Influence of Gibberellic Acid Concentrations and Dipping Durations on Growth and Yield of Bitter Gourd
Influence of Gibberellic Acid Concentrations and Dipping Durations on Growth and Yield of Bitter Gourd
Aliya Ayaz, Gohar Ayub, Maqsood Khan* and Syed Aizaz Ali Shah
The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | This research was carried out at Horticulture Research Farm, The University of Agriculture Peshawar, during 2016. The experimental design was Randomized Complete Block Design (RCBD) with two factors, replicated three times. Factor A was gibberellic acid concentrations i.e. 0, 20, 40 and 60 mgL-1, while factor B was comprised of dipping durations i.e. 0, 6, 12, and 18 hours respectively. Gibberellic acid concentrations and dipping durations had significant effect on the growth and yield of bitter gourd. Gibberellic acid concentration at @ 60 mgL-1 significantly reduced days to emergence and days to first flower, while increased germination percentage, number of fruits plant-1, fruit length, fruit weight, plant height, yield plot-1, total yield and survival percentage. In case of dipping durations, seeds dipped for 18 hours significantly reduced days to emergence and days to first flower, while increased germination percentage, number of fruits plant-1, fruit length, fruit weight, plant height, yield plot-1 and total yield. Interaction was found non-significant for all the studied parameters except days to emergence, germination percentage and survival percentage. Seeds dipped in gibberellic acid at a level of 60 mgL-1 for 18 hours significantly reduced days to emergence, while increased germination percentage and survival percentage. From results it is concluded that seeds dipped for 18 hours in 60 mgL-1 of gibberellic acid concentrations showed best results on growth and yield of bitter gourd under the agro-climatic conditions of Peshawar.
Received | March 20, 2018; Accepted | March 10, 2019; Published | May 12, 2019
*Correspondence | Maqsood Khan, The university of agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Ayaz, A., G. Ayub, M. Khan* and S.A.A. Shah. 2019. Influence of gibberellic acid concentrations and dipping durations on growth and yield of bitter gourd. Sarhad Journal of Agriculture, 35(2): 587-593.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.2.587.593
Keywords | Bitter gourd, Seed priming, Gibberellic acid, Fruit yield, Peshawar climate
Introduction
Bitter gourd (Memordica charantia L.), commonly called karela, balsam pear or bitter cucumber, belongs to cucurbitaceae family. Bitter gourd is monoecious, having pistillate and staminate flowers at different nodes in same plant. Bitter gourd is initiated in eastern Asia (Minraj et al., 1993) and is extensively distributed in China, Malaysia, India, Pakistan, Bangladesh and tropical Africa (Baloch, 1994). Its total area under cultivation is 6080 hectares, whereas its overall production is 56239 tons. In Khyber Pakhtunkhwa, the area under cultivation is 894 hectares, where the production is 6483 tons during 2008-09 (MINFAL, 2009). Likewise, nutritional values of other cucurbits, bitter gourd is exceptional in vitamins and considered to be a rich source of vitamin A and C (Palada and Chang, 2003). It is also considered as a valued vegetable because of its high healthful value. Its fruit stem, leaves and roots have been used in different medicines to treat hyperlipidemia microbial infections, digestive disarray (Yibchok et al., 2006). Bitter gourd lowers the blood sugar and is considered to be useful for diabetic treatment. It is also used for treatment of cancer because of its anti-noxious possessions (Grover and Yadav, 2004). Bitter gourd is generally known as annually grown plant but can be grown as perennial in frost free areas. In plain areas, summer season crop can be grown from January to June. As long as the soil medium is concerned, it tolerates a wide range of soil such as well-drained sandy loam soil and alkaline soils. The ideal soil pH for bitter gourd is 6.0-6.7. Bitter gourd needs a balance of organic and inorganic nutrients. However, the rates of nutrients depend upon the soil medium, level of fertility and the organic contents of soil (Palada and Chang, 2003). The major problems of restrictive yield of bitter gourd are poor seedling germination, hindered emergence and slow growth rate due to seed coated embryo. However, temperature 25-28 0C is ideal for germination of bitter gourd seeds (Peter et al., 1998). Seed soakings in various solutions with high osmotic potential enhances the metabolic processes required for germination (Nawaz et al., 2013). Priming is a technique in which seeds are presoaked before planting to enhance germination. The benefits of priming include activation of enzymes, softening of seed coat, breaking of seed dormancy. Priming decrease the time necessary for germination (Nawaz et al., 2013). The germination rate can be enhanced by these treatments (Khan, 2005). A plant hormone called Gibberellic acid (GA3) plays a very important function in plant growth and development like germination of seed, elongation of stem and development of flower. Different kinds of stimuli such as developmental, environmental and hormonal regulates GA3 biosynthesis. Soaking seeds with suitable concentrations of GA3 have positive effect on germination, growth and producing various plant varieties (Davies, 1995). The current research has been devised in the light of importance of seed soaking and dipping treatments to overcome the limiting yield and growth of bitter gourd.
Materials and Methods
The land was prepared with suitable moisture conservation before sowing the seed. The land was divided into experimental units according to the experimental layout. Recommended basal dose of NPK @120: 80: 80 kg ha-1 was applied to the soil. For Solution preparation calculated concentration of gibberellic acid was dissolved in 5% ethanol and then raised the volume up to one liter. All the solutions were prepared by the same procedure. Seeds of bitter gourd were dipped in GA3 concentrations (20, 40 and 60 mgL-1) for 0, 6, 12 and 18 hours respectively. The seeds were then dried in tissue folds and sown at next day. All the treated seeds of bitter gourd cultivar (Jaunpuri) were sown 30 cm apart on the ridges. Two to three seeds at a depth of 2.5cm were sown. Thinning was also done to keep one plant after seedling emergence. Row to row distance was kept 2 meters. All the cultural practices were practiced in the field uniformly and timely. Randomized Complete Block Design (RCBD) with two factors, replicated three times was used as experimental design. Factor A was GA3 Concentrations (G1 = control, G2 = 20 mg/L, G3 = 40 mg/L and G4 = 60 mg/L), while factor B was Dipping durations (D1= Control, D2= 6hrs, D3= 12hrs and D4= 18hrs). The data of following experimental parameters was calculated. Number of days taken to emergence was recorded from sowing to emergence of seedlings. Similarly, germination percentage was recorded by dividing the number of germinated seeds on total seed sown. The formula is as under:
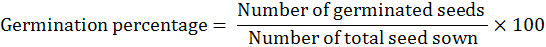
Furthermore, number of days to first flower was calculated by counting the number of days from sowing to first flower appearance of randomly selected five plants and their average was calculated. While numbers of fruits plant-1 of randomly selected five plants were recorded and their average was calculated. Average fruit length was calculated using the fruit picked from randomly selected five plants. In case of fruit weight (g), average fruit weight of randomly selected five plants was recorded and their average was calculated. Similarly, plant height (cm) was determined by selecting randomly five plants from each treatment and their average was calculated. Furthermore, Average fruit yield plant-1 of randomly selected five plants was measured and their averages were calculated. Then total fruit yield plot-1 was recorded and the yield (tons ha-1) was computed by the following formulae:
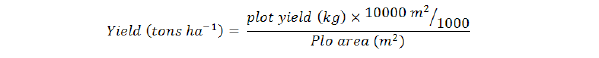
While Survival percentage (%) was obtained by using the following formulae:
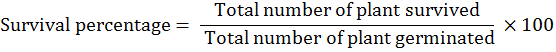
Statistical analysis
For statistical analysis Statistix 8.1 was used and for mean comparison LSD- test was applied (Steel et al., 1997).
Results and Discussions
Days to emergence
The interactive effects of GA3 concentration and dipping duration of seed significantly reduced days to emergence. Maximum earliest emergence was observed in the seeds dipped in 90 ppm GA3 for 18 hours (Figure 1). Regarding GA3 concentrations mean table shows that minimum days to emergence (7.15) were observed in the seeds dipped in 60 mgL-1 while maximum (8.67) were observed in seeds dipped in distilled water. While comparing means of different dipping duration’s minimum days to emergence (7.50) were observed in seeds dipped for 18 hours while maximum (8.39) was observed in untreated seeds. Regarding interaction, minimum days to emergence (6.88) were observed when the seeds were dipped in 60 mgL-1 for 18 hours while maximum (10.33) were observed when seeds were kept untreated. The success of crop is viewed from its emergence. Seed viability has an important contribution in emergence. The result of this study is in line with the work of Arif et al. (2005), Bradford (1998), who reported that the possible reason for the primed seeds early emergence might be the conclusion of pre-germinative metabolic activities. Priming stimulates enzymes like amylase and lipase which activate storage materials in seeds. These results are in agreement with the findings of Farooq et al. (2005). Every specie needs a specific amount of time for germination in lag phase. (Pazdera and Hosnedl, 2002).
Germination percentage (%)
The interactive effects of GA3 concentration and dipping duration of seed significantly improved germination percentage. Maximum germination of seeds was observed when seed dipped in 90 ppm GA3 for 18 hours (Figure 2). Regarding GA3 concentrations, maximum germination percentage (79.1%) was observed when seeds were dipped in 60 mgL-1 solution, while minimum germination percentage (54.0) was observed when seeds were dipped in distilled water. In case of Dipping durations, maximum germination percentage (75.1%) was observed in seeds dipped for 18 hours and minimum germination percentage (59.0 %) was recorded in unprimed seeds. regarding interaction maximum (88.9%) germination percentage was observed in seeds soaked for 18 hours in 60 mgL-1solution of gibberellic acid while the minimum germination percentage (44.7%) was recorded in un-primed seeds. The induction of membrane integrity and quantitative changes of seeds biochemical content is due to priming. During seed germination, the physiological activities are also enhanced because of priming. These results have been justified the reason for higher germination percentage was the pre-soaking treatment with GA3 as pre-soaking stimulates biochemical processes such as hydrolysis, metabolism of growth inhibitors, enzymes activation required for germination of plant. Also, rehydration causes early germination because all pre-germinative processes have already been occurred. The reason for increased germination with increase in dipping duration is because of that each specie needs an optimum level of water in germination lag phase where all pre-germinative processes taken place (Pazdera and Hosnedl, 2002).
Days to first flower
Regarding GA3 concentrations, mean table shows that minimum days to first flower (36.8) was observed in the seeds dipped in 60 mgL-1 while maximum (43.3) was observed when seeds dipped in distilled H2O. While comparing means of different dipping durations, minimum days to first flower (36.6) was observed when seeds were dipped for 18 hours while maximum (43.0) were observed in untreated seeds. Days to flowering determines the period of maturity (Table 1). It is highly linked with physiological maturity. Days to flowering can be measured as the extinction of vegetative cycle and initiation of reproductive cycle. The seeds dormancy is broken in chemical priming. Growth hormones are secreted and the speed of growth becomes much more rapid than normal, likewise reported by Murungo et al. (2004). During their experiments, they found that primed crop emerges fast, flower earlier and give high yield. Mauromicale et al. (2000) investigated seed osmopriming as a mean to get early flowering, and thus increase the yield of summer squash (Cucurbita pepo L). The results of this study are also interrelated with Harris et al. (2000) who showed that rice priming causes earlier flowering in the crops and hence improved the yield.
Table 1: Days to emergence, Germination %age, Days to 1st flower, No of fruits and Fruit Length as affected by seed priming of bitter gourd as affected by GA3 concentrations and dipping duration.
| GA3 Concentrations | Days to emergence | Germination %age | Days to 1st flower | No of fruits | Fruit Length |
| 0 | 8.67 a | 54.0 d | 43.3 a | 10.6 d | 13.1 d |
| 20 | 7.97 b | 68.0 c | 41.1 b | 12.6 c | 14.1 c |
| 40 | 7.61 b | 74.3 b | 39.2 c | 15.1 b | 15.1 b |
| 60 | 7.15 c | 79.1 a | 36.8 d | 17.5 a | 16.5 a |
| LSD at 5% | 0.45 | 3.21 | 1.69 | 0.63 | 0.54 |
| Dipping Duration | |||||
| 0 | 8.39 a | 59.0 c | 43.0 a | 12.9 c | 13.2 d |
| 6 | 7.97 ab | 70.0 b | 41.0 b | 13.8 b | 14.2 c |
| 12 | 7.54 bc | 71.3 b | 39.8 b | 14.1 b | 15.2 b |
| 18 | 7.50 c | 75.1 a | 36.6 c | 15.0 a | 16.3 a |
| LSD at 5% | 0.45 | 3.21 | 1.69 | 0.63 | 0.54 |
Number of fruits
According to the mean values of GA3 concentrations, maximum number of fruits (17.5) were observed when seeds were dipped in 60 mgL-1solution, while minimum number of fruits (10.6) when seeds were dipped in distilled water, were recorded. In case of Dipping durations, maximum number of fruits (15.0) was observed in plots in which seeds were dipped for 18 hours followed by (14.1) when seeds were dipped for 12 hours while the minimum number of fruits (12.9) was recorded in untreated seeds (Table 1). Number of fruits per plant is a main factor for final yield determination. Number of fruits and number of leaves has direct connection. The results are in line with the findings of Ullah et al. (2002) which shows that yield parameters such as number of primary branches per plant, no of fruits per plant are increased because of priming. The possible reason for increase in fruits is subjected to improved emergence and better seedlings growth as accepted by several researchers (Harris et al. 2000).
Table 2: Fruit Weight, Plant height, Yield plot, Yield ton hec-1 and Survival %age as affected by seed priming of bitter gourd as affected by GA3 concentrations and dipping duration.
| GA3 Concentrations | Fruit Weight | Plant height | Yield plot | Yield ton per hec | Survival %age |
| 0 | 86.2 d | 194.3 d | 4.6 d | 12.7 d | 69.5 d |
| 20 | 96.3 c | 200.8 c | 6.1 c | 16.9 c | 77.0 c |
| 40 | 105.9 b | 209.5 b | 8.0 b | 22.2 b | 83.1 b |
| 60 | 115.0 a | 216.2 a | 10.1 a | 28.0 a | 88.2 a |
| LSD at 5% | 3.43 | 3.9 | 0.4 | 1.12 | 2.72 |
| Dipping Duration | |||||
| 0 | 96.0 c | 196.3 d | 6.3 c | 17.6 c | 73.6 c |
| 6 | 99.8 b | 200.4 c | 7.0 b | 19.4 b | 79.6 b |
| 12 | 101.3 b | 209.1 b | 7.3 b | 20.2 b | 80.5 b |
| 18 | 106.3 a | 215.1 a | 8.1 a | 22.5 a | 84.1 a |
| LSD at 5% | 3.43 | 3.9 | 0.4 | 1.12 | 2.72 |
Fruit length (cm)
According to the mean values of GA3 concentrations, maximum fruit length (16.5cm) was observed when seeds were dipped in 60 mgL-1 solution and minimum fruit length (13.1cm) was recorded when seeds were dipped in distilled water. In case of dipping durations, maximum fruit length (16.3cm) was observed in seeds dipped for 18 hours, while the minimum fruit length (13.2cm) was recorded in un-soaked seeds. This increase in fruit length is due to better accumulation of carbohydrates due to greater photosynthesis which caused the fruit to increase in length. These results are supported by the findings of (Uddain and Hossain, 2009). The longer fruits under GA3 might be due increased cell division and cell elongation which would have special uptake of water and nutrients. A similar effect with gibberellic acid application was reported by Singh et al. (2006) (Table 1). The increase in fruit length is due to activating cell division and cell elongation along with increasing the metabolic activity. Prabhu and Natarajan (2006) recorded maximum fruit length in Ivy gourd when GA3 and NAA were applied. Similarly, Ashrafuzzaman et al. (2010), Rajasekar (2015) also reported higher fruit length in bitter gourd.
Fruit weight (gm)
According to the mean values of GA3 concentrations, maximum fruit weight (115.0g) was observed when seeds were dipped in 60 mgL-1 solution, whereas minimum fruit weight (86.2g) was recorded when seeds were dipped in distilled water. In case of Dipping durations, maximum fruit weight (106.3 g) was recorded in seeds dipped for 18 hours and minimum fruit weight (96.0 g) was observed in un- primed seeds (Table 2). This increase in fruit weight is assigned to GA3, since by its distinctiveness asset (cell elongation) it has promoted the growth of all vegetative parts and as a result more food substances for fruit development was created by such plants and fruits with superior weight were obtained. The increasing fruit weight as a result of GA3 application has also been obtained by (Uddain and Hossain, 2009). Results related observation was also reported by Nagamani et al (2015), Ashrafuzzaman et al (2010), Rajasekar (2015) in bitter gourd.
Plant height (cm)
According to the mean values of GA3 concentrations, maximum plant height (216.2 cm) was observed when seeds were dipped in 60 mgL-1 solution and minimum plant height (194.3 cm) was observed when seeds were dipped in distilled water. In case of Dipping durations, maximum plant height (215.1 cm) was observed in seed dipped for 18 hours, while the minimum plant height (196.3 cm) was recorded in untreated seeds (Table 2). The possible reason could be faster growth rate of seedlings which generate the competency amongst plants for nutrients, light, water and space and resulted in taller plants. The results are in conformity with Rashid et al. (2004), who evaluated that seed priming improves the plant growth and yield.
Yield plot-1 (kg)
According to the mean values of GA concentrations, maximum yield plot-1 (10.1 kg) was observed when seeds were dipped in 60 mgL-1 solution, whereas minimum yield plot-1 (4.6 kg) when seeds were dipped in distilled water. In case of Dipping durations, maximum yield plot-1 (8.1 kg) was observed in seeds dipped for 18 hours and minimum yield plot-1 (6.3 kg) was recorded in untreated seeds. The probable reason could be increase in number of fruits, fruit length and weight. Yield is concluding production of any crop. It depends upon various factors such as genetic makeup, soil types and environmental factors. The improved yield due to priming in spinach is due to early seedling growth and better plant nutrition, Zhang et al. (1998). Uniform and vigorous seedling growth improves yield of primed seeds and ultimately results higher grain yield as reported by Harris et al. (2000). The findings of the current research are in line with Basra et al. (2003) who found that priming treatment appreciably enhanced and plant weight unlike with control.
Yield (tons ha-1)
According to the mean values of GA3 concentrations, maximum yield (28.0 tons) was observed when seeds were dipped in 60 mgL-1 solution, while minimum yield (12.7 tons) when seeds were dipped in distilled water, was recorded. In case of Dipping durations, maximum yield (22.5 tons) was observed in seeds dipped for 18 hours and minimum yield (17.6 tons) was recorded in un-soaked seeds. Early seedling growth and better plant diet results superior yield due to priming in spinach as reported by Zhang et al. (1998). The improved yield of primed seed plants is due to consistent and substantial growth of seeds, well developed root system and competent successive growth. This led to higher grain yield as reported by Harris et al. (2000). The results are similar with the findings of Basra et al. (2003) who investigated that priming treatment noticeably improved plant weight as compared with control.
Survival percentage (%)
The interactive effects of GA3 concentration and dipping duration of seed significantly increased survival percentage. Maximum survival of plants was observed when seed dipped in 90 ppm GA3 for 18 hours (Figure 3). According to the mean values of GA3 concentrations, maximum survival percentage (88.2%) were observed when seeds were dipped in 60 mgL-1 solution, while minimum survival percentage (69.5%) when seeds were dipped in distilled water. In case of Dipping durations, maximum survival percentage (84.1%) was observed in plots having seeds dipped for 18 and minimum survival percentage (73.6 %) was obtained in untreated seeds. However according to their interaction, maximum (97.0%) survival percentage was observed in plots with seeds soaking for 18 hours in 60 mgL-1 solution of gibberellic acid while the minimum survival percentage (63.2%) was recorded in un-primed seeds. Survival percentage depends upon the quality of seeds. Seeds are mostly infected by different pathogens. Due to infection, seed germination percentage is decreased and chances of plant survival are reduced. Phosphorus inactivates the pathogens to avoid early seedling infection resulting more survival percentage of primary seeds. The results of this research study are aligned with Musa et al. (2001) who worked on seed priming in chickpea in which the damage caused by collar rot (Sclerotium rolfsii) is significantly reduced. Recent work of Rashid et al. (2004) also studies mungbean (Vigna radiate). These mungbean grown from seed primed in water. It showed serious symptoms of infection due to Mungbean Yellow Mosaic Virus.
Conclusions and Recommendations
It is concluded that seeds of bitter gourd give best results after priming with gibberellic acid (GA3) for 18 hours. So, it should be practiced to boost the production of bitter gourd in Peshawar valley.
Author’s Contribution
Aliya Ayaz perform the experiment. Gohar Ayub supervised the experiment. Maqsood Khan and Syed Aizaz Ali Shah did data collection, statistical analysis and paper writing.
References
Arif, M., S. Ali, A. Shah, N. Javed and A. Rashid. 2005. Seed priming of maize for improving emergence on seedling growth. Sarhad. J. Agric. 21(4): 539-543.
Ashrafuzzaman., M. Razi., K.M.A. Fazal, M.K. Uddin and A. Pardan. 2010. Effect of GABA application on the growth and yield of Bitter Gourd. Int. J. Agric. Bio. 12: 129-132.
Baloch. A.F. 1994. Vegetable crops in horticulture. national book foundation Islamabad. pp. 522-523.
Basra, S.M.A., Ehsanullah, E.A. Warraich, M.A. Cheema and I. Afzal. 2003. Effect of Storage on growth and yield of primed canola (Brassica napus L.) seeds. Int. J. Agric. Bio. 117-120.
Bradford, K.J., D.M. May, B.J. Hoyle, Z.S. Skibinski and K.B. Tyle. 1998. Seed and soil treatment to improve emergence of muskmelon from cold or crusted soils. Crop Sci. 28:1001-1005. https://doi.org/10.2135/cropsci1988.0011183X002800060028x
Davies, P.J. 1995. Plant hormones, physiology, biochemistry and molecular biology. Kluwer Acad. Publ. Dordrecht.
Farooq, M., S.M.A. Basra, K. Hafeez, S.A. Asad and N. Ahmad. 2005. Use of commercial fertilizers as osmotic for rice priming. J. Agric. and Soc. Sci. 1: 172-175.
Grover, J.K. and S.P. Yadav. 2004. Pharmacological actions and potential uses of Momordica charantia. J. Ethnopharmacol. 93: 123-132. https://doi.org/10.1016/j.jep.2004.03.035
Harris, D., R.S. Tripathi and A. Joshi. 2000. On-farm seed priming to improve crop establishment and yield in dry direct-seeded rice. Paper presented at the workshop on dry seeded rice Technology, Bangkok, Thailand.
Khan, A.A., J.D. Magure and G.S. Abawi. 2005. Matric conditioning of vegetables seed to improve stand establishment in early field planting. J. Am. Soc. Hort. Sci. 117: 41-47. https://doi.org/10.21273/JASHS.117.1.41
Mauromicale, G., V. Cavallaro, P.J. Stoffella, D.J. Cantliffe and G. Damato. 2000. Effects of seeds osmopriming on the harvest time and yield of summer squash. Bari. Italy. 15-18. Acta. Hort. 533: 83-88. https://doi.org/10.17660/ActaHortic.2000.533.8
MINFAL. 2009. Fruit, vegetables and condiments statistics of Pakistan. GoP, Minist. Food Agric. Islamabad. pp. 11-18.
Miniraj, N., K.P. Prasanna and K.V. Peter. 1993. Bitter gourd Momordica spp. Genetic improvement of vegetable plants. pp. 239-246.
Murungu, F.S., C. Chiduza, P. Nyamugafata, L.J. Clark and W.R. Whalley. 2004. Effect of on farm seed priming on emergence, growth and yield of cotton and maize in a semi-arid area of Zimbabwe. Exp. Agric. 40: 23-36. https://doi.org/10.1017/S0014479703001509
Musa, A.M., D. Harris, C. Johansen and J. Kumar. 2001. Short duration chickpea to replace fallow after aman rice: the role of on-farm seed priming in the High Barind Tract of Bangladesh. Exp. Agric. 37(04): 509-521. https://doi.org/10.1017/S0014479701000448
Nagamani. S., S. Basu, S. Singh, S.K. Lala, T.K. Behera, S.K. Chakrabarty and A. Talukdar. 2015. Effect of plant growth regulators on sex expression, fruit setting, seed yield and quality in the parental lines for hybrid seed production in bitter gourd (Momordica charantia). India. J. Agric. Sci. 85 (9): 1185–91.
Nawaz, J., M. Hussain, A. Jabbar, G.A. Nadeem, M. Sajid, M.U. Subtain and I. Shabbir. 2013. Seed priming a technique. Int. J. Agric. Crop Sci. 6(20): 1373.
Palada, M.C. and L.C. Chang. 2003. Suggested cultural practices for bitter gourd. AVRDC Int. Cooperators Guide. 03: 547.
Pazdera, J. and V. Hosnedl. 2002. Effect of hydration treatments on seed parameters of different lettuce (Lactuca sativa L.) seed lots. Hort. Sci. 29(1): 12-16. https://doi.org/10.17221/4464-HORTSCI
Peter, K.V., M.K. Sadhu, M. Raj and K.P. Prasanna. 1998. Improvement and cultivation of bitter gourd, snake gourd, pointed gourd and ivy gourd. Sci. Publishers Inc., Enfield, NH, USA. pp. 187-195.
Prabhu. M. and S. Natarajan. 2006. Effect of growth regulators on fruit characters and seediness in ivy gourd (coccinia grandis l). Dep. of Veg Crops, Hort. College and Research Insti, Tamil Nadu Agric. Univ. Coimbatore. 641 003, India. Agric. Sci. Digest. 26(3): 188 - 190.
Rajasekar, M. and V. Swaminathan. 2015. Impact of pre-harvest chemical spray on yield and yield parameters of bitter gourd (Momordica charantia L.) cultivars. Int. J. Agric. Sci. Res. (IJASR) Vol. 5. pp: 185-192.
Rashid, A., D. Harris, P. Hollington and S. Ali. 2004. On-farm seed priming reduces yield losses of mungbean (Vigna radiata) associated with mung bean yellow mosaic virus in the North West Frontier Province Pak. Crop Prot. 23(11): 1119-1124. https://doi.org/10.1016/j.cropro.2004.04.002
Singh, N.P., D.K. Singh and V. Kumar. 2006. Vegetable seed production technology. 1st Ed. International book distributing. Co. Lucknow. Pp. 143-145.
Steel, R.G.D., J.H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics: A biometrical approach. 3rd ed. McGraw Hill Book Co. Inc. New York: 400-428
Udden, J., K.M.A. Hossain, M.G. Mostafa and M.J. Rahman. 2009. Effect of different plant growth regulators on growth and yield of tomato. Int. J. Sust. Agric . 1 (3): 58-63.
Ullah, H., J.G. Chen, S. Wang and A.M. Jones. 2002. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 129 (2): 897-907. https://doi.org/10.1104/pp.005017
Yibchok, A.S., S. Adisakwattana, C.Y. Yao, P. Sangvanich, S. Roengsunran and W.H. Hsu. 2006. Slow acting protein extract from fruit pulp of Momordica charantia with insulin mimetic activities. J. Biol. Pharm. Bull. 29: 1126-1131. https://doi.org/10.1248/bpb.29.1126
Zhang, M., M. Nyborg and W.B. McGill. 1998. Phosphorous imbibed by barley seed and location within the seeds and assimilation by seedlings. J. Seeds Sci. Tech. 26: 325-332.
To share on other social networks, click on any share button. What are these?








