Optimization of Ultrasonic Assisted Extraction of Bioactive Compounds from Almond Hull
Research Article
Optimization of Ultrasonic Assisted Extraction of Bioactive Compounds from Almond Hull
Nabila Khan1, Imran Ahmad2 and Muhammad Bilal Sadiq1*
1School of Life Sciences, Forman Christian College (A Chartered University), Lahore, 54600, Pakistan; 2Food Agriculture and Biotechnology Innovation Lab (FABIL), Florida International University, Biscayne Bay Campus, North Miami, Florida, USA.
Abstract | Ultrasonic assisted extraction (UAE) of total phenolic content (TPC) from almond hull was optimized by response surface methodology (RSM). TPC and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity were used as response variables. Almond hull extract was chemically characterized by Fourier transform infrared spectrometer (FTIR) and gas chromatography mass spectrometer (GCMS). The extraction conditions were optimized by using independent extraction parameters; sample to solvent ratio (1:20-1:40 w/v), solvent concentrations (20-80%, v/v) and time (10-30 min), whereas, TPC and DPPH were used as response variables. At optimized extraction conditions (40 ml/g, 22.5 min and 80% of ethanol), the experimental values for TPC and DPPH inhibition were 110.17 ± 3.44 mg of GAE/g of extract and 87.45 ± 1.28%, respectively. For DPPH inhibition assay, IC50 value of almond hull extract was 51.64 μg/ml which was lower than the ascorbic acid (180 μg/ml). Almond hull extract showed antimicrobial effect against Escherichia coli, Staphylococcus aureus and Salmonella typhimurium. β-sitosterol (44 %), was identified as major phytoconstituent in almond hull extract. Due to antioxidant and antimicrobial potential, almond hull extract can be utilized as a functional food ingredient, and natural preservative.
Received | September 12, 2021; Accepted | November 15, 2021; Published | April 28, 2022
*Correspondence | Muhammad Bilal Sadiq, School of Life Sciences, Forman Christian College (A Chartered University), Lahore, 54600, Pakistan; Email: [email protected]
Citation | Khan, N., I. Ahmad and M.B. Sadiq. 2022. Optimization of ultrasonic assisted extraction of bioactive compounds from almond hull. Sarhad Journal of Agriculture, 38(2): 676-684.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.2.676.684
Keywords | Almond hull, Optimization, Antioxidant, Phenolic content, Food by-products
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Almond tree (Prunus amygdalus) is popular nut trees worldwide and belongs to family Rosaceae. In 2019, global almond production was 3.53 million tons and United States of America, Australia, Iran and Italy were recognized as major almond producers (FAO, 2020). Almond hull is the main byproduct of almonds and comprised of 35-62% of fresh almond weight (Prgomet et al., 2017). Almond hulls are assumed as a low economic value byproduct and are mainly used in animal feed. However, almond hulls are rich TPC and can be used for the development of functional food products (Kahlaoui et al., 2019). Almond hull contains antioxidants and exhibits free radical-scavenging potential (Wijeratne et al., 2006). The efficient utilization of agriculture byproducts helps to reduce the environmental hazards associated with organic waste and improved the economic returns (Prgomet et al., 2019a). The food commodities have diverse profile of phenolic compounds and extraction of these polyphenols is dependent on extraction method and extraction parameters. Solvent type, temperature, sample to solvent proportion and time of extraction can greatly influence the phenolic extraction from food matrices (Iglesias-Carres et al., 2018; Zitka et al., 2011). The conventional methods require long extraction time, high temperature and large proportion of solvents, which leads to the degradation of heat sensitive bioactive compounds. Therefore, the concept of modern extraction techniques is gaining interest, such as extraction by ultrasonication and microwave assisted extraction (Guglielmetti et al., 2017). These advance extraction techniques offer less solvent consumption, extraction at low temperature, high yield and purity of target compounds. UAE is inexpensive and simple technique which is achieved at lower temperature and short time by creating acoustic cavitation which disrupts plant cell walls and release of internal cellular constituents into the extraction matrix (Piyalungka et al., 2019). Due to simple, inexpensive and reproducible technique UAE is effective at industrial scale (Sitthiya et al., 2018). The aim of this study was to optimize the UAE of bioactive compounds from almond hull and evaluation of their antioxidant and antimicrobial potential against foodborne pathogens.
Materials and Methods
Sample preparation
Almond fruits were harvested from Hunza valley of Pakistan and hulls were separated manually followed by oven drying for 48 h at 50°C. The samples were ground by mechanical grinder (Philips Co. Ltd., China) and stored at 4°C till further use (Hiranrangsee et al., 2016).
Extraction process
TPC from the almond hull were extracted by UAE and conventional extraction methods.
Conventional extraction
The powdered sample (5 g) was added to ethanol (45 ml; 80%, v/v) and placed in a at 25℃ and 300 rpm in a shaker. The extraction was carried out for 24 and 48 h, separately. The extracts were filtered and stored at 4 °C (Sadiq et al., 2015).
Ultrasonic assisted extraction (UAE)
UAE of almond hull was optimized by RSM using Design-Expert® software (Minneapolis, MN, USA) at a fixed frequency of 20 kHz. UAE was optimized by independent extraction variables, which were sample to solvent ratio (1:20, 1:30 and 1:40 w/v), solvent concentrations (20, 50 and 80%, v/v) and time (10, 20 and 30 min) at fixed temperature of 45°C. TPC and DPPH inhibition were used as response variables (R1 and R2, respectively). The sample was added to beaker containing the extraction solvent and subjected to ultrasonic processor (LSP-500, Industrial Sonomechanics, USA).
TPC and antioxidant activity
TPC was determined using Folin–Ciocalteau regent (Sigma-Aldrich, USA), by following the method described by Sadiq et al. (2015). TPC was estimated by using gallic acid standard curve and presented as mg of gallic acid equivalent (GAE) per gram of raw sample.
Antioxidant potential of almond hull extract was estimated by DPPH inhibition assay by following Sadiq et al. (2015). The extract (50 µl) was added to 5 ml of DPPH in ethanol (40 ppm). The mixture was kept for half an hour in dark at 25℃. After incubation absorbance was measured at 517 nm using UV-Vis spectrophotometer. % DPPH inhibition was calculated using equation 3.
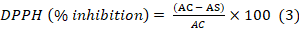
AC: Absorbance of the control (DPPH without extract); AS: Absorbance of sample.
Optimization of UAE extraction
UAE extraction of almond hull was optimized using response surface methodology and optimized run was selected using 0.994 desirability. The optimized extraction conditions were 1:40 g/ml sample to solvent proportion, 22.5 min time of extraction and 80% ethanol (v/v). The optimized extract was lyophilized (Christ Alpha 1-2 LD plus, Germany) to obtain powdered extract.
Characterization of optimized extract
TPC and antioxidant activity: The stock solution (1 mg/ml) of dried extract was diluted with deionized water to 100 μg/ml and TPC was determined using Folin–Ciocalteau regent. Antioxidant potential of optimized extract was determined by DPPH inhibition and FRAP assay. For %DPPH inhibition, different concentrations (31.25-2000 µg/ml) of extract were prepared and %DPPH inhibition was estimated by using equation 3. IC50 values were determined by non-linear regression using GraphPad Prism® version 7. (San Diego, US) (Sadiq et al., 2017). For FRAP assay, almond hull extract (50 μl of 1 mg/ml) was mixed with FRAP reagent (1.5 ml) and incubated at 37°C for 4 min, followed by reading the absorbance at 593 nm. Ferrous sulfate solution (100–1000 μM) was used to develop a standard curve. Ascorbic acid was used as positive control.
Antibacterial activity: Antibacterial activity of almond hull extract was evaluated against Escherichia coli (ATCC 8739), Salmonella typhimurium (ATCC 14028) and Staphylococcus aureus (ATCC 25923). The sterilized cotton swab was used to spread the bacteria (108 CFU/ml) on the surface of nutrient agar plates. The extract (100 µl) in different concentrations (1.56-50 mg/ml) was added into the 8 mm wells made on agar plates. After 24 h incubation at 37 ºC, the inhibition zone diameter was measured. All experiments were performed in triplicates (Taye et al., 2011). MIC of almond hull extract (1.56-50 mg/ml) was estimated by broth macrodilution method following Kubo et al. (2004). After incubation period, the lowest concentration without any visible growth was marked as MIC. MBC was measured by sub-culturing 100 µl of each extract concentrations that had no visible growth on nutrient agar. After 24 h incubation, the minimum concentration without detectable growth on nutrient agar plates was considered as the MBC.
Fourier transform infrared spectroscopy analysis
The optimized extract was characterized by FTIR spectrometer (Agilent technologies, USA). The spectra were recorded (4000-650 cm-1) with a resolution of 4cm-1 using absorbance mode.
GCMS analysis
The optimized extract was analyzed by using GC-MS system (GC-7890A/MS-5975C, Agilent Technologies, Santa Clara, CA, USA) with HP-5 MS capillary column. Helium gas was used as carrier (1.0 ml/min) and sample injection was programmed at 200°C. All data were acquired within the range 50–600 a.m.u. The compounds were identified by using NIST 05 spectral library (Gaithersburg, MD, USA).
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey’s HSD tests were carried out to find significant (p < 0.05) differences among mean observations by using SPSS statistical software package (SPSS, version 23.0).
Table 1: Optimization of ultrasound assisted extraction of almond hull and effect of extraction parameters on response variables.
|
Experiments |
Independent variables |
Response variables |
|||
|
Sample to solvent ratio (g/ml) |
Time (min) |
Ethanol (%) |
DPPH inhibition (%) |
TPC (mg GAE/g of raw sample) |
|
|
1 |
1:20 |
20 |
20 |
68.18 ± 2.1 |
5.82 ± 0.11 |
|
2 |
1:40 |
20 |
20 |
72.56 ± 2.48 |
11.13 ± 0.5 |
|
3 |
1:30 |
20 |
50 |
70.07 ± 0.51 |
8.39 ± 0.2 |
|
4 |
1:40 |
20 |
80 |
86.27±3.41 |
11.31 ± 0.19 |
|
5 |
1:30 |
30 |
80 |
82.10 ± 1.66 |
7.94 ± 0.12 |
|
6 |
1:30 |
30 |
20 |
73.27 ± 1.48 |
7.10 ± 0.35 |
|
7 |
1:20 |
20 |
80 |
81.26 ± 2.31 |
4.92 ± 0.5 |
|
8 |
1:30 |
10 |
80 |
73.20 ± 0.92 |
8.02 ± 0.193 |
|
9 |
1:40 |
30 |
50 |
83.66 ± 2.77 |
9.75 ± 0.4 |
|
10 |
1:30 |
20 |
50 |
76.01 ± 1.68 |
7.50 ± 0.36 |
|
11 |
1:30 |
20 |
50 |
76.15 ± 0.51 |
7.44 ± 0.31 |
|
12 |
1:40 |
10 |
50 |
75.72 ± 0.51 |
9.76 ± 0.32 |
|
13 |
1:20 |
30 |
50 |
77.65 ± 0.35 |
5.31 ± 0.12 |
|
14 |
1:30 |
10 |
20 |
67.66 ± 3.31 |
7.29 ± 0.4 |
|
15 |
1:20 |
10 |
50 |
68.91 ± 0.76 |
5.09 ± 0.11 |
Results and Discussions
UAE and conventional extraction of almond hull
UAE and conventional solvent extractions were used for the extraction of TPC. The conventional extraction resulted in TPC and %DPPH inhibition of 2.67 ± 0.17 mg GAE/g of raw almond hull and 63.89 ± 0.45%, respectively after 24 h of extraction, whereas, after 48 h of extraction the TPC and %DPPH inhibition were 2.56 ± 0.27 mg GAE/g and 64.34 ± 1.97%, respectively. UAE extraction of almond hull was optimized by using Box-Behnken design (Table 1). The optimal extraction conditions were 1:40 sample to solvent ratio, 80% ethanol and 20 min of extraction time which corresponded to TPC and %DPPH inhibition of 11.31 ± 0.19 mg GAE/g and 86.03 ± 3.41%, respectively. As compared to conventional solvent extraction, UAE was found to be more effective and rapid for TPC extraction. The reported difference in yield of TPC by conventional and UAE extractions might also be influenced by different extraction conditions such as extraction time, sample to solvent ratio and extraction temperature. UAE is based on the application of sound waves to disrupt plant membranes, which facilitates the solvent penetration and enhance the high extraction yield (Mala et al., 2021). Similarly, He et al. (2016) found high yield of TPC and antioxidant activity in blueberry wine pomace after UAE in comparison to conventional extraction.
Effect of UAE extraction parameters on TPC
TPC of almond hull extract was in the range of 4.92 ± 0.5 to 11.31 ± 0.19 mg GAE/g of raw material. Quadratic model was used to evaluate the effect of extraction parameters on response variables. The sample to solvent proportion significantly effect (p < 0.05) TPC in comparison to solvent concentration and UAE extraction time. The polynomial equation for TPC is presented as equation 1.
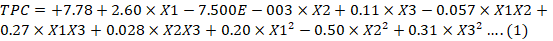
X1: Sample to solvent ratio; X2: Extraction time; X3: Solvent concentration.
The p-value of 0.0042 and R2 (coefficient of determination) value of 0.9641 indicated that the model was significant. Ingawale et al. (2018) reported that the hydroalcoholic solvent was more effective for the extraction of TPC than alcohol alone and increase in concentration of alcohol resulted in an increase in TPC. Simsek et al. (2012) reported that TPC was increased with an increase in extraction time of sour cherry pomace to optimal time and further increase in time, decreased the TPC. Extended extraction time might enhance the exposure of phenolics to oxygen and light, which resulted in degradation of the antioxidants. The extraction of TPC from Murtus communis L. leaves and milled berries was reported to increase with an increase in sample to solvent ratio (Cacace et al., 2003; Dahmoune et al., 2015). The increase in sample to solvent proportion results in an increase mass transfer, hence extraction yield is improved (Pinelo et al., 2007).
Effect of UAE extraction parameters on %DPPH inhibition
Antioxidant activity of almond hull extract was in the range of 67.54 ± 0.51 to 86.03±3.41%. Quadratic model was used to evaluate the effect of extraction parameters on %DPPH inhibition. All the extraction parameters (sample to solvent proportion, extraction time and solvent concentration) had significant effect (p < 0.05) on %DPPH inhibition. The sample to solvent proportion, extraction time and concentration of solvent were reported to significantly influence the antioxidant activity (Belwal et al., 2016; Chavan and Singhal, 2013). The polynomial equation for %DPPH inhibition is presented as equation 2.
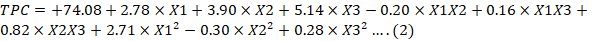
X1: Sample to solvent ratio; X2: Extraction time; X3: Solvent concentration.
The p-value (0.0420) and R2 (coefficient of determination) value of 0.9035 indicated that the model was significant.
Optimization of UAE extraction
UAE extraction conditions were optimized by using optimization function of Design expert and optimized extraction conditions (1:40 g/ml, sample to solvent ratio, 22.5 min extraction time and 80% ethanol) with desirability of 0.994 were used for TPC extraction. The optimized extract was evaluated for its bioactive potential.
Characterization of optimized extract
TPC and DPPH inhibition: TPC of optimized extract was 110.17 ± 3.44 mg of GAE/g of dried extract. Sfahlan et al. (2009) reported TPC of almond hull extract as 78.2 mg GAE/g, which was lower than the current investigation. Prgomet et al. (2019a) reported TPC of almond hull extract in the range of 91.76-138.9 mg GAE/g of extract. The reported variations in TPC depends on plant species, environmental conditions, postharvest processing, maturity of fruit and collection season (Sadiq et al., 2015). The antioxidant activity increased with concentration and the highest DPPH inhibition (87.45 ± 1.28%) was observed at 2000 µg/ml, whereas, the lowest DPPH inhibition (44.17 ± 1.91%) was observed at 31.25 µg/ml (Figure 1). Positive control (vitamin C) exhibited 95.03 ± 0.81% DPPH inhibition at 2000 µg/ml. The corresponding IC50 values for almond hull and vitamin C were 51. 64 and 180 μg/ml, respectively. Qureshi et al. (2019) reported IC50 value of almond hull extract as 167.11 and 76.04 μg/ml for 70% ethanol extract and n-butanol fraction, respectively. FRAP values of optimized almond hull extract and vitamin C were 3625.5 ± 66 and 3530 ± 96 μM of Fe (II)/g, respectively. Tlili et al. (2019) reported that almond hulls contain bio-antioxidants which can be used in food and feed industries.
Antimicrobial activity: The antimicrobial activity of almond hull extract was increased with the increase in extract concentration (Table 2). The maximum diameter of inhibition zones was 19.66 ± 0.58, 19.67 ± 0.58 and 19.33 ± 1.15 mm against E. coli, S. typhimurium and S. aureus, respectively, at the highest test concentration (50 mg/ml). MIC value of almond hull extract was 12.5 mg/ml against all test bacteria. MBC value of almond hull extract was 12.5 mg/ml for E. coli and S. aureus, whereas it was 25 mg/ml for S. typhimurium. Polyphenolic compounds present in almond hull were reported to exhibit antimicrobial potential (Prgomet et al., 2019b). Antibacterial mechanism of polyphenolic compounds is associated with their ability to form hydrogen bonding with cell membrane proteins, destruction of electron transport chain and disruption of membranes (Liaqat et al., 2019).
Table 2: Antimicrobial activity of almond hull extract.
|
Concentration |
Diameter of zone of inhibition against pathogens |
||
|
mg/ml |
Escherichia coli (mm) |
Salmonella typhimurium (mm) |
Staphylococcus aureus (mm) |
|
50 |
19.66 ± 0.58a |
19.67 ± 0.58a |
19.33 ± 1.15a |
|
25 |
18.33 ± 1.53a |
15.33 ± 1.53b |
16.33 ± 1.53a |
|
12.5 |
12.66 ± 1.15b |
13.67 ± 1.53bc |
15.33 ± 1.53ab |
|
6.25 |
10.66 ± 1.53b |
11.33 ± 2.08c |
11 ± 2.65b |
|
3.12 |
- |
- |
- |
|
1.56 |
- |
- |
- |
Where, - indicate that there was no inhibition. Different superscript letters (a-c) indicate significant differences (p < 0.05) among mean observations within columns.
FTIR analysis of almond hull extract
FTIR spectrum of extract was summarized in Table 3 and Figure 2. The peaks in the range of 2936-2913 cm-1, 1618-1498 cm-1, 1377-1233 cm-1, 900-700 cm-1 and 1040-1030 cm-1 were attributed to aliphatic compounds, aromatic compounds, carboxylic acid, aromatic hydrocarbons and aliphatic ethers, respectively (Geetha et al., 2019). The presence of several hydroxyl groups on aromatic ring provides ability to polyphenolic compounds to donate a proton to a radical and act as an antioxidant or chain breaking molecule upon secondary oxidation (Franco et al., 2008).
GCMS analysis of almond hull extract
The major phytoconstituents identified in almond hull extract were β-sitosterol (44 %), diisooctyl phthalate (12%), benzene, 1,3-bis (1,1-dimethylethly) (9.9%), phenol, 2,4-bis (1,1-dimethylethly) (8.7%), n-hexadecanoic acid (8%), 9-octadecenamide, (Z) (7%), spirost (5.20%) and lupeol (4.9%). β-sitosterol and its glucoside derivatives were reported for lowering cholesterol, cancer prevention, antimutagenic and anti-inflammatory effects (Villasenor et al., 2002). Lupeol and hexadecanoic acid were previously reported as strong antioxidants (Jiang et al., 2017; Srivastava et al., 2013).
Table 3: Assignment of FTIR peaks to the functional groups present in almond hull extract.
|
Range (cm-1) |
Group and class of compound |
Assignment and remarks |
Almond hull extract (cm-¹) |
|
2936-2913 |
CH3—CH2 In Aliphatic compounds |
CH3—CH2— Anti-symmetric stretch |
2929.7 |
|
1697 |
C=O |
—C=O stretch |
1688.5 |
|
1600-1520 |
NH3+ In NH4OH |
NH3 Deformation |
1580.4, 1524.5 |
|
1618-1498 |
Benzene ring in aromatic compounds |
C=C Aromatic ring stretch |
1580.4, 1524.5 |
|
1550-1475 |
N—O Nitro compounds |
N—O Asymmetric stretch |
1524.5 |
|
1427 |
O—H In carboxylic acid |
O—H stretch |
1440.6 |
|
1233-1377 |
C—O In carboxylic acid |
C—O stretch |
1390.3, 1284.1 |
|
1246.92 |
O—H In carboxylic acid |
O—H stretch |
1248.7 |
|
1040-1030 |
C—O—C In aliphatic ethers, Si—O In silicates |
C—O—C Asymmetric stretch, Si—O stretch |
1041.8 |
|
900-700 |
=CH In aromatic hydrocarbons |
=C—H out of plane bending |
877.79, 820.01, 765.97 |
Parvez et al. (2018) reported that phytoconstituents such as β-sitosterol and lupeol exhibit antimicrobial and antioxidant potential.
Conclusions and Recommendations
This study was aimed to optimize the conditions that provide the maximum yield of TPC and high DPPH inhibition of almond hull extract. The optimized ultrasonic assisted extraction conditions for almond hull extract were with 80% ethanol for 20 min and 1:40 solid to solvent ratio to achieve the maximum output response. Almond hulls were found good source of phenolics, exhibited high DPPH inhibition and showed considerable inhibition of food borne pathogens. Due to antioxidative and antimicrobial potential, almond hull extract can be used as a natural source of preservative for food and feed applications.
Acknowledgements
The manuscript has not been submitted or published anywhere. All authors agree contribute significantly and agree to submit manuscript to Sarhad Journal of Agriculture.
Novelty Statement
The research study highlights the importance of almond hull which is byproduct of almond processing, as a potential functional ingredient in food and feed.
Authors’ Contributions
Nabila Khan: Performed all the experiments.
Muhammad Bilal Sadiq: Supervised the research and designed the experimental plan.
Imran Ahmad: Performed data analysis and manuscript drafting.
Conflicts of interest
The authors have declared no conflict of interest.
References
Belwal, T., Dhyani, P., Bhatt, I.D., Rawal, R.S. and Pande, V. 2016. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem., 207: 115-124. https://doi.org/10.1016/j.foodchem.2016.03.081
Cacace, J.E. and Mazza, G. 2003. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng., 59(4): 379-389. https://doi.org/10.1016/S0260-8774(02)00497-1
Chavan, Y. and Singhal, R.S. 2013. Ultrasound-assisted extraction (UAE) of bioactives from arecanut (Areca catechu L.) and optimization study using response surface methodology. Innov. Food Sci. Emerg. Technol., 17: 106-113. https://doi.org/10.1016/j.ifset.2012.10.001
Dahmoune, F., Nayak, B., Moussi, K., Remini, H. and Madani, K. 2015. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem., 166: 585-595. https://doi.org/10.1016/j.foodchem.2014.06.066
Food and Agriculture Organization of the United Nations [FAO]. 2020. Corporate Statistical Database. Rome: Food and Agriculture Organization of the United Nations.
Franco, D., Sineiro, J., Rubilar, M., Sánchez, M., Jerez, M., Pinelo, M., Costoya, N. and Núñez, M.J. 2008. Polyphenols from plant materials: extraction and antioxidant power. Elec. J. Environ. Agric. Food Chem., 7(8): 3210-3216.
Geetha, N., Harini. K., Joseph. M., Sangeetha, R. and Venkatachalam, P. 2019. A Comparison of Microwave Assisted Medicinal Plant Extractions for Detection of Their Phytocompounds Through Qualitative Phytochemical and FTIR Analyses. Iran J. Sci. Technol. Trans. A Sci., 43(2): 397-407. https://doi.org/10.1007/s40995-017-0424-5
Guglielmetti, L., Jaspard, M., Le, Dû. D., Lachâtre, M., Marigot-Outtandy, D., Bernard, C., Veziris, N., Robert, J., Yazdanpanah, Y., Caumes, E. and Fréchet-Jachym, M. 2017. Long-term outcome and safety of prolonged bedaquiline treatment for multidrug-resistant tuberculosis. Eur. Respir. J., 49(3): 1601799; https://doi.org/10.1183/13993003.01799-2016
He, B., Zhang, L.L., Yue, X.Y., Liang, J., Jiang, J., Gao, X.L. and Yue, P.X. 2016. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem., 204: 70-76. https://doi.org/10.1016/j.foodchem.2016.02.094
Hiranrangsee, L., Kumaree, K.K., Sadiq, M.B. and Anal, A.K. 2016. Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by Ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. J. food Sci. Technol., 53(10): 3806-3813. https://doi.org/10.1007/s13197-016-2368-8
Iglesias-Carres, L., Mas-Capdevila, A., Sancho-Pardo, L., Bravo, F.I., Mulero, M., Muguerza, B. and Arola-Arnal, A. 2018. Optimized Extraction by Response Surface Methodology Used for the Characterization and Quantification of Phenolic Compounds in Whole Red Grapes (Vitis vinifera). Nutrients, 10(12): 1931. https://doi.org/10.3390/nu10121931
Ingawale, A.S., Sadiq, M.B., Nguyen, L.T. and Ngan, T.B. 2018. Optimization of extraction conditions and assessment of antioxidant, α-glucosidase inhibitory and antimicrobial activities of Xanthium strumarium L. fruits. Biocatal. Agric. Biotechnol., 14: 40-47. https://doi.org/10.1016/j.bcab.2018.02.004
Jiang, T., Kaal, J., Liang, J., Zhang, Y., Wei, S., Wang, D. and Green, N.W. 2017. Composition of dissolved organic matter (DOM) from periodically submerged soils in the Three Gorges Reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC–MS and thermally assisted hydrolysis and methylation. Sci. Total Environ., 603: 461-471. https://doi.org/10.1016/j.scitotenv.2017.06.114
Kahlaoui, M., Borotto, D.V.S., Giovine, F., Ben, H.K.H., Bouzouita, N., Barbosa, P.L. and Zeppa, G. 2019. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants, 8(12): 647. https://doi.org/10.3390/antiox8120647
Kubo, I., Fujita, K.I., Kubo, A., Nihei, K.I. and Ogura, T. 2004. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J. Agric. Food Chem. 52(11): 3329-3332. https://doi.org/10.1021/jf0354186
Liaqat, A., Zahoor, T., Atif, R.andhawa M. and Shahid, M. 2019. Characterization and antimicrobial potential of bioactive components of sonicated extract from garlic (Allium sativum) against foodborne pathogens. J. Food Process Preserv., 43(5): e13936. https://doi.org/10.1111/jfpp.13936
Mala, T., Sadiq, M.B. and Anal, A.K. 2021. Comparative extraction of bromelain and bioactive peptides from pineapple byproducts by ultrasonic and microwave-assisted extractions. J. Food Process Eng., 44(6): e13709. https://doi.org/10.1111/jfpe.13709
Parvez, M.K., Alam, P., Arbab, A.H., Al-Dosari, M.S., Alhowiriny, T.A., Alqasoumi, S.I. 2018. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm. J. 26(5): 685-693. https://doi.org/10.1016/j.jsps.2018.02.022
Pinelo, M., Tress, A.G., Pedersen, M., Arnous, A. and Meyer, A.S. 2007. Effect of cellulases, solvent type and particle size distribution on the extraction of chlorogenic acid and other phenols from spent coffee grounds. Am. J. Food Technol., 2(7): 641-651. https://doi.org/10.3923/ajft.2007.641.651
Piyalungka, P., Sadiq, M.B., Assavarachan, R. and Nguyen, L.T. 2019. Effects of osmotic pretreatment and frying conditions on quality and storage stability of vacuum-fried pumpkin chips. Int. J. Food Sci. Technol., 54(10): 2963-2972. https://doi.org/10.1111/ijfs.14209
Prgomet, I., Gonçalves, B., Domínguez-Perles, R., Pascual-Seva, N. and Barros, A.I. 2017. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules, 22(10): 1774. https://doi.org/10.3390/molecules22101774
Prgomet, I., Gonçalves, B., Domínguez-Perles, R., Pascual-Seva, N. and Barros, A.I. 2019a. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-products. Food Anal Methods, 12(9): 2009-2024. https://doi.org/10.1007/s12161-019-01540-5
Prgomet, I., Gonçalves, B., Dominguez-Perles, R., Santos, R., Saavedra, M.J., Aires, A., Pascual-Seva, N., Barros, A. 2019b. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly) phenols. Ind Crops Prod., 132: 186-196. https://doi.org/10.1016/j.indcrop.2019.02.024
Qureshi, M.N., Numonov, S. and Aisa, H.A. 2019. Chemical and Pharmacological Evaluation of Hulls of Prunus dulcis Nuts. Int. J. Anal. Chem. 2019. https://doi.org/10.1155/2019/5861692
Sadiq, M.B., Hanpithakpong, W., Tarning, J. and Anal, A.K. 2015. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind Crops Prod. 77: 873-882. https://doi.org/10.1016/j.indcrop.2015.09.067
Sadiq, M.B., Tarning, J., Aye, C.ho T.Z. and Anal, A.K. 2017. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. against multidrug-resistant Escherichia coli and Salmonella. Molecules, 22(1): 47. https://doi.org/10.3390/molecules22010047
Sfahlan, A.J., Mahmoodzadeh, A., Hasanzadeh, A., Heidari, R. and Jamei, R. 2009. Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chem. 115(2): 529-533. https://doi.org/10.1016/j.foodchem.2008.12.049
Simsek, M., Sumnu, G. and Sahin, S. 2012. Microwave assisted extraction of phenolic compounds from sour cherry pomace. Sep. Sci. Technol., 47(8): 1248-1254. https://doi.org/10.1080/01496395.2011.644616
Sitthiya, K., Devkota, L., Sadiq, M.B. and Anal, A.K. 2018. Extraction and characterization of proteins from banana (Musa Sapientum L.) flower and evaluation of antimicrobial activities. J. Food Sci. Technol. 55(2): 658-666. https://doi.org/10.1007/s13197-017-2975-z
Srivastava, S., Sonkar, R., Mishra, S.K., Tiwari, A., Balramnavar, V., Mir, S., Bhatia, G., Saxena, A.K., Lakshmi, V. 2013. Antidyslipidemic and antioxidant effects of novel Lupeol-derived chalcones. Lipids, 48(10): 1017-1027. https://doi.org/10.1007/s11745-013-3824-0
Taye, B., Giday, M., Animut, A. and Seid, J. 2011. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac. J. Trop. Biomed., 1(5), 370-375. https://doi.org/10.1016/S2221-1691(11)60082-8
Tlili, N., Kirkan, B. and Sarikurkcu, C. 2019. LC–ESI–MS/MS characterization, antioxidant power and inhibitory effects on α-amylase and tyrosinase of bioactive compounds from hulls of Amygdalus communis: The influence of the extracting solvents. Ind. Crops Prod., 128: 147-152. https://doi.org/10.1016/j.indcrop.2018.11.014
Villaseñor, I.M., Angelada, J., Canlas, A.P. and Echegoyen, D. 2002. Bioactivity studies on β‐sitosterol and its glucoside. Phytotherapy Research: Int. J. Devoted Pharmacol. Toxicol. Evaluat. Nat. Prod. Derivatives, 16(5): 417-421. https://doi.org/10.1002/ptr.910
Wijeratne, S.S., Abou-Zaid, M.M. and Shahidi, F. 2006. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem., 54(2): 312-318. https://doi.org/10.1021/jf051692j
Zitka, O., Sochor, J., Rop, O., Skalickova, S., Sobrova, P., Zehnalek, J., Beklova, M., Krska, B., Adam, V. and Kizek, R. 2011. Comparison of various easy-to-use procedures for extraction of phenols from apricot fruits. Molecules, 16(4): 2914-2936. https://doi.org/10.3390/molecules16042914
To share on other social networks, click on any share button. What are these?







