010; Batool et al., 2014). Seed germination is negatively affected under salt stress due to lack of fresh water availability, reduction in stored reserves mobility and alterations in structural organization of seed proteins (Machado et al., 2004; James et al., 2006; Sayar et al., 2010). Seeds need ample water to imbibe and germinate but under the saline conditions the excessive accumulation of salts in the seeds causes the osmotic stress by decreasing the availability of water for imbibitions and germination. Grain shriveling occurs due to water stress (Kazmi et al., 2003). The interactive effect of specific ion and osmotic stress causes a decline in the number of germinated seeds as well as cause the retardation in the rate of germination.
Linseed (Botanical name; Linum usitatissimum L.) commonly known as flax, is an economically important dual purpose (fiber and oil) medicinal crop. It has great adaptability and product diversity that provides fiber (flax) from stem and oil from seed (linseed). It is widely grown as a minor crop for different types of products all over the world (Ebtihal et al., 2012). During last few decades, several studies have been conducted in Australia, North America, Europe and Asia to get various linseed products. Linseed has significant variations for salinity tolerance but in Pakistan, little work is done on native linseed genotypes in Pakistan and contradictory claims regarding salt tolerance in linseed are reported. For instance, Ashraf and Fatima (1994) reported that extent of salt stress tolerance of linseed remains same with growth stage and Na+ inclusion in the shoot is the functional trait of salt stress tolerance in the crop. Similarly, Muhammad and Hussain (2010) evaluated the physiological responses of some medicinal plants including linseed and concluded that linseed was moderately tolerant in salinity tolerance and can be grown on saline soils to obtain some biomass. These contradictory reports convinced us to conduct comprehensive study of linseed regarding its various aspects from germination to maturity under saline conditions. Thus after screening of native linseed germ plasm (Qayyum et al., 2015), current experiments were designed to evaluate the response of selected linseed genotypes (S-907, C-99-3-115, 637-72 and NO-303) for NaCl stress at germination and seedling stage.
Materials and Methods
Seed collection and germination
A seed germination trial was conducted in laboratory conditions at Saline Agriculture Research Centre, Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan. Healthy seeds of four linseed genotypes namely: S-907, C-99-3-115 (salt sensitive) and 637-72, NO-303 (salt tolerant) were sown in trays containing pure sand. Four replicates of 50 seeds were germinated in covered, sterilized filter paper moistened with distilled water (control, T1), 100 mM NaCl (T2) and 200 mM of NaCl (T3) solutions. Petri dishes were sealed with para film to prevent evaporation and minimize the changes in the concentration of solutions. Seeds were placed in a growth chamber at 20 ±1 ºC. Seeds with emerged radicals were taken as germinated. Counting of germinated seeds was done on each day until the last 10th day of germination period. Seeds with germinated radicals were discarded from petri dishes and moisture was maintained by adding 5 ml prepared salt solutions after every three days period.
Nursery raising
The aqua culture experiment was conducted in the rain protected wire house of Saline Agriculture Research Centre (SARC), University of Agriculture, Faisalabad, Pakistan during September 2012. Healthy seeds of four linseed genotypes namely: S-907, C-99-3-115 (salt sensitive) and 637-72, NO-303 (salt tolerant) selected from the previous experiment (Qayyum et al., 2015), were sown in separate sand filled trays. Trays were sprinkled daily with good quality water to maintain optimum moisture for seed germination.
Seedling transplanting
Nine iron tubs of 200L capacity were filled with distilled water and half strength Hoagland’s solution was added in each tub. Seedlings at four leaf stage were uprooted from sand trays, wrapped with foam at root shoot junction and shifted in holes on thermo pore sheets (sheets of water insulating material used in hydroponic experiment to transplant seedlings of test crop, bought from Pakistan scientific store, Faisalabad) which were placed on 200 L volume iron tub containing half strength Hoagland’s solution (Hoagland and Arnon, 1950). Plant’s roots were aerated by providing air to the nutrient solution for 8 hours a day by air pump and the solution was changed twice a week during four weeks duration of trial. The factorial trial was arranged with complete randomized design using three replications.
Treatment application
After one week of transplanting, NaCl solution was prepared with control (T1),100 mM NaCl (T2) and 200 mM NaCl (T3). The control i.e., tub containing half strength Hoagland’s nutrient solution without added salts, had an EC of 1.17 dS m-1. The required level of treatments in each tub was developed by applying NaCl salts in three increments while control was kept without salt. The low level of stress (100 mM NaCl) and high level of stress (200 mM NaCl) were maintained throughout the duration of experiment. The pH of growth medium was kept to 6.5±0.5 during the growing period with 1 M NaOH or HCl as required.
Measurements of plant growth and biomass
After four weeks of salt exposure, , plans were harvested and washed thoroughly with distilled water. Plant growth was measured in terms of measuring fresh and dry weights of roots and stems of linseed genotypes. Fresh weight was taken immediately after harvesting of plants, however, for the determination of dry weight, plants were parted into roots, stems and leaves and dried at 65 ± 5oC for 48 hours to constant weight in a forced air driven oven (Jones and Case, 1990).
Relative growth rate (RGR)
For the determination of relative growth rate (RGR, g-1 d-1), dry weight of two consecutive harvests was used. Three plants were harvested from each tub after two weeks interval. Relative growth rate was determined by the following formula:
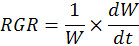
Where; W= plant dry weight at first harvest; dW= difference in plants dry weightat two harvests and dt= time interval between two consecutive harvests.
Determination of Na+ and K+ contents
After 30 days of salt exposure, three plants from each treatment were harvested, thoroughly washed with salt free distilled water and dried with blotting paper. At harvesting, roots, stem and leaves were parted and oven dried at 65±2 oC till constant weight to determine Na+ and K+. For the analysis of Na+ and K+, 50 mg of well ground dried portion of root, stem and leaves was digested separately in 10 mL of di-acids (HNO3:HClO4) mixture. After filtration with Whatman No. 1 filter paper, filtrate was used for Na+ and K+ determination through flame photometer (Jenway PFP7) (Jones and Case, 1990).
Statistical analysis
Data taken in the study comprises of three replicates. Analysis of variance (ANOVA) was executed through a statistical package, Statistix 8.1. Significance among treatment means was evaluated at the P ≤ 0.05 levels. (Steel et al., 1997).
Results and Discussion
Seed germination and survival
Lab experiment regarding the germination of linseed genotypes revealed that an increase in NaCl concentration adversely affected seed germination and seedling survival percentage of four linseed genotypes. It was noticed that seed germination was 100% under non-saline conditions (control) but at 200 mM NaCl seed germination reduced by84% in salt tolerant genotypes (673-72 and NO-303) while in salt sensitive genotypes, this percentage was even less (78% in S-907 and 80% in C-99-3-115) (Table 1). These findings clearly indicated that seed germination of linseed was inhibited by the manifestation of Na salts in the growing medium. The results also demonstrated that the genotypic variation in seed germination occurred in all linseed genotypes under salinity stress. The genotype NO-303 achieved the maximum germination percentages at all levels of salt stress. The decline in seed germination under salt stress might be either due to the decreased rate of water uptake by the seed coat (osmotic shock) and/or alterations of enzymatic or hormonal activities by certain toxic ion accumulation (Sayar et al., 2010; Batool et al., 2014; Wu et al., 2015). Salt sensitive genotypes were affected more by imposing salt stress in terms of reduction in seed germination when compared with salt tolerant genotypes. Guo et al. (2012) reported the profound reduction in linseed germination under salt stress where absolute growth relative to control was taken as basis of salt tolerance under given salt stress level.. Comparable findings were noted by Khan et al. (2017) in wheat where seed germination decreased with increasing salinity. Seedling survival of all the genotypes was also considerably influenced by NaCl and reduced by50% in salt tolerant genotypes (673-72 and NO-303) and by 40% in salt sensitive genotypes (S-907 and C-99-3-115) (Table 1). This reduction in survival of linseed seedling was highly correlated to germination percentage of seeds (Figure 1). The variation in seedling growth and survival might also be due to seed reserves as greater seed weight was stemmed in vigorous seedling growth as speculated by Kaya et al. (2008) in chickpea and Kaya and Day (2008) in sunflower.
Relative growth rate and biomass production
To make more reliable and applicable comparisons of plant growth among genotypes under salt stress, relative growth rate is taken as a very critical parameter than absolute growth. The findings of our work clearly showed the response of linseed genotypes exposed to increasing NaCl concentrations as RGR and shoot fresh weight reduced significantly. This reduction in RGR and root and shoot biomass could be owing to toxicity of specific ions or declined osmotic potential in addition to reduced extensibility of cell wall (Zorb et al., 2015; Feng et al., 2016). There are several reports on osmotic stress and ion toxicity resulted from salt stress in linseed (El-Beltagi et al., 2008; Muhammad and Hussain, 2010; Kaya et al., 2012). Relative growth rate (RGR) of salt sensitive genotypes was severely reduced by the application of salinity and it reduced to 38% of respective control at 200 mM NaCl (Table 2). This reduction in RGR might reduce the root and shoot biomass production in linseed genotypes at higher levels of salinity and severe reduction in root biomass was noted as compared to shoot biomass at higher level of salinity especially in salt sensitive genotypes (Table 3). These outcomes are identical to the results of Ashraf and Fatima (1994) and Khan et al. (2007) who reported the same results in linseed. Similarly, declined RGR and plant root and shoot biomass during salt stress was observed in several other species (Bakht et al., 2006; Turan et al., 2009; Kaya et al.,2012; Rab et al., 2017; Zaman et al., 2015; Ali et al., 2019; Shafique et al., 2019). This decline in growth is triggered by salt induced drought stress. Drought reduces the metabolic activity of plants, leading to the stunted growth and development (Maqbool et al., 2015; Jatoi et al., 2011).
Sodium and potassium percentage in roots, stems and leaves of linseed
Salt tolerant genotypes had high potential to check Na+ entry to the upper parts of plant and bound more Na+ in their roots in comparison of salt sensitive genotypes (Table 4, Figure 2). This potential indicates the availability of more carriers leading to faster ion uptake in tolerant genotypes (Almeida et al., 2017; Gupta and Huang, 2014). Thus salt tolerant genotypes had low Na+ accumulation in stems as well as leaves. In addition, tolerant genotypes proved themselves as the high accumulator of K+ in stems (Table 5, 6). Root Na+ and K+ contents did not show significant interaction with dry matter production. Interaction of stem and leaf Na+ contents with stem dry weight was less than that of stem and leaf K+ contents.
Potassium to sodium ratio in roots, stems and leaves of linseed
K+/Na+ratio of root, stem and leaves showed even better interaction with shoot dry weight of linseed genotypes and proved important criteria for salt stress tolerance. Increased salinity reduced K+/Na+ ratio in salt sensitive genotypes while salt tolerant genotypes possessed higher K+/Na+ ratio in roots, stems and leaves. Stem K+/Na+ ratio of linseed genotypes was more than that of roots and leaves (Table 7, Figure 3). Wu et al. (2015) reported the same results in sunflower while Kaya et al. (2012) also observed that salt tolerant genotypes of linseed possessed lesser Na+/ K+
Table 1: Effect of different levels of NaCl (mM) on germination and survival of linseed genotypes.
| Genotypes | Germination (%). | Survival (%) | ||||
| Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | |
| S-907 | 100±1.73 | 86 ±2.03 | 78±1.45 | 100±1.15 | 80 ±2.31 | 40 ±1.53 |
| C-99-3-115 | 100±2.31 | 88 ±2.65 | 80 ±2.89 | 100 ±1.73 | 90±2.52 | 60±2.08 |
| 637-72 | 100±2.31 | 93 ±2.08 | 84±1.86 | 100 ±1.73 | 90 ±1.15 | 60 ±2.89 |
| NO-303 | 100±2.89 | 94 ±2.65 | 84±3.06 | 100 ±3.21 | 80±2.31 | 50 ±3.46 |
Each value is an average of 3 replications ± SE.
Table 2: Relative growth rate, stem fresh and dry weight of linseed genotypes at different salinity levels.
| Genotypes |
RGR (g g-1 d-1) |
SFW (g plant-1) |
SDW (g plant-1) |
||||||
| Control | NaCl (100 mM) |
NaCl (200 mM) |
Control | NaCl (100 mM) |
NaCl (200 mM) | Control | NaCl (100 mM) |
NaCl (200 mM) |
|
| S-907 | 0.80±0.15 | 0.55± 0.13 (68%) |
0.34± 0.11 (42%) |
8.27±0.32 | 5.07 ± 0.28 (61%) |
3.43 ±0.24 (41%) | 0.97± 0.18 |
0.54± 0.12 (56%) |
0.29± 0.13 (30%) |
| C-99-3-115 | 0.84±0.14 | 0.59± 0.13 (70%) |
0.32± 0.09 (38%) |
7.87±0.27 | 4.80 ± 0.42 (61%) |
2.90 ±0.21 (37%) | 0.92± 0.17 |
0.51± 0.11 (55%) |
0.27± 0.07 (29%) |
| 637-72 | 0.90±0.16 | 0.73± 0.13 (81%) |
0.50± 0.10 (55%) |
13.30±0.34 | 11.20± 0.25 (84%) |
6.55 ±0.28 (49%) | 1.11± 0.12 |
1.03± 0.13 (92%) |
0.51± 0.10 (46%) |
| NO-303 | 0.94±0.18 | 0.80± 0.16 (86%) |
0.50± 0.13 (53%) |
11.61±0.26 | 10.21± 0.18 (88%) |
5.02 ±0.48 (43%) | 1.05± 0.16 |
0.85± 0.10 (81%) |
0.33± 0.13 (32%) |
Each value is an average of 3 replications ± SEand values in parenthesis are the percent (%) of their respective control.
Table 3: Root fresh and dry weight of linseed genotypes at different salinity levels.
| Genotypes |
RFW (g plant-1) |
RDW (g plant-1) |
||||
| Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | |
| S-907 | 9.77±0.17 | 4.18±0.13 (43%) | 3.07±0.35 (31%) | 0.83±0.19 | 0.29±0.09 (35%) | 0.19±0.08 (23%) |
| C-99-3-115 | 9.03±0.10 | 4.60±0.29 (51%) | 4.03±0.15 (45%) | 0.67±0.13 | 0.32±0.11 (48%) | 0.23±0.12 (35%) |
| 637-72 | 9.59±0.20 | 7.42±0.10 (77%) | 5.54±0.20 (58%) | 0.74±0.15 | 0.50±0.13 (67%) | 0.37±0.10 (51%) |
| NO-303 | 10.51±0.25 | 10.03±0.26 (95%) | 9.39±0.21 (89%) | 1.28±0.10 | 1.06±0.15 (83%) | 0.71±0.13 (55%) |
Each value is an average of 3 replications ± SE and values in parenthesis are the percent of their respective control.
Table 4: Effect of different levels of NaCl (mM) on Na+ and K+ contents in roots of salt sensitive (S-907 and C-99-3-115) and salt tolerant (637-72 and NO-303) genotypes of linseed.
| Genotypes |
Na+ (%) |
K+ (%) |
||||
| Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | |
| S-907 | 1.45±0.10 | 5.04±0.07 | 7.99±0.02 | 2.86±0.06 | 1.30±0.03 | 0.45 ±0.05 |
| C-99-3-115 | 1.43±0.05 | 5.62±0.09 | 10.11±0.06 | 2.88±0.07 | 1.69 ±0.05 | 0.55 ±0.08 |
| 637-72 | 1.36±0.07 | 7.06±0.09 | 12.63±0.02 | 3.14±0.03 | 2.03±0.08 | 1.19 ±0.03 |
| NO-303 | 1.38±0.05 | 5.75±0.08 | 10.31±0.06 | 3.16±0.07 | 2.26± 0.08 | 2.46 ±0.05 |
Each value is an average of 3 replications ± SE.
Table 5: Effect of different levels of NaCl (mM) on Na+ and K+ contents in stems of salt sensitive (S-907 and C-99-3-115) and salt tolerant (637-72 and NO-303) genotypes of linseed.
| Genotypes |
Na+ (%) |
K+ (%) |
||||
| Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | |
| S-907 | 0.51±0.02 | 3.13±0.04 | 4.12±0.08 | 1.64±0.03 | 1.17±0.09 | 0.49 ±0.06 |
| C-99-3-115 | 0.46±0.03 | 3.80±0.07 | 4.51±0.06 | 1.65±0.05 | 1.06 ±0.07 | 0.88 ±0.06 |
| 637-72 | 0.45±0.03 | 2.97±0.01 | 3.74±0.06 | 2.01±0.07 | 1.71±0.02 | 2.57 ±0.08 |
| NO-303 | 0.44±0.02 | 3.09±0.03 | 3.35±0.03 | 2.03±0.09 | 1.53± 0.05 | 2.23 ±0.02 |
Each value is an average of 3 replications ± SE.
Table 6: Effect of different levels of NaCl (mM) on Na+ and K+ contents in leaves of salt sensitive (S-907 and C-99-3-115) and salt tolerant (637-72 and NO-303) genotypes of linseed.
| Genotypes |
Na+ (%) |
K+ (%) |
||||
| Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | |
| S-907 | 0.14± 0.03 | 3.55±0.05 | 4.51±0.03 | 1.80 ± 0.06 | 0.76±0.03 | 0.40 ±0.05 |
| C-99-3-115 | 0.18± 0.03 | 3.42±0.03 | 4.51±0.02 | 1.81±0.05 | 0.68 ±0.02 | 0.21 ±0.03 |
| 637-72 | 0.22± 0.09 | 3.37±0.04 | 4.51±0.01 | 1.87±0.02 | 1.67±0.04 | 1.89 ±0.06 |
| NO-303 | 0.14± 0.06 | 3.44±0.05 | 3.34±0.02 | 1.88±0.03 | 1.68± 0.01 | 2.00 ±0.03 |
Each value is an average of 3 replications ± SE.
Table 7: Effect of different levels of NaCl (mM) on K+: Na+ ratios in roots, stems and leaves of salt sensitive (S-907 and C-99-3-115) and salt tolerant (637-72 and NO-303) genotypes of linseed.
| Genotypes |
K+:Na+ ratio in roots |
K+:Na+ ratio in stems |
K+:Na+ ratio in leaves |
||||||
| Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | Control | NaCl (100 mM) | NaCl (200 mM) | |
| S-907 | 1.98± 0.11 | 0.26±0.04 | 0.06±0.01 | 3.23± 0.19 | 0.38±0.03 | 0.12±0.01 | 15.07± 4.48 | 0.21±0.04 | 0.09±0.01 |
| C-99-3-115 | 2.02± 0.12 | 0.30±0.05 | 0.05±0.01 | 3.62± 0.17 | 0.28±0.04 | 0.19±0.02 | 10.72± 1.59 | 0.20±0.03 | 0.05±0.01 |
| 637-72 | 2.32± 0.09 | 0.29±0.02 | 0.10±0.01 | 4.51± 0.17 | 0.57±0.02 | 0.69±0.05 | 11.51± 3.66 | 0.50±0.06 | 0.42±0.01 |
| NO-303 | 2.30± 0.08 | 0.39±0.02 | 0.24±0.01 | 4.62± 0.02 | 0.49±0.05 | 0.67±0.03 | 18.55± 6.88 | 0.49±0.05 | 0.60±0.01 |
Each value is an average of 3 replications ± SE.
ratio when compared with their sensitive competitors. Our results are in contradiction with that of Ashraf and Fatima (1994) who found that high K+/Na+ ratio was the characteristics of salt sensitive accessions of linseed. The healthier growth of salt tolerant genotypes might have provided adequate energy for active uptake of K+ and for active removal of Na+ at root level across the plasma membrane and tonoplast.
Conclusions and Recommendations
In conclusion, the findings of conducted experimentrevealed thatsalt tolerant genotypes of linseed showed a relatively good seed germination, seedling survival ion selectivity and high interaction of ionic parameters especially K+/Na+ ratio in stems and leaves and can be used as useful criteria of salt tolerance in linseed genotypes. The ability of genotypes to accumulate Na+ at root levels and to restrict the entry of this ion to upper parts of the plant seems to be one of the distinct features of salt tolerant linseed genotypes. This trait can be manipulated by plant breeders for developing salt tolerant varieties of linseed which can be grown on saline soils.
Acknowledgments
The authors acknowledge the financial support provided by Higher Education Commission (HEC) of Pakistan to conduct this project under 5000 Indigenous PhD fellowship program. Authors extend thanks to the reviewers for improving the manuscript.
Novelty Statement
This research indicates the potential of salt tolerance in native linseed genotypes and potential sodium accumulation in root, stem and leaves for conferring salt tolerance during salt stress conditions.
Author’s Contributions
Dr. Muhammad Abdul Qayyum designed and executed the experiment. Farhat Bashir analysed the soil, water and plant samples during experiment. Dr. Muhammad Mudassar Maqbool and Dr. Anser Ali helped in agronomic aspects of experiment, Dr. Saqib Bashir and Dr. Qaiser Abbas technically reviewed the manuscript.
References
Ali, S., M.J. Khan, Z. Shah, Naveedullah and A. Jalal. 2019. Genotypic screening of maize (Zea mays L.) for salt tolerance at early growth stage under different salinity levels. Sarhad J. Agric. 35(1): 208-215. https://doi.org/10.17582/journal.sja/2019/35.1.208.215
Almeida, D.M., M. Oliveira and J.M. Saibo. 2017. Regulation of Na and K homeostasis in plants: towards improved salt stress tolerance in crop plants. Genetics Mol. Biol. 40(1): 326-345. https://doi.org/10.1590/1678-4685-gmb-2016-0106
Al-Taisan, W.A. 2010. Comparative effects of drought and salt stress on germination and seedling growth of Pennisetum divisum (Gmel.) Henr. Am. J. Appl. Sci. 7(5): 640-646. https://doi.org/10.3844/ajassp.2010.640.646
Ashraf, M. and H. Fatima. 1994. Intra-specific variation for salt tolerance in linseed (Linum usitatissimum L.). J. Agron. Crop Sci. 173:193-203. https://doi.org/10.1111/j.1439-037X.1994.tb00554.x
Bakht, T., A. Basir, M. Shafi, M.J. Khan. 2006. Effect of various levels of salinity on sorghum at early seedling stage in solution culture. Sarhad J. Agric. 22: 17-21.
Batool, N., A. Shahzad and N. Ilyas. 2014. Plants and Salt Stress. Ant. J. Agric. Crop Sci. 7(14): 1439-1446.
Berrichi, A., R. Tazi, A. Bellirou, N. Kouddane and A. Bouali. 2010. Role of salt stress on seed germination and growth of jojoba plant Simmondsia chinensis (link) Schneider. IUFS J. Biol. 69(1):33-39.
Ebtihal, M., A. El-Hamid and M.S. Sadak. 2012. Performance of flax cultivars in response to exogenous application of salicylic acid under salinity stress. J. Appl. Sci. Res. 8(10):5081-5088.
El-Beltagi, H.S., Z.A. Salama and D.M. El Hariri. 2008. Some biochemical markers for evaluation of flax cultivars under salt stress conditions. J. Nat. Fibers. 5(4):316-330. https://doi.org/10.1080/15440470802252487
El-Naim, A.M., K.E. Mohammed, E.A. Ibrahim and N.N. Suleiman. 2012. Impact of salinity on seed germination and early seedling growth of three sorghum (Sorghum bicolor L. Moench) cultivars. Sci. Tech. 2(2):16-20. https://doi.org/10.5923/j.scit.20120202.03
Feng, W., H. Linder, N.E. Robbins II and J.R. Dinneny. 2016. Growing out of stress: The roll of cell and organic scale growth control in plant water stress responses. Plant Cell. 28: 1769-1782. https://doi.org/10.1105/tpc.16.00182
Guo, R., W. Hao and D.Z. Gong. 2012. Effects of water stress on germination and growth of linseed seedlings (Linum usitatissimum L), photosynthetic efficiency and accumulation of metabolites. J. Agric. Sci. 4(10):253-265. https://doi.org/10.5539/jas.v4n10p253
Gupta, B. and B. Huang. 2014. Mechanism of salinity tolerance in plants: Physiological, Biochemical and Molecular Characterization. Int. J. Genomics. 2014: 701596. https://doi.org/10.1155/2014/701596
Hoagland, D.R. and D.I. Arnon. 1950. The water culture method for growing plants without soil. Calif. Agr. Expt. Sta. Circ. 347.
James, R.A., R. Munns and S. Von Caemerer. 2006. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na, K and Cl in salt-affected barley and Durum wheat. Plant Cell Environ. 29: 2185-297. https://doi.org/10.1111/j.1365-3040.2006.01592.x
Jatoi, W.A., M.J. Baloch, M.B. Kumbharm N.U. Khan and M.I. Kerio. 2011. Effect of water stress on physiological and yield parameters at anthesis stages in elite spring wheat cultivars. Sarhad J. Agric. 27(1): 59-65.
Jones, J.R.J. and V.W. Case. 1990. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis, R.L. Westerman (ed.). SSSA. Madison, WI, USA. pp. 389-428.
Kandil, A.A., A.E. Sharief, W.A.E. Abido and M.M. Ibrahim. 2012. Effect of salinity on seed germination and seedling characters of some forage sorghum cultivars. Int. J. Agric. Sci. 4(7): 306-311. https://doi.org/10.9735/0975-3710.4.7.306-311
Kaya, M.D. and S. Day. 2008. Relationship between seed size and NaCl on germination, seed vigor and early seedling growth of sunflower (Helianthus annuus L.). Afr. J. Agric. Res. 3: 787-791.
Kaya, M., G. Kaya, M.D. Kaya, M. Atak, S. Saglam, K.M. Khawar, and C.Y. Ciftci. 2008. Interaction between seed size and NaCl on germination and early seedling growth of some Turkish cultivars of chickpea (Cicer arietinum L.). J. Zhejiang Univ. Sci. B. 9: 371-377. https://doi.org/10.1631/jzus.B0720268
Kaya, M.D., S. Day, Y. Cikili and N. Arslan. 2012. Classification of some linseed (Linum usitatissimum L.) genotypes for salinity tolerance using germination, seedling growth and ion content. Chilean J. Agric. Res. 72 (1): 27-32. https://doi.org/10.4067/S0718-58392012000100005
Kazmi, R.H., M.Q. Khan and M.K. Abbasi. 2003. Effect of water stress on the performance of wheat grown under controlled conditions at Rawalakot, Azad Jamu and Kashmir. Sarhad J. Agric. 19 (1): 61-68.
Keshavarzi, M.H.B., M.S.O. Rafsanjani, S. Mohsen, Moussavinik and A.P. Lak. 2011. Effect of salt (NaCl) stress on germination and early seedling growth of spinach (Spinacia oleracea L.). Ann. Biol. Res. 2(4): 490-497.
Khan, M.N., H.S. Manzar, F. Mohammad, M. Masroor, A. Khan and M. Naeem. 2007. Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in linseed genotypes. World J. Agric. Sci. 3 (5):685-695.
Khan, A., M. Shafi, J. Bakht and S. Anwar. 2017. Effect of Salinity and Seed Priming on Growth Characters of Wheat Varieties. Sarhad J. Agric. 33 (3): 435-446. https://doi.org/10.17582/journal.sja/2017/33.3.435.446
Machado Neto, N.B., S.M. Saturnino, D.C. Bomfim, and C.C. Custodio. 2004. Water stress induced by Mannitol and Sodium chloride in Soybean cultivars. Braz. Arch. Biol. Tech. 47 (4): 521-529. https://doi.org/10.1590/S1516-89132004000400004
Maqbool, M.M., A. Ali, T. Haq, M.N. Majeed and D.J. Lee. 2015. Response of spring wheat (Triticum aestivum L.) to induced water stress at critical growth stages. Sarhad J. Agric. 31(1): 53-58.
Munns, R., R.A. James and A. Lauchi. 2006. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 57: 1025-1043. https://doi.org/10.1093/jxb/erj100
Muhammad, Z. and F. Hussain. 2010. Vegetative growth performance of five medicinal plants under NaCl salt stress. Pak. J. Bot. 42(1): 303-316.
Muhammad, Z. and F. Hussain. 2010b. Effect of NaCl salinity on the germination and seedling growth of some medicinal plants. Pakistan J. Bot. 42(2): 889-897.
Moosavi, S.G., M.J. Seghatoleslami, Z. Jouyban and H. Javadi. 2013. Effect of salt stress on germination and early seedling growth of Nigella sativa L. Int. J. Trad. Herb. Med. 1(2):45-48.
Qayyum, M.A., J. Akhtar, Z.A. Saqib and S.M.A. Basra. 2015. Phenotyping of linseed (Linum usitatissimum L) genotypes for potential against NaCl stress. Soil Environ. 34(2): 200-206.
Rab, A., M. Sajid, N. Ahmad, K. Nawab and S.G. Ali. 2017. Foliar calcium application ameliorates salinity-induced changes of tomato crop grown in saline conditions. Sarhad J. Agric. 33 (4):540-548. https://doi.org/10.17582/journal.sja/2017/33.4.540.548
Ratnakar, A. and A. Rai. 2013. Effect of Sodium Chloride Salinity on seedgermination and early seedling growth of Trigonella forenum-gracecum L. Var. Peb. Octa J. Environ. Res. 1(4): 304-309.
Sayar, R., H. Bchini, M. Mosbahi and M. Ezzine. 2010. Effects of salt and drought stresses on germination, emergence and seedling growth of Durum wheat (Triticum durum Desf.). J. Agric. Res. 5(15): 2008-2016.
Shafique, M., N.N. Elahi, M. Rashid, A. Farooq and K.H. Shah. 2019. Application of PGPR enhances development and nodulation of Vigna Radiata L. grown under salt stress. Sarhad J. Agric. 35(3): 763-769. https://doi.org/10.17582/journal.sja/2019/35.3.763.769
Sikha, S., P. Sunil, J. Arti and B. Sujata. 2013. Impact of water deficit and salinity stress on seed germination and seedling growth of Capsicum annuum ‘Solan Bharpur. Int. Res. J. Biol. Sci. 2(8):9-15.
Steel, R.G.D., J.H. Torrie and D.A. Dickey. 1997. Principles and Procedures of Statistics: A Biometrical Approach, 3rd Edition, McGraw Hill Co., New York, USA.
Turan, M.A., A.H.A. Elkarim, N. Taban and S. Taban. 2009. Effect of salt stress on growth, stomatal resistance, proline and chlorophyll concentrations on maize plant. Afr. J. Agric. Res. 4(9): 893-897.
Wu, G.Q., Q. Jiao and Q.Z. Shui. 2015. Effect of salinity on seed germination,seedling growth and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.). Plant Soil Environ. 61 (5): 220-226. https://doi.org/10.17221/22/2015-PSE
Zaman, M.S., G.M. Ali, A. Muhammad, K. Farooq and I. Hussain. 2015. In vitro screening of salt tolerance in potato (Solanum tuberosum L.) varieties. Sarhad J. Agric. 31(2): 106-113. https://doi.org/10.17582/journal.sja/2015/31.2.106.113
Zorb, C., K. H. Muhling, U. Kutschera and C.M. Gelifus. 2015. Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: Is the epidermis growth restricting? PLoS One. 10(3): 1-15. https://doi.org/10.1371/journal.pone.0118406
To share on other social networks, click on any share button. What are these?








