Performance Evaluation of Different Insecticides against Thrips (Frankliniella occidentalis) on Jasminum sambac
Research Article
Performance Evaluation of Different Insecticides against Thrips (Frankliniella occidentalis) on Jasminum sambac
Iftikhar Ahmad1, Tahir Saeed1, Umair Faheem2*, Qaisar Abbas2, Muhammad Saleem Akhtar Khan3, Mussurrat Hussain2, Tanveer Ahmad4, Gulzar Akhtar4, Asifa Hameed5, Muhammad Hasnain6 and Muhammad Jamil7
1Horticultural Research Sub-Station for Floriculture and Landscaping, Multan, Pakistan; 2Entomological Research Sub-Station, Multan, Pakistan; 3Directorate of Floriculture Training and Research, Lahore, Pakistan; 4Department of Horticulture, MNS-University of Agriculture, Multan, Pakistan; 5Mango Research Institute, Multan, Pakistan; 6Cotton Research Station, Faisalabad, Pakistan; 7Cotton Research Station, Vehari, Pakistan.
Abstract | The quality and marketing of horticultural crops is affected by the feeding of thrips on plant tissues. Thrips commonly feed upon the gladiolus and different vegetative and floral parts of gladioli are attacked by them. Application of chemical insecticides provides effective control of insect pest in short period of time. The present investigation was carried out on Jasminum sambac against flower thrips for efficacy of insecticides. To conduct this experiment, six insecticides viz., imidacloprid 20Sl, Spinosad 240SC, Spintoram 120SC, Chlophenapyre, Imidacloprid+fipronil and abamectin were applied. Thrips population was observed on jasmine flower before application of insecticides and thrips mortality data was noted after 24, 72 and 168 hours of insecticides spray. The maximum mortality of thrips was recorded in Imidacloprid+fipronil (68.45%) and Spinosad (65.45%) after 24 hours of insecticides spray. The lowest mortality of thrips was counted in abamectin and imidacloprid. Population of thrips was reduced after 72 and 168 hours as compared to 24 hours after insecticides spray. It is concluded from the findings of investigation that the insecticide (Imidacloprid+fipronil) was found effective against the population of thrips after 24, 72 and 168 hours in years, 2021 and 2022, respectively when compared to other insecticides.
Received | May 31, 2023; Accepted | October 16, 2023; Published | November 10, 2023
*Correspondence | Umair Faheem, Entomological Research Sub-Station, Multan, Pakistan; Email: [email protected]
Citation | Ahmad, I., T. Saeed, U. Faheem, Q. Abbas, M.S.A. Khan, M. Hussain, T. Ahmad, G. Akhtar, A. Hameed, M. Hasnain and M. Jamil. 2023. Performance evaluation of different insecticides against thrips (Frankliniella occidentalis) on Jasminum sambac. Sarhad Journal of Agriculture, 39(4): 861-867.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39.4.861.867
Keywords | Jasminum sambac, Insecticides, Western flower thrips, Efficacy, Dose
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Jasmine (Jasminum sambac L.) belongs to an important group of plants and it is commercially grown in different countries of the world. The jasmine belongs to family “Oleaceae” and the Genus Jasminum consists of about 200 species (Taj and Naik, 2013), which are distributed in the slightly hot areas of Europe, Asia, Africa and the Pacific region (Bhattacharjee, 1980).
Different species of jasmine are cultivated in Pakistan for the production of loose flower. J. sambac is also used for the production of essential oil in perfume industry and its oil is used in cosmetic and medicinal industry. Flowers of jasmine are used for flavoring purpose in China. Jasmine flowers are widely used in the preparation of garland, making bouquet and hair adornment on different social and religious occasions (Gao et al., 2011).
Jasmine plant is attacked by 50 insect pest species having a place within excess of eight orders harboring fluctuated microhabitats (Hemalatha, 2009).
The yield of jasmine is reduced by numerous factors but the attack of insect pests cause major and main hindrance in the quality production of jasmine flowers. The major insect pests damaging jasmine are jasmine bud worm (Hendecasis duplifascialus Hampson), leaf webworm (Nausinea geometralis Guenee), gallery worm (Elasmopalpus jasminophagus Hampson), leaf roller, blossom midge (Contarinia maculipennis Felt), (Glyphodes nionalis Hubner), and western flower thrips Frankliniella occidentalis (Pergande).
Western flower thrips is a most destructive pest of floricultural plants found globally (Mouden et al., 2017). Western flower thrips attacks on more than 250 species of plants with 60 numerous families (Tommasini and Maini, 1995). Thrips causes damage by two ways either direct or indirect way (Harrewijn et al., 1996; Pappu et al., 2009). Both adults and young one feed directly on plants by inserting their mouth-parts (Harrewijn et al., 1996; Hunter and Ullman, 1989). Symptoms of direct damage is related with vegetative and reproductive part of with silvering appearance (Childers, 1997; Cloyd, 2009). In case of indirect damage, adult act as vector for transmission of viral diseases e.g., the tospo viruses, Tomato spotted wilt virus and Impatiens necrotic spot virus (Daughtrey et al., 1997; Kirk, 2002; Pappu et al., 2009). Due to both kind of damages including direct and indirect reduce the production ornamental plants which lead to apparent economic loss (Goldbach and Peters, 1994; Reitz and Funderburk, 2012).
Western flower thrips damage is usually controlled effectively by the use of different broad-spectrum insecticides on ornamental and horticultural crops because they have less resistant against thrips (Cloyd, 2009; Mouden et al., 2017; Reitz and Funderburk, 2012). It has been recorded that the insecticide application plays a vital role in controlling the western flower thrips on Jasminum sambac.
The damage of thrips is vulnerable to ornamental as well as agricultural crops. Western flower thrips sucks the cell sap of different body parts of plants and deteriorates the shape of plants and nymphs feed by piercing plant tissues with their needle-shaped mandible and consumes the contents of damaged tissues (Kirk, 1997). The quality and marketing of horticultural crops is affected by the feeding of thrips on plant tissues (Childers, 1997). Thrips commonly feeds the gladiolus (Milevoj et al., 2008). Different vegetative and floral parts of gladioli are attacked by the thrips. Application of chemical insecticides provides effective control of insect pest in short period of time. During a study Confidor was observed to be the most deleterious against thrips and the minimum efficacy was documented in situation of Actara (Ullah et al., 2010). Confidor and Mospilan found to be the best insecticides for thrips (Aslam et al., 2004). Shivanna et al. (2011) testified that Dimethoate was best on thrips after three days of application. Uddin et al. (2019) proved that Thiodan is best followed by Curacron and Mospilan. The present studies were conducted with the objective to compare the efficacy of various insecticides available in market in order to control thrips population on Jasminum sambac.
Materials and Methods
Insecticidal trial
Field study was carried out during April, 2021 and 2022 at Horticultural Research Sub-station for Floriculture and Landscaping, Multan to determine the efficacy of six insecticides against population of thrips. The experiment was laid out under Randomized Complete Block design with three replications. Average population of thrips was taken from 25 plants in each plot. The insecticides were comprised of Imidaclopid 20Sl, Spinosad 240SC, spinetoram 120SC, Chlorfenapyre, Imidacloprid+fipronil and Abamectin. When the population of thrips reached at Economic Threshold Level (ETL) (10 thrips/flower), the insecticides were sprayed on thrips. The mortality of thrips was observed after application of insecticides. Data regarding the population of thrips were observed before spray and after spray at 24, 72 and 168 h. Percent population reduction was calculated by the following formula:
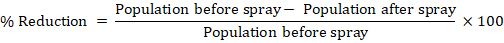
Statistical analysis
The data regarding mortality % age of thrips were analyzed by using ANOVA and treatments means were compared by Tukey’s test.
Treatments
|
Treatments |
Trade name |
Common name (formulation) |
Dose/100L |
|
T1: |
Confidor® |
Imidacloprid 20Sl |
240ml |
|
T2: |
Tracer® |
Spinosad 240SC |
50ml |
|
T3: |
Radiant® |
Spinetoram 120SC |
60ml |
|
T4: |
Dominex® |
Chlophenapyre |
120ml |
|
T5: |
Lasenta® |
Imidacloprid+fipronil |
45g |
|
T6: |
Shogun® |
Abamectin |
250ml |
|
T7: |
Control |
- |
Results and Discussion
Mortality of Thrips during 2021
Percent mortality of Thrips 24 hours after spray during 2021: The data on the efficacy of insecticides against population reduction of thrips after 24 hours of spray for the year 2021 is presented in Table 1. The maximum percentage mortality of thrips after 24 hours of insecticides application was observed in plots treated with Imidacloprid + fipronil (68.45%), Spinosad (65.45%), Spinetoram (53.40%), Chlorfenapyr (40.70%) followed by Abamectin (32.50%), and Imidacloprid (25.30%) (Figure 1).
Percent mortality of Thrips 72 hours after sp
ray during 2021: The data pertaining to performance of numerous insecticides for the control of thrips after 72 hours of spray for the year, 2021 is listed in Table 1. The highest percentage mortality of thrips after 72 hours of insecticides application was recorded in plots treated with Imidacloprid + fipronil (72.70%), Chlorfenapyr (71.20%), Spinosad (70.00%), spinetoram (58.60%) followed by Abamectin (48.20%) and Imidacloprid (24.60%). Different insecticides performed effectively against mortality of thrips (Figure 2).
Percent mortality of Thrips 168 hours after spray during 2021: The data concerning to mortality % of thrips after 168 hours of spray depicted a highly significant difference among the insecticides in 2021 (Table 1). The maximum percentage mortality of thrips after 168 hours of insecticides application was counted in Imidacloprid+fipronil (64.25%), Spinosad (63.00%), Chlorfenapyre (62.60%) and Spintoram (49.45%). Minimum mortality was recorded in abamectin (39.40%) and imidacloprid (18.00%). The population of thrips was highly affected by application of insecticides on Jasminum sambac (Figure 3).
Mortality of Thrips during 2022
Percent mortality of Thrips 24 hours after spray during 2022: The data regarding the mortality % of thrips after 24 hours of spray for the year, 2022 is presented in Table 2. Among the insecticides the maximum percentage mortality of thrips was seen
Table 1: Mean comparison of percent mortality of thrips 24, 72 and 168 hr after spraying insecticides during 2021.
|
Treatments |
Common name |
Population |
After 24 hr |
After 72 hr |
after 168 hr |
|||
|
Before spray |
24 hr |
72 hr |
168 hr |
|||||
|
T1: |
Imidacloprid 20Sl |
11.60 |
8.66 |
8.74 |
9.51 |
25.30F |
24.60F |
18.00F |
|
T2: |
Spinetoram 120SC |
10.70 |
4.98 |
4.42 |
5.41 |
53.40C |
58.60D |
49.45D |
|
T3: |
Spinosad 240SC |
13.40 |
4.62 |
4.02 |
4.95 |
65.45B |
70.00C |
63.00B |
|
T4: |
Imidacloprid+fipronil |
10.50 |
3.31 |
2.86 |
3.75 |
68.45A |
72.70A |
64.25A |
|
T5: |
Chlorfenapyre |
12.40 |
7.35 |
3.57 |
4.63 |
40.70D |
71.20B |
62.60C |
|
T6: |
Abamectin |
10.80 |
7.29 |
5.59 |
6.54 |
32.50E |
48.20E |
39.40E |
|
T:7 |
Control |
11.50 |
13.75 |
15.40 |
17.20 |
- |
- |
- |
|
LSD value@ 5% |
0.34 |
0.44 |
0.46 |
|||||
Means sharing similar letters are not significantly different by Tukey’s Test at P = 0.05
Table 2: Mean comparison of percent mortality of thrips 24, 72 and 168 hr after spray during 2022.
|
Treatments |
Common name |
Population |
After 24 hr |
After 72 hr |
After 168 hr |
|||
|
Before spray |
24 hr |
72 hr |
168 hr |
|||||
|
T1: |
Imidacloprid 20SL |
10.90 |
7.80 |
7.49 |
8.46 |
28.40F |
31.30F |
22.30F |
|
T2: |
Spinetoram 120SC |
12.50 |
5.22 |
4.68 |
5.81 |
58.20C |
62.50D |
53.45D |
|
T3: |
Spinosad 240SC |
9.90 |
3.02 |
2.52 |
3.36 |
69.50B |
74.50B |
66.00B |
|
T4: |
Imidacloprid+fipronil |
13.20 |
3.84 |
3.06 |
4.30 |
70.90A |
76.80A |
67.35A |
|
T5: |
Chlofenapyre |
10.18 |
5.43 |
2.88 |
4.03 |
49.70D |
73.30C |
62.60C |
|
T6: |
Abamectin |
12.40 |
8.69 |
6.63 |
7.87 |
29.90E |
46.50E |
36.50E |
|
T:7 |
Control |
11.90 |
12.80 |
14.60 |
16.90 |
- |
- |
- |
|
LSD value |
0.37 |
1.00 |
0.96 |
|||||
Means sharing similar letters are not significantly different by Tukey’s test at P = 0.05
with Imidacloprid+fipronil (70.90%) and Spinosad (69.50%) respectively followed by Chlorfenapyre (58.20%) and Spinetoram (49.70%). The mortality % of thrips was statistically at par in insecticides Abamectin (29.90%), and Imidacloprid (28.40 %) respectively. The population of thrips was significantly reduced in different insecticides on Jasminum sambac (Figure 4).
Percent mortality of Thrips 72 hours after spray during 2022: The data regarding mortality % of thrips for the year, 2022 after 72 hours of spray is presented in Table 2. The maximum percentage mortality of thrips was recorded in insecticides Imidacloprid+ fipronil (76.80%), Spinosad (74.50%), Chlofenapyre (73.30%), Spintoram (62.50%) followed by Abamectin (46.50%) and Imidacloprid (31.30%). All insecticides significantly decreased the population of thrips on Jasminum sambac (Figure 5).
Percent mortality of Thrips 168 hours after spray during 2022: The effect of various insecticides against mortality % thrips after 168 hours of spray for the year, 2022 is presented in Table 2. The highest mortality % of thrips was noted with Imidacloprid + fipronil (67.35%), Spinosad (66.0%), Spintoram (62.60%), Chlorfenapyre (53.45%) followed by Abamectin (36.50%) and Imidacloprid (22.30%). The mortality % of thrips was greatly influenced in all insecticides on Jasminum sambac (Figure 6).
The use of insecticides against insect pests is an effective tool to provide an immediate solution to control and seems to be most important pest management strategy in enhancing agricultural produce (Saini et al., 2010). The research trial was carried out to screen out the most suitable insecticide for the control of Western Flower Thrips on Jasminum sambac flower. Following insecticides i.e., Imidacloprid 20Sl, Spinosad 240SC, Spintoram 120SC, Chlorphenapyre, Imidacloprid+fipronil and abamectin were tested against thrips. The result indicated that maximum mortality 24 hour after application was recorded due to Imidacloprid+fipronil i.e. (68.45% and 70.90) followed by Spinosad (65.45% and 69.50), Spintoram (53.40% and 58.20) and Chlorfenapyar (40.70% and 49.70) during both years of studies in 2021 and 2022, respectively. Our results are in accordance with the study of Pandey et al. (2013) recorded that lowest thrips population by applying fipronil. The other workers also reported that fipronil and imidachlorprid reduced the thrips damage severity (Ullah et al., 2010; Gachu et al., 2012).
During 72 and 168 hours of application maximum mortality was recorded by application of Imidacloprid+Fipronil i.e., 64-76% followed by Spinosad i.e., 63-74% followed by Chlorfenapyre i.e., 62-73%. However least mortality was observed by applying abamectin i.e., 36-46% during both years of studies i.e., 2021-2022, respectively. Our results are in accordance with Jadhao et al. (2016) concluded that Spinosad 45 SC @ 0.018% against thrips was more effective and it decreased the population of thrips about (67.3%). Prasad and Ahmad (2009) studied that population of Scirtothrips dorsalis was effectively inhibited by Spinosad. Meena and Raju (2014) examined that thrips population was reduced to a greater extent by application of fipronil 5% SC on chilli. Sumitha et al. (2008) investigated that population of Scirtothrips dorsalis was controlled effectively by fipronil 5 SC @ 0.01%. Ravikumar et al. (2016) observed that maximum yield (30050 kg ha-1) was recorded by spray of Spinosad 45 SC @ 0.01 % against thrips.
Conclusions and Recommendations
From the findings of present studies, it is concluded that Imidacloprid + Fipronil and Spinosad insecticides are highly effective against Jasminum sambac thrips as compared to other insecticides. These insecticides can be recommended to the growers to manage the thrips population below economic threshold level on Jasminum sambac.
Acknowledgements
This research was funded from regular funds of research provided by Department of Agriculture, Government of Punjab, Pakistan. Authors are thankful to Entomological Research Sub Station, Multan for their technical support.
Novelty Statement
Studies were carried out to find out effective insecticides against flower thrips on jasmine to recommend the farmers of jasmine crop.
Author’s Contribution
Iftikhar Ahmad: Prepared initial draft, worked in field collected data and prepared initial manuscript.
Tahir Saeed: Critically reviewed the manuscript, analyzed the data and prepared graphs.
Qaisar Abbas: Conceived idea and designed studies.
Umair Faheem: Critically reviewed the manuscript and plotted graphs.
Muhammad Saleem Akhtar Khan, Mussurrat Hussain, Tanveer Ahmad, Gulzar Akhtar, Asifa Hameed and Muhammad Jamil: worked in field and collected data. All authors have read and approved the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Aslam, M., M. Razaq, S.A. Shah and F. Ahmad. 2004. Comparative efficacy of different insecticides against sucking pests of pulses. J. Res. Sci., 15(1): 53-58.
Bhattacharjee, S.K., 1980. Native Jasmine of India. Indian Perfumer, 24(3): 126-133.
Cathey, M.H., 1980. Phosphon and CCC for controlling height of chrysanthemum. Floriculture Ex., 135: 12-13.
Childers, C.C., 1997. Feeding and oviposition injuries to plants. Thrips as Crop Pests. Lewis, Tedn, CAB International, New York, pp. 505-537.
Childers, C.C., 1997. Feeding and oviposition in-juries to plants, In: T. Lewis (ed.). pp. 505–537.
Cloyd, R.A., 2009. Western flower thrips (Frank-liniella occidentalis) management on ornamen-tal crops grown in greenhouses: Have we reached an impasse? Pest Tech., 3: 1-9.
Daughtrey, M.L., R.K. Jones, J.W., Moyer, M.E. Daub and J.R. Baker. 1997. Tospo viruses strike the greenhouse industry: INSV has become a major pathogen on flower crops. Plant Dis., 81: 1220-1230. https://doi.org/10.1094/PDIS.1997.81.11.1220
Gachu, S.M., J.W. Muthomi, R.D. Narla, J.H. Nderitu, F.M. Olubayo and J.M. Wagacha. 2012. Management of thrips (Thrips tabaci) in bulb onion by use of vegetable intercrops. Int. J. Agric. Sci., 2: 393-402.
Gao, Y., N. Hu, X.Y. Han, T. Ding, C. Giffen, A.M. Goldstein and P.R. Taylor. 2011. Risk factors for esophageal and gastric cancers in Shanxi Province. China: A case-control study. Cancer Epidemiol., 35(6): 91-99. https://doi.org/10.1016/j.canep.2011.06.006
Goldbach, R. and D. Peters. 1994. Possible causes of the emergence of tospo virus diseases. Semin. Virol., 5: 113-120. https://doi.org/10.1006/smvy.1994.1012
Harrewijn, P., W.F. Tjallingii and C. Mollema. 1996. Electrical recording of plant penetration by western flower thrips. Entomol. Exp. Appl., 79: 345-353. https://doi.org/10.1111/j.1570-7458.1996.tb00842.x
Hemalatha, G., 2009. Biorational management of key pests of Jasmine (Jasminum sambac). College of Horticulture, Vellanikkara.
Hunter, W.B. and D.E. Ullman. 1989. Analysis of mouthpart movements during feeding of Frankliniella occidentalis (Pergande) and F. schultzei (Trybom) (Thysanoptera: Thripi-dae). Int. J. Insect Morphol. Embryol., 18: 161-171. https://doi.org/10.1016/0020-7322(89)90024-X
Jadhao, A.V., S.K. Patil and S.R. Kulkarni. 2016. Evaluation of insecticides against Chilli thrips, Scirtothrips dorsalis (Hood). Ann. Plant Prot. Sci., 1: 27-30.
Kirk, W.D.J., 1997. Distribution, abundance, and population dynamics. Thrips as crop pests (Ed. Lewis T), CAB International, Wallingford, UK. pp. 217-258.
Kirk, W.D.J., 2002. The pest of the west: Frank-liniella occidentalis. Thrips and Tospoviruses: Proc. 7th Intl. Symp. Thysanoptera, 2: 33-42.
Meena, L.K. and S.V.S. Raju. 2014. Bio-efficacy of insecticides against whitefly and leaf hopper of tomato under field conditions. Ann. Plant Prot. Sci., 22: 14-17.
Milevoj, L., M. Zdesar and S. Trdan. 2008. Susceptibility to gladiolus thrips (Thrips simplex [Morison]) in four different coloured gladiolus cultivars. Acta Phytopathol. Hun., 43(2): 323-327. https://doi.org/10.1556/APhyt.43.2008.2.16
Mouden, S., K.F. Sarmiento, P.G. Klinkhamer and K.A. Leiss. 2017. Integrated pest management in western flower thrips: Past, present and future. Pest Manage. Sci., 73: 813-822. https://doi.org/10.1002/ps.4531
Mouden, S., K.F. Sarmiento, P.G. Klinkhamer and K.A. Leiss. 2017. Integrated pest management in western flower thrips: Past, present and future. Pest Manag. Sci., 73: 813-822. https://doi.org/10.1002/ps.4531
Pandey, S., B.K. Singh and R.P. Gupta. 2013. Effect of neem based botanicals, chemicals and bio-insecticides for the management of thrips in onion. Indian J. Agric. Res., 47: 545-548.
Pappu, H., R.A.C. Jones and R.K. Jain. 2009. Global status of tospovirus epidemics in di-verse cropping systems: Successes achieved and challenges ahead. Virus Res., 141: 219-236. https://doi.org/10.1016/j.virusres.2009.01.009
Prasad, N. and A. Khalid. 2009. Efficacy of Spinosad 45 SC against thrips Scirtothrips dorsalis (Hood) and pod borer, Spodoptera exigua (Hubner) on Chillies. Pestic. Res. J., 21(1): 49-51.
Ravikumar, A., C. Chinniah, S. Manisegaran, S. Irulandi and P. Mohanraj. 2016. Effect of biorationals against the thrips, Scirtothrips dorsalis (Hood) infesting chilli. Int. J. Plant Prot., 9(1): 158-161. https://doi.org/10.15740/HAS/IJPP/9.1/158-161
Reitz, S.R., 2009. Biology and ecology of western flower thrips (Thysanoptera: Thripidae): The making of a pest. Fla. Entomol., 92: 7-13. https://doi.org/10.1653/024.092.0102
Reitz, S.R. and J. Funderburk. 2012. Management strategies for western flower thrips and the role of insecticides. In: F. Perveen (ed.). Agric. Biol. Sci. Insecticides-Pest Engineering. InTech, Rijeka, Croatia. pp. 355–384.
Saini, R.K., Y. Kumar and K.K. Dahiya. 2010. Bioefficacy of tolfenpyrad (PII 405 15% EC) against cotton leaf hopper, Amrasca biguttula biguttula (Ishida) on cotton. In: Proceedings of National Conference on Plant protection in agriculture through eco-friendly techniques and traditional farming practices. February 18-20, 2010 held at ARS, Durgapura., 215.
Shivanna, B.K., B.N. Gangadhara, R. Nagaraja, M.K. Basavaraja, K. Swamy and C. Karegowda. 2011. Bio efficacy of new insecticides against sucking insect pests of transgenic peas. Int. J. Sci. Nat., 2(1): 79-83.
Sumitha, N.D., S.B. Jagginavar, D.R. Patil, D.N. Kambrekar. 2008. Management of thrips complex in grape ecosystem. Ann. Plant Prot. Sci., 16: 83-86.
Taj, A. and B.H. Naik. 2013. Performance of gerbera genotypes under protected cultivation. Indian society of ornamental horticulture. 16: (3 and 4), 164. Thrips as crop pests. CAB International, New York.
Tommasini, M. and S. Maini. 1995. Frankliniella occidentalisand other thrips harmful to vegetable and ornamental crops in Europe. Wageningen Agric. Univ. Pap., 95: 1–42.
Uddin, A., M. Yousuf, M. Khan, K. Ahmed, A.G. Khoso, S. Ahmed and Z.U. Haq. 2019. Efficacy of different insecticides against onion thrip, Thrips tabaci in Awaran District. Int. J. Acata Multidis. Res., 3(6): 14-17.
Ullah, F., U.M. Maraj, F. Abid, Q.S. Muhammad and S. Shahid. 2010. Population dynamics and chemical control of onion Thrips (Thrips tabaci, Lindemann). Pak. J. Zool., 42(4): 401–406.
To share on other social networks, click on any share button. What are these?







