Plant Taste Affects Diet Selection by Plateau Zokors (Eospalax baileyi)
Plant Taste Affects Diet Selection by Plateau Zokors (Eospalax baileyi)
Shou-Dong Zhang1,2,3, Dao-Xin Liu1,2, Dan Mou1, Tong-Zuo Zhang2, Jian-Ping Su2 and Jiu-Xiang Xie1*
1State Key Laboratory of Plateau Ecology and Agriculture, College of Agriculture and Animal Husbandry, Qinghai University, Xining, 810016, China
2Key Laboratory of Evolution and Adaptation of Plateau Biota, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining, 810008, China
3Ministry of Education Key Laboratory for Biodiversity Science and Ecological Engineering, Coastal Ecosystems Research Station of the Yangtze River Estuary, Shanghai Institute of Eco-Chongming, Fudan University, Shanghai, 200433, China
ABSTRACT
Diet selection can be influenced by many factors and is crucial for survival. In this study, we measured the diet selection (Ei index) of plateau zokors (Eospalax baileyi) by comparing the composition of plant species present in overwinter caches and inside nearby quadrats of zokor burrow systems on the Qinghai-Tibet Plateau. Based on human volunteer taste-testing, or assignment using the Chinese Materia Medica Monographs, we divided the collected plant species into four different taste groups (sweet, bitter, other-taste, and tasteless) and then compared Ei values to determine whether plant taste can affect zokor diet selection. A total of 124 burrow systems were analyzed and 103 plants were used in the analyses. Kruskal-Wallis tests revealed statistically significant deviations (from the mean) in Ei values among the four taste groups (χ2 = 199.033, df =3, p = 0.000). Pairwise Mann-Whitney U tests showed that, at the p = 0.05 or higher significance level, the Ei values of the four taste groups could be ranked as: sweet > bitter = other-taste > tasteless. Using bootstrapping analyses, we detected ten sweet and five bitter plants that were positively selected by zokors (p < 0.05). Taken together, these findings indicate that plant taste plays an important role in the dietary choices of plateau zokors. Moreover, our results suggest that there were obvious trade-offs between choosing to gain nutrients and avoiding disadvantageous secondary plant metabolites.
Article Information
Received 22 January 2019
Revised 13 May 2020
Accepted 28 September 2020
Available online 21 February 2022
(early access)
Published 23 September 2022
Authors’ Contribution
JXX and SDZ conceived and designed the experiments. SDZ, JXX, DXL, DM and TZZ performed the experiments. SDZ analyzed the data. JXX and TZZ contributed reagents/materials/analysis tools. SDZ and JXX wrote the paper.
Key words
Overwinter cache, Diet selection, Taste, Plant secondary metabolites, Eospalax baileyi
DOI: https://dx.doi.org/10.17582/journal.pjz/20190122140145
* Corresponding author: [email protected]
0030-9923/2022/0006-2973 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Diet selection underlies all aspects of the ecology of animal species (Hughes, 1993). In herbivores, the relationship between plant nutrient content and preference is believed to be the major behavioral mechanism that leads to diet choice. How herbivores overcome plant defenses to gain access to their nutrients has been the subject of significant research attention (Ginane et al., 2005). Herbivores are thought to learn about the nutrient and toxic properties of food plants by associating sensory characteristics (e.g. taste and smell) with post-ingestive consequences (Provenza, 1995), and this is considered to be an important mechanism influencing diet selection (Provenza and Balph, 1990). Many experiments have shown that the taste of sweet or umami is related to a high content of sugar and protein, while a bitter taste is related to the presence of toxins (Nelson and Sanregret, 1997). As such, the preference for a food item is often related to its hedonic taste perception. The intake of food items with sweet or umami tastes can increase after their infusion with sources of energy or protein (Villalba and Provenza, 1997a, b), or decrease after their infusion with toxins (Provenza, 1995, 1996). However, such research has mainly been conducted on cattle, mice and humans. Little information is available for wild small mammals, especially subterranean rodents.
The plateau zokor (Rodentia, Spalacidae, Eospalax baileyi) is a typical subterranean rodent species. Its subterranean life style has three characteristics: high energy budget, burrow-limited elbow room, and seasonal limited foraging time. Limited by these three characteristics, subterranean rodents must collect all relevant food species to survive (Heth et al., 1989). While some studies suggest that subterranean rodents are dietary generalists, examples of selective foraging have also been documented (Xie et al., 2014). Indeed, our previous studies have shown that plateau zokors demonstrate obvious diet selection when collecting overwintering food materials. However, the behavioral mechanisms leading to diet choice are not clear.
In this study, we collected plant species from overwinter caches and in nearby quadrats of the burrow systems of plateau zokors and measured their composition. We then measured taste and calculated the food selection indices of each plant species, and subsequently tested the relationship between diet selection and plant taste.
Materials and methods
The study was conducted in Datong County, Qinghai, China (37°6’ N, 101°36’ E, elevation 3,025m above sea level). During October 1st-20th 2012, we excavated zokor caches and collected all food items in each of the burrow systems. Caches collected in the same burrow system were merged. In addition, along each burrow system, three 50cm×50cm quadrats were sampled. The three-quadrat samples were in locations where zokors had recently foraged. Plants, along with the soil from each quadrat, were dug up and packed into fiber bags. All plants were washed and air-dried. Plant samples obtained from the three quadrats of the same burrow system were merged. Each plant was identified according to Flora Qinghaiica (Liu et al., 1997), dried at 60ºC, and weighed using an analytical balance with a readability of 0.01 g. The biomass of each plant species sampled from the quadrats of a burrow system was referred to as ‘available plants in the vicinity’, and the total biomass cached in the same burrow system was referred to as selected plants. In order to derive more robust conclusions, we also included the data and plant materials collected from another 57 burrowing systems obtained from Menyuan County, Qinghai, China (37°30’N, 101°13’E, elevation 3200m above sea level) during our previous studies (which were conducted in another location but with the same collection processes) (Xie et al., 2014).
The taste of most of the plants collected was determined according to Chinese Materia Medica Monographs (China Pharmacopoeia Committee, 2000). For those plant species not recorded in the monographs, their taste was determined by a taste test: each plant was independently tasted by five healthy human volunteer subjects without a history of smoking or alcohol abuse, and the taste with the highest frequency was termed as the “true” taste. Tastes were classified into 4 groups: sweet, bitter, other-tastes (including pungent taste, sour taste, and other difficult tastes to describe) and tasteless.
As in our previous studies (Xie et al., 2014), we applied Ivlev’s electivity index (Ei) (Ivlev, 1961) to measure diet selection. Ei was defined as:
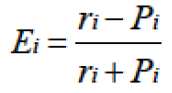
Where ri is the relative abundance of a plant species in a zokor’s diet and Pi is the plant species’ relative abundance in the burrow system. According to Ivlev’s selection index, Ei ranges from -1 to +1, and Ei in the interval of -1 to 0 means negative selection, while Ei in the interval of 0 to +1 means positive selection.
In order to test the selection status of each plant species based on Ei, we performed bootstrap descriptive statistics, with 1000 bootstrap replications, and used 90% confidence intervals to distinguish the preferred food items from the avoided food items, based on Ei. Using this method, if the lower limit of the 90% confidence interval of a given plant’s Ei is greater than 0, this species is recognized as positively selected dietary material at a 5% statistical significance level (one-tailed test). Similarly, if the upper limit of the 90% confidence interval of a given plant’s Ei is less than 0, this species is recognized as negatively selected dietary material at a 5% statistical significance level. Other species with a 90% confidence interval of Ei passed through the line Ei = 0 and were referred to as diets lacking a definite preference by zokors.
We first tested the normality of the data using the One-Sample Kolmogorov-Smirnov Test and found that the dependent variable (Ei) data significantly deviated from normality (Kolmogorov-Smirnov Z = 13.085, N = 3,019, p = 0.000). Therefore, we used non-parametric statistical methods for our analyses. We then divided the sampled plants into four groups according to the taste of each plant: sweet, bitter, other-taste, and tasteless. Kruskal-Wallis tests were used to show if there were significant universal deviations of Ei values from the mean value among the four taste groups. Finally, the Mann-Whitney U Test was applied to compare the pairwise difference between each set of two groups. All statistical analyses were executed in SPSS 20.0.
Results and discussion
A total of 67 burrow systems were found to have been newly excavated and caches from these were collected. In addition to the 57 samples formerly collected in our previous studies, there were therefore a total of 124 burrow systems to be analyzed. We found a total of 103 different plant species in the caches and vicinity, 89 of which were found in the caches alone. Bootstrap descriptive statistics based on a total of 70 plant species (33 plant species having been excluded from testing because their sample sizes were n < 10), revealed 15 positively selected and 37 negatively selected plants. A further 18 lacked a definite preference. About 1/3 of the 31 sweet plants and 1/5 of the 26 bitter plants were positively selected, while none of the 6 other-taste plants nor any of the tasteless plants were positively selected (Fig. 1 and Supplementary Table S1).
Of the original 103 plants, 38, 43, 13, and 9 were determined to be sweet, bitter, other-taste and tasteless, respectively. The Kruskal-Wallis Test revealed statistically significant universal deviations of Ei values from the mean among the four taste groups (χ2 = 199.033, df = 3, p = 0.000). The sweet group had the largest mean rank, followed by other-taste, and bitter (these two groups had a similar mean rank value), while the tasteless group had the smallest mean rank (Table I). The pairwise Mann-Whitney U Test showed that, at the p = 0.05 or higher significance level, there were statistically significant differences between each two-paired comparison group, except for the comparison between the bitter and other-taste groups where there was no significant difference (Table II). Taken together, the Ei values of the four groups could be ranked as sweet > bitter = other-taste > tasteless.
Table I. Kruskal-wallis test comparing Ei values among the taste groups.
|
Taste groups |
N |
Mean rank |
|
Sweet |
1282 |
1712.81 |
|
Bitter |
1067 |
1454.66 |
|
Other-taste |
189 |
1511.32 |
|
Tasteless |
481 |
1091.70 |
|
Total |
3019 |
χ2 =199.033, p= 0.000 |
Our findings indicate that plant taste plays an important role in the diet selection of plateau zokors. Zokors preferred sweet plants, followed by bitter or other-taste plants, and rejected plants in the tasteless group. As a general rule, molecules that serve as energy carriers, such as sugars, are perceived as sweet by humans and tend to be favored by both humans and animals (Pfaffman, 1975). Many experiments have shown that the taste of sweet or umami is related to a high content of sugar and protein (Nelson and Sanregret, 1997). Meanwhile, sweet plants are excellent forage (Guo et al., 1987) are reported to be nutritious and to tend to be innocuous or have low toxicity (Wang, 1998). As a typical subterranean rodent, the majority of the plateau zokors’ activities (foraging, migration, mating, etc.) are often accompanied by a large number of excavation activities. Digging for food and shelter is an energetically demanding process, utilizing more energy than that required by above ground animals (Vleck, 1979). Therefore, it is not surprising that sweet plants containing high-energy nutrients are preferred by zokors.
Table II. Mann-whitney test comparing pairwise difference between taste groups.
|
Pairwise comparison |
Z value |
p value |
|
Sweet vs. bitter |
-6.947 |
0.000 |
|
Sweet vs. other-taste |
-2.911 |
0.004 |
|
Sweet vs. tasteless |
-14.380 |
0.000 |
|
Bitter vs. other-taste |
-0.805 |
0.421 |
|
Bitter vs. tasteless |
-7.163 |
0.000 |
|
Other-taste vs. tasteless |
-5.803 |
0.000 |
Interestingly, zokors preferred bitter and other-taste plants to tasteless plants. Previous studies have shown that a bitter taste is generally related to the presence of toxins (Nelson and Sanregret, 1997). Most of the bitter or pungent and sour plant species tend to be unnourishing, fibrous or lignified, or poisonous (Galef, 1996). However, in this study, many bitter and other-taste plants were not rejected by zokors. In fact, five bitter plants were positively selected. For example, Stellera chamaejasme, an important poisonous plant of the alpine meadow habitat (Shi, 1997) that causes cattle to vomit, scour, or even die (Song et al., 2008), was found (through bootstrap analysis) to be the favorite food item among the 26 bitter tasting plants. Polygonum viviparum, which is high in phenols and tannins (Zhang et al., 2008) that reduce nutritional digestibility (Foley et al.,1999), was also found to be positively selected by zokors. In contrast, Gramineae plants, which are foraged by most herbivores (e.g. ungulates, rabbits, etc.), were found to be largely rejected by zokors in our study. It should be mentioned that a few of the bitter or other-taste plants, such as P. viviparum and S. chamaejasme, have thick and succulent roots, indicating that they are more nutritious and more easily collected and stored. However, all the tasteless plants are slender and shriveled with a high content of cellulose and lignin, indicating that they contain little energy and are not easily collected and stored. Hence, we hypothesize that zokors select diets based on trade-offs between the nutritional quality and the avoidance of disadvantageous secondary plant metabolites. Of note, previous studies have shown that zokors have a considerable ability to deal with toxic (terpenes) and digestion-reducing (tannins) secondary metabolites (Lin et al., 2012).
It should also be mentioned that zokors are blind. They collect food by digging in dark underground burrows and therefore (in addition to plant odor) taste must be an important cue for food recognition. Tasteless plants give weak flavor profiles and may be difficult to distinguish from inorganic substances, which would help explain why plateau zokor almost totally rejected tasteless plants in this study. It should be noted that in the current study the different plant tastes were identified by humans, not by the zokor themselves (i.e. the “human tastes” may not be equal to the “zokor tastes”). Whilst we may not ever know which tastes are truly recognized by zokors, there are many similarities between the taste systems of humans and other mammals. Most mammals have relatively conserved systems of the five basic tastes: sweet, salty, sour, bitter, and umami (Li and Zhang, 2014). For instance, rats detect similar taste dimensions compared to humans (Burn, 2008). Moreover, although there are considerable differences in bitter plant selection among mammals, a recent study from our laboratory has revealed that there are approximately 26 intact bitter taste receptor (tas2r) genes in plateau zokor (unpublished) that are very similar the 25 intact tas2r genes found in humans (Li and Zhang, 2014). We therefore suggest that the effect of “human taste” on zokor diet selection in this study mimics to some degree the real relationships between “zokor tastes” and zokor diet selection.
Acknowledgments
We thank Gary Brierley for his help in improving the English of this manuscript. We thank Wei-ping Li, Wei-You Ou, Yun-Chen, Li-Ni Su, Yue Zheng, Wen-Li Quan, and Na Hu for their help in collecting and separating the plant species and the testing of plant tastes. This study was financially supported by the National Natural Science Foundation of China (31760622).
There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.pjz/20190122140145
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Burn, C.C., 2008. Appl. Anim. Behav. Sci., 112: 1-32. https://doi.org/10.1182/blood.V112.11.sci-32.sci-32
China Pharmacopoeia Committee., 2000. China Chemical Industry Press, Beijing.
Foley, J.W., Iason, G.R. and Mcarthur, C., 1999. In: Nutritional ecology of herbivores (eds. H.G. Jung and G.C. Fahey). American Society of Animal Science, Illinois. pp. 130-209.
Galef, B.G., 1996. Neur. Biobehav. Rev., 20: 67-73. https://doi.org/10.1016/0149-7634(95)00041-C
Ginane, C., Duncan, A.J., Young, S.A., Elston, D.A. and Gordon, I.J., 2005. Anim. Behav., 69: 541-550. https://doi.org/10.1016/j.anbehav.2004.06.008
Guo, B.Z., Zhang, H.Z., Pan, J.T., Yang, Y.C., Wu, Z.L. and He, T.N., 1987. Flora of Qinghai economic plants. The Qinghai People Press, Xining.
Heth, G., Golenberg, E.M. and Nevo, E., 1989. Oecologia., 79: 496-505. https://doi.org/10.1007/BF00378667
Hughes, R.N., 1993. Diet selection. Blackwell Scientific, Oxford.
Ivlev, V.S., 1961. Experimental ecology of the feeding of fishes. Yale University Press, New Haven.
Li, D. and Zhang, J., 2014. Mol. Biol. Evol., 31: 303-309. https://doi.org/10.1093/molbev/mst219
Lin, G.H., Xie, J.X., Cui, X.F., Nevo, E., Su, J.P. and Zhang, T.Z., 2012. Ann. Zool. Fenn., 49: 371-377.
Liu, S.W., Lu, S.L., Wu, Z., He, T.N., Zhou, L., Huang, R.F. and Pan, J., 1997. Flora Qinghaiica. Qinghai People’s Press, Xining.
Nelson, S.L. and Sanregret, J.D., 1997. Chem. Senses, 22: 129-132. https://doi.org/10.1093/chemse/22.2.129
Pfaffman, C., 1975. In: Olfaction and taste (eds. D.A. Denton and J.P. Coghlan). Academic Press, NY.
Provenza, F.D. and Balph, D.F., 1990. In: Behavioural mechanisms of food selection (ed. R.N. Hughes) Springer-Verlag, Berlin. pp. 423-459. https://doi.org/10.1007/978-3-642-75118-9_22
Provenza, F.D., 1995. J. Range. Manage., 48: 2-17. https://doi.org/10.2307/4002498
Provenza, F.D., 1996. J. Anim. Sci., 74: 2010-2020. https://doi.org/10.2527/1996.7482010x
Shi, Z., 1997. Important poisonous plants of China Grassland. Chinese Agriculture Press, Beijing.
Song, R., Hasagawa, N., Li, G., Xu, N., Cai, G. and Zhang, Q., 2008. J. Domest. Anim. Ecol., 29: 31-35.
Villalba, J.J. and Provenza, F.D., 1997a. Br. J. Nutr., 77: 287-297. https://doi.org/10.1079/BJN19970030
Villalba, J.J. and Provenza, F.D., 1997b. Br. J. Nutr., 78: 545-561. https://doi.org/10.1079/BJN19970174
Vleck, D., 1979. Physiol. Zool., 52: 122-135.
Wang, K.,1998. J. Qingh. U (Nat. Sci.), 16: 5-6.
Xie, J.X., Lin, G.H., Zhang, T.Z. and Su, J.P., 2014. Pol. J. Ecol., 62: 173-182. https://doi.org/10.3161/104.062.0116
Zhang, X., Long, R., Dan, R. and Ding, X., 2008. J. Gansu. Agric. U., 1: 126-129.
To share on other social networks, click on any share button. What are these?









