Population Structure and Habitat Utilization of Migratory Birds at Bakhira Bird Sanctuary, Uttar Pradesh, India
Population Structure and Habitat Utilization of Migratory Birds at Bakhira Bird Sanctuary, Uttar Pradesh, India
Himanshu Mishra, Vikas Kumar and Ashish Kumar*
Animal Diversity and Ecology Laboratory, Department of Zoology, University of Lucknow, Lucknow-226007 U.P., India
ABSTRACT
An attempt has been made for the very first time to study the population structure and habitat utilization of migratory birds at Bakhira bird sanctuary, district Sant Kabir Nagar, Uttar Pradesh, India from October 2017 to March 2018. Thirty two species of birds were observed during the field investigation. The line transect method was employed for population estimates. During the field survey, we recorded a significantly higher number of migratory birds at the end of early winter (December) and at the commencement of middle winter (January). Red crested pochard (Netta rufina), Common coot (Fulica atra) and Gadwall (Mareca strepera) were the most populated species in the present study. The number of birds was not significantly different among winter months except between October/December and October/January wherein, we found significant variation in the number of birds at the Bakhira tal. Of the five main habitat types viz. lowland vegetation, upland vegetation, emergent vegetation, open water and agricultural fields, the most utilized were lowland vegetation and the emergent vegetation in the early and middle wintering stage. While in late winter, the emergent vegetation was the most utilized habitat. The findings of the present study provide the baseline information about the population of migratory birds and the rate of habitat utilization at the Bakhira bird sanctuary.
Article Information
Received 20 November 2018
Revised 11 May 2019
Accepted 20 June 2019
Available online 30 October 2019
Authors’ Contribution
AK planned this research. HM and VK performed field surveys, bird identification and population census. HM analysed the data and wrote the article.
Key words
Bakhira bird sanctuary, Habitat utilization, Migratory birds, Population structure, Wetland
DOI: https://dx.doi.org/10.17582/journal.pjz/2020.52.1.247.254
* Corresponding author: [email protected]
0030-9923/2020/0001-0247 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INETRODUCTION
Wetlands can be broadly defined as intermediary land between terrestrial and aquatic ecosystems, where the water table is typically at or near the surface or the land is enclosed by shallow water (Mitsch and Gosselink, 1986). Wetlands cover an area of 58.2 million hectares in India (Prasad et al., 2002). Of the 9702 bird species reported worldwide (Sibley and Monroe, 1990), 1313 species have been recorded from the Indian subcontinent (Grimmett et al., 2011). India has more than 1200 species of birds which is over 13% of the world’s avifauna (Kumar et al., 2005). According to Kumar et al. (2005), 310 species are known to depend particularly on wetlands, of which 107 species are winter migrants. Among the, migratory birds waterfowl are one of the main components of global biodiversity (Li and Mundkur, 2004). Many important migratory bird species visit India from October to March (Rahmani et al., 1997). Wetlands support the highest number of water birds during the winter. Wetlands are considered important conservation sites due to the extensive food chain and rich biodiversity they hold (Getzner, 2002).
Migration is a common phenomenon in eukaryotes. It is the consequence of complex interaction between intrinsic (genetic, physiological and behavioural) and extrinsic factors (weather, habitat condition, food availability, predation, topography (Akesson and Hedenstrom, 2007). Migration in birds is an inclination that has fascinated researchers and naturalists all over the world. The identification of effects and timing of limiting factors for migratory birds is rather difficult, because of the momentary interconnected nature of migration conditions throughout the annual cycle (Sillett and Holmes, 2002).
Habitat use is defined as the way in which an individual or species uses the habitat to fulfill the life history needs (Jones, 2001). Three factors are important in habitat selection by birds viz. food availability, protection from predators and constraints imposed by morphological characteristics (Hilden, 1965). In recent years, due to the over-exploitation of resources, wetland deprivation has become a serious issue that has resulted in habitat shrinkage for migratory water birds. According to a group of researchers food resource utilization is regarded as the interspecific competition among wintering waterbirds, that may be intensified by food shortages (Xiang and Wang, 2005; Wang et al., 2011). Food, space and other resources are extremely limited in degraded wetlands, particularly during cold and severe winters. The competitive intensity of waterfowl generally peaks at this time (Oksanen, 1987).
The review of literature suggests that so far, no research has been carried out on population structure and habitat utilization of migratory birds at Bakhira bird sanctuary, district Sant Kabir Nagar, Uttar Pradesh, India. Therefore, the present study was undertaken to collate comprehensive information about the population structure and habitat utilization of migratory birds in the study area. In India some researchers have reported pattern of habitat utilization in wetland birds particularly in Kerala and Chhattisgarh (Sharma et al., 2014; Roshnath and Shruthi, 2015).
MATERIALS AND METHODS
Study area
Bakhira bird sanctuary also known as Bakhira Tal (N 26°54’ E 83°06’) located to the west of the Rapti river bank, is a shallow-water, river-connecting wetland, declared a bird sanctuary in 1990 by the Forest and Wild life Department, Uttar Pradesh, India vide order number 822/14-3-60/1989, dated 14/5/1990 (Fig. 1a). The google image of Bakhira bird sanctuary has been placed in Figure 1b while Figure 1c shows the representative image. It is part of the natural floodplain in Uttar Pradesh, expanding over an area of 29 km2. The landscape and terrain of the wetland are approximately flat having an average elevation of 100 meters above mean sea level representing a typical terai landscape. The vegetation in the wetland mainly consists of Typha angustifolia, Phragmites karka, Eichhornia crassipes, Hydrilla verticillata, Vallisneria spiralis and Lemna minor (Mishra and Narain, 2010). It facilitates the wintering and staging ground for many migratory waterfowl and breeding ground for resident (and summer migrating) birds (Mishra et al., 2016). We identified five habitat types viz upland vegetation, lowland vegetation, emergent vegetation, open water and agricultural fields. An exhaustive study was carried out from October 2017 to March 2018 to determine the population structure and habitat utilization of migratory birds.
The wintering season was divided into three stages according to hydrological variations in the wetland as follows: (1) The early phase of wintering season from October to late December. During this period, the wetland is still at a high water but starts to retreat; (2) The middle stage from early January to late February in the year, when the water level drops and large areas of the wetland shore become exposed; (C) The late phase from late February to the end of March, in which the wetland shore becomes drier and the water level begins to decline.
Population survey
Birds were counted using 15-minute line transect count (3 observations/hour×3 hour per day ×4 days/ month
with the settlement period of 5 minutes between 2 observation sessions) in which we traversed a line and reordered birds as the target objects method (Burnham et al., 1980). During each visit birds were counted three times from 6.0-7.0 am, 11.0 am-12.0 hours and 4.0-5.0 pm respectively. A total of 216 (36/month×6) observations were recorded to estimate mean number of migratory birds.
Migratory bird survey was conducted randomly at 5-6 day intervals in a month. A total of 36 surveys (6 surveys per month) were undertaken during the study period. The counting of migratory birds was done by 5-6 main observers to avoid double counting. The bird identification was carried out with the aid of key reference books Ali (2002); Grewal et al. (2002) and Grimmett et al. (2007).
Habitat utilization
The habitat utilization rates (U) of all habitat types by these migratory birds was calculated as per Zhao et al. (2013).
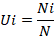
Where Ui is the utilization rate of the ith habitat type by water birds; Ni the number of water birds in the ith habitat type and N the total number of water birds in all habitat types.
Statistical analysis
Population structure of migratory birds was analyzed by Analysis of Variance (One way ANOVA) followed by Tukey´s post hoc test. All the statistical analyses were carried out using PAST (version 3.12) and Graph Pad Prism (version 5.01).
RESULTS
Population structure
Population status of migratory birds was significantly (p=0.030) high at the end of early winter (December) and commencement of middle winter (January) (Table Ι). Red crested pochard (Netta rufina) (46.5±9.79), Common coot (Fulica atra) (45.1±8.42) and Gadwall (Mareca strepera) (35.6±9.13) outnumbered other species (Table Ι). Tukey´s post hoc test showed that there was no significant difference (p>0.05) in the number of birds among winter months (Table ΙΙ) although significant variation (p<0.05) was apparent in the number of birds between October/December and October/January (Table ΙΙ).
Habitat utilization
In order to estimate habitat utilization rate, there were eight (8) observations (4 observations/month/ wintering stages) each were recorded for each wintering stage. The most utilized habitat type was lowland vegetation (p=0.0267) at the early wintering stage, with a utilization rate 0.427±0.021 followed by 0.254±0.027 for the emergent vegetation. The utilization rates of open water areas, upland vegetation and agricultural fields were relatively low, i.e., 0.152±0.028, 0.134±0.169 and 0.058±0.022 respectively. The most frequently utilized habitats at the middle wintering stage were lowland vegetation (p=0.0124) and emergent vegetation (p=0.0124) with a utilization rate of 0.351±0.028 and 0.346±0.023 followed by 0.136±0.025, 0.131±0.019 and 0.062±0.020 for agricultural fields, upland vegetation and open water respectively. The utilization rate at the late wintering stage of emergent vegetation was 0.58±0.220, which was evidently higher (p=0.032) than that of other habitats. The utilization rate was 0.337±0.031, 0.156±0.022, 0.059±0.021 and 0.052±0.023 for shallow-water areas, open water areas, agricultural fields and upland vegetation respectively.
DISCUSSION
Population structure
The findings of the study revealed a diverse migratory bird population in the study area. In the present study, 32 species (8 orders and 11 families) of migratory birds were recorded which is in agreement with the finding of Harisha and Hosetti (2018) at Komaranahalli Lake, Davanagere District, Karnataka, India (November 2012 to October 2013). The peak population of migratory birds was recorded during the months of December and January. Almost all of them left the wetland by the end of March.
Table Ι. Population structure of migratory birds during winter season in 2017-2018.
|
Order |
Family |
Species |
Scientific name |
Oct |
Nov |
Dec |
Jan |
Feb |
Mar |
Mean ±SD |
|
Mean±SD |
||||||||||
|
Acciptri-formes |
Accipi-tridae |
Oriental honey buzzard |
Pernis ptilorh-yncus |
25 (0.81) |
26 (1.29) |
24 (0.84) |
24 (0.75) |
22 (1.71) |
19 (2.8) |
23.3± 2.50 |
|
Black baza |
Aviceda leuphotes |
2 (0.54) |
3 (0.83) |
3 (0.74) |
2 (0.68) |
1 (0.32) |
0 (0) |
1.8± 1.16 |
||
|
Egyptian vulture |
Neophron percno-pterus |
0 (0) |
2 (0.58) |
3 (0.79) |
3 (0.35) |
2 (0.21) |
2 (0.17) |
2± 1.09 |
||
|
Tawny eagle |
Aquila rapax |
5 (0.75) |
7 (1.14) |
7 (1.12) |
9 (1.21) |
8 (1.16) |
4 (0.85) |
6.6± 1.86 |
||
|
Hen harrier |
Circus cyaneus |
8 (1.25) |
12 (2.63) |
14 (1.67) |
14 (1.25) |
12 (0.59) |
9 (0.78) |
11.5± 2.50 |
||
|
Black winged kite |
Elanus caeruleus |
15 (1.50) |
18 (2.24) |
20 (2.06) |
14 (1.41) |
13 (1.90) |
10 (1.65) |
15 ± 3.57 |
||
|
Greater spotted eagle |
Clanga clanga |
0 (0) |
1 (0.52) |
1 (0.63) |
2 (0.32) |
2 (0.47) |
1 (0.41) |
1.16 ±0.75 |
||
|
Lesser spotted eagle |
Clanga pomarina |
2 (0.73) |
3 (0.89) |
2 (0.53) |
2 (0.58) |
1 (0.35) |
1 (0.42) |
1.8 ±0.75 |
||
|
Anseri-formes |
Anati-dae |
Common teal |
Anas crecca |
20 (3.01) |
30 (2.60) |
34 (2.15) |
36 (2.65) |
36 (2.17) |
30 (1.53) |
30.66667 |
|
Spot billed duck |
Anas poecilo-rhyncha |
14 (1.33) |
18 (1.28) |
22 (2.14) |
24 (2.11) |
23 (2.34) |
21 (2.16) |
20.3± 3.72 |
||
|
Mallard |
Anas platyrhnchos |
15 (0.85) |
25 (2.16) |
29 (1.95) |
30 (1.39) |
29 (2.24) |
22 (2.11) |
25± 5.76 |
||
|
Gadwall |
Mareca strepera |
29 (2.36) |
32 (2.17) |
44 (1.78) |
48 (3.26) |
37 (1.23) |
24 (1.86) |
35.6 ±9.13 |
||
|
Eurasian wigeon |
Mareca penelope |
4 (1.49) |
8 (1.59) |
15 (1.79) |
11 (2.86) |
8 (1.98) |
5 (1.98) |
8.5 ±4.03 |
||
|
Garganey |
Spatula querquedula |
16 (1.85) |
34 (2.68) |
40 (2.73) |
42 (2.15) |
35 (3.96) |
21 (2.33) |
31.3 ±10.50 |
||
|
Tufted pochard |
Aythya fuligula |
12 (1.22) |
16 (2.59) |
22 (1.19) |
17 (3.36) |
13 (1.65) |
8 (0.75) |
14.6 ±4.80 |
||
|
Ruddy shelduck |
Tadorna ferruginae |
0 (0) |
2 (0.52) |
6 (0.21) |
7 (0.36) |
6 (0.55) |
4 (0.17) |
4.1± 2.71 |
||
|
Northern shoveler |
Spatula clypeata |
0 (0) |
14 (2.31) |
15 (0.89) |
16 (1.38) |
15 (1.88) |
12 (1.22) |
12± |
||
|
Cotton pygmy goose |
Nettapus coromand-elianus |
12 (1.85) |
17 (2.31) |
19 (2.15) |
24 (2.11) |
22 (2.55) |
18 (2.67) |
18.6± 4.18 |
||
|
Graylag goose |
Anser anser |
25 (3.19) |
38 (3.98) |
44 (2.66) |
42 (3.26) |
41 (3.45) |
29 (2.14) |
36.5± 7.71 |
||
|
Northern pintail |
Anas acuta |
0 (0) |
12 (1.55) |
14 (1.48) |
15 (1.11) |
12 (0.96) |
11 (1.38) |
10.6± 5.42 |
||
|
Bar headed goose |
Anser indicus |
14 (1.62) |
18 (1.65) |
28 (2.46) |
30 (2.28) |
25 (2.46) |
21 (1.97) |
22.6± 6.12 |
||
|
Red crested pochard |
Netta rufina |
40 (2.86) |
45 (2.15) |
56 (2.76) |
58 (3.10) |
48 (3.26) |
32 (2.67) |
46.5± 9.79 |
||
|
Cuculi-formes |
Cucu-lidae |
Jacobin cuckoo |
Clamator jacobinus |
5 (0.56) |
6 (0.31) |
6 (0.12) |
8 (0.98) |
5 (0.34) |
4 (0.19) |
5.7± 1.36 |
|
Columbi-formes |
Colum-bidae |
Oriental turtle dove |
Streptopelia orientalis |
4 (0.25) |
8 (0.68) |
8 (0.45) |
12 (0.15) |
11 (1.21) |
8 (0.49) |
8.5± 2.81 |
|
Scolop-acidae |
Wood snipe |
Gallinago namoricola |
0 (0) |
0 (0) |
5 (0.46) |
4 (0.13) |
4 (0.16) |
2 (0.01) |
2.5± 2.16 |
|
|
Common redshank |
Tringa totanus |
26 (2.78) |
29 (2.96) |
32 (2.44) |
34 (2.59) |
25 (2.03) |
21 (1.97) |
27.8± 10.08 |
||
|
Little stint |
Calidris minuta |
24 (2.02) |
28 (2.36) |
30 (3.14) |
28 (3.26) |
23 (2.19) |
19 (2.05) |
25.3± |
||
|
Larid-ae |
Common tern |
Sterna hirundo |
16 (2.14) |
19 (2.32) |
28 (3.25) |
27 (2.99) |
24 (2.13) |
22 (2.56) |
22.6± 4.63 |
|
|
Ciconii-formes |
Cicon-iidae |
European white stork |
Ciconia ciconia |
18 (1.53) |
19 (1.29) |
25 (2.12) |
24 (2.39) |
22 (1.37) |
21 (1.04) |
21.5± 2.73 |
|
Gruifo-rmes |
Rall-idae |
Common coot |
Fulica atra |
39 (3.27) |
41 (3.39) |
54 (2.23) |
58 (2.96) |
50 (3.92) |
35 (2.91) |
45.1± 8.42 |
|
Passeri-formes |
Dicru-ridae |
Fork tailed drongo |
Dicrurus adsimilis |
8 (0.36) |
8 (0.25) |
10 (1.20) |
12 (1.14) |
11 (0.96) |
7 (0.38) |
9.3± 1.96 |
|
Pelecan-iformes |
Arde-idae |
Grey heron |
Ardea cinerea |
12 (0.96) |
14 (1.23) |
21 (1.26) |
20 (1.49) |
18 (1.53) |
12 (1.11) |
16.1± 4.02 |
Table ΙΙ. Tukey´s multiple comparisons test among all months during winter season 2017-2018. A-October, B-November, C- December, D-January, E- February, F-March.
|
Tukey's Multiple Comparison Test |
Mean difference |
q |
Significant? P < 0.05? |
Summary |
95% CI of diff |
|
Column A vs Column B |
-4.949 |
2.51 |
No |
ns |
-13.02 to 3.123 |
|
Column A vs Column C |
-8.949 |
4.538 |
Yes |
* |
-17.02 to -0.8773 |
|
Column A vs Column D |
-9.128 |
4.63 |
Yes |
* |
-17.20 to -1.057 |
|
Column A vs Column E |
-6 |
3.043 |
No |
ns |
-14.07 to 2.071 |
|
Column A vs Column F |
-1.333 |
0.6762 |
No |
ns |
-9.405 to 6.738 |
|
Column B vs Column C |
-4 |
2.029 |
No |
ns |
-12.07 to 4.071 |
|
Column B vs Column D |
-4.179 |
2.12 |
No |
ns |
-12.25 to 3.892 |
|
Column B vs Column E |
-1.051 |
0.5332 |
No |
ns |
-9.123 to 7.020 |
|
Column B vs Column F |
3.615 |
1.834 |
No |
ns |
-4.456 to 11.69 |
|
Column C vs Column D |
-0.1795 |
0.09103 |
No |
ns |
-8.251 to 7.892 |
|
Column C vs Column E |
2.949 |
1.495 |
No |
ns |
-5.123 to 11.02 |
|
Column C vs Column F |
7.615 |
3.862 |
No |
ns |
-0.4560 to 15.69 |
|
Column D vs Column E |
3.128 |
1.587 |
No |
ns |
-4.943 to 11.20 |
|
Column D vs Column F |
7.795 |
3.953 |
No |
ns |
-0.2765 to 15.87 |
|
Column E vs Column F |
4.667 |
2.367 |
No |
ns |
-3.405 to 12.74 |
The basic need of migratory water birds at their wintering sites includes adequate food and shelter (Lakshmi, 2006). The study indicated a stable or increasing migratory bird trend at Bakhira bird sanctuary which was found to be in agreement with stable or increasing status of their major food-bases. Waterbird abundances is intricate to interpret because different factors often act simultaneously, confounding the effects of individual ones (Chalfoun and Martin, 2007). The correlation between waterbirds and wetland variables may be simple reflection of some decisive habitat features, frequently correlated with food-bases, which are not readily measured (Terborgh, 1985). Relative abundance of birds depends upon wetland features such as size, water level, quality of water, availability and distribution of food resources and the presence of appropriate roosting and nursery sites (Terborgh, 1985).
Skagen et al. (1999) are of the view that interior wetland complexes are endowed with critical refueling resources along the direct routes between summering and wintering grounds. Migratory birds face tremendous ecological and physiological challenges during long-distance migrations.
In addition to the vigorous expenditure flight, birds must find periodic stopovers to rest and refuel and to cope with unfavorable weather, uncertainties of resource abundance and accessibility, intra and inter-specific competition, and predation pressures, all within the context of unfamiliar environments (Moore et al., 2005). These challenges, in concert with wide-scale anthropogenic changes in habitats and landscapes along historical migratory pathways, espouse that intercontinental migration poses formidable hardships to many birds.
Habitat utilization
A variety of foraging habitat types and feeding techniques are used by migratory birds during the wintering season (Mishra et al., 2019). The most utilized habitat types were lowland vegetation and the emergent vegetation in the early and middle winter stages. Such condition of the particular sites of water bodies supporting greater densities and diversity of macro-benthic invertebrates has been well acknowledged by Kaminski and Prince (1981) who suggested that this usually occurred when the ratio of emergent vegetation and open water was almost equal (50:50). Moreover, macro-benthic invertebrates which correspond to the major diet of waterfowl (Chick and McIvor, 1994) were more intimately associated with aquatic vegetation than the barren substrate (Swanson et al., 1974; Joyner, 1980) as crumbling of the vegetation provided supplementary nutrients and surface for the proliferation and habitation of macro-invertebrates (Murkin et al., 1982).
Distinguished researchers such as Bellrose (1980) and Jones and Drobney (1986) also reported that waterbirds foraged profoundly on benthic macro-invertebrates. This confirms the present observation. The utilization rate of open water areas, upland vegetation and agricultural fields was quite low. The utilization rate of emergent vegetation was evidently higher than that of other habitats at the late wintering stage. Due to the decline in water level in late winter, low land vegetation was less utilized than emerging one. Habitat selection of wintering birds is influenced by prey availability and accessibility (Nagarajan and Thiyagesan, 1996). Availability of microhabitats and various food resources is the determining factors that controlled seasonal changes of bird species composition. The distribution of food is one of the most significant factors influencing the selection of feeding sites (Grant and Grant, 1987).
Water birds have to select appropriate habitats while facing constantly changing habitats during the winter (Warnock and Takekawa, 1995; Beerens et al., 2011). In many cases the decline in population involves groups of species that share the same habitat and have analogous ecological requirements, representing underlying causes for these population changes rather than a unique explanation for each species. Food preference of waterbirds can differ significantly among species, even among those within the same group (Zhu and Zou, 2001).
Waterbirds use different foods, including seeds (dabbling ducks, geese, cranes), leaves (geese), tubers and rhizomes (geese, swans), invertebrates (shorebirds, waterfowl), and some vertebrates for example fish and amphibians (wading birds). The amount, composition, and spatio-temporal dynamics of these foods primarily affect the use of foraging habitats by waterbirds and considered to be imperative indicators of habitat quality (Taft and Haig, 2005; Hartke et al., 2009). Wetlands could be important foraging habitats for waterbirds, particularly during wintering periods (Masero et al., 2000).
CONCLUSIONS
In conclusion, 32-species of migratory waders were recorded at Bakhira bird sanctuary. We concluded that all habitat types were efficiently utilized by migratory waders in all the wintering stages. Low vegetation was most frequently utilized habitat type in early winter stage. Low vegetation and emergent vegetation were evidently utilized habitat types in middle winter stage. Finally, emergent vegetation was suggested to utilize more prominently in late winter stage. The outcome of our study recommended that, Bakhira bird sanctuary may provides an efficient foraging and nesting ground for migratory birds as well as resident birds. We suggested some conservation measures for migratory waders as well as resident birds. The land use for human habitations should be discouraged to avoid agricultural drain off posing pollution threat. Control of aquatic microphytes is necessary in order to maintain optimal habitats. The weeds especially the emergent and floating types obstruct the waterways which should be thinned or removed by mechanical or manual measures. The wetland habitat being a famed and crowded bird sanctuary more effective steps from control of poaching should be recommended. For habitat preservation, the bird sanctuary should be fenced to afford a disturbance-free environment for birds and afford complete protection from illegal activities.
ACKNOWLEDGEMENTS
The authors would like to articulate their gratefulness to the Head, Department of Zoology, University of Lucknow, for providing facilities and administrative support. Himanshu Mishra is thankful to the University Grants Commission (UGC), New Delhi for Senior Research Fellowship [Ref. No. 21/12/2014 (ii) EU-V]. The authors are thankful to Ms. Farah bano, for her assistance in statistical analysis of the data. The authors are also grateful to the staff, Forest Department, Sohagibarwa, District Sant Kabir Nagar, Uttar Pradesh for their valuable support during field visits.
Statement of conflict of interest
The author declares that they have no conflict of interests.
REFERENCES
Akesson, S. and Hedenstrom, A., 2007. How migrants get there: migratory performance and orientation. AIBS Bull., 57: 123-133. https://doi.org/10.1641/B570207
Ali, S., 2002. The book of Indian birds. Bombay Natural History Society, 13th edition. https://doi.org/10.5962/bhl.title.43949
Beerens, J.M., Gawlik D.E., Herring, G. and Cook, M.I., 2011. Dynamic habitat selection by two wading bird species with divergent foraging strategies in a seasonally fluctuating wetland. Auk, 128: 651–662. https://doi.org/10.1525/auk.2011.10165
Bellrose, F.C., 1980. Ducks, geese and swans of North America. Stackpole Books, Harrisburg, Pennsylvania, USA.
Burnham, K.P., Anderson, D.R. and Laake, J.L., 1980. Estimation of density from line transect sampling of biological populations. Wildl. Monogr., 72: 3-202.
Chalfoun, A.D. and Martin, T.E., 2007. Assessment of habitat preferences and quality depend on spatial scale and metrics of fitness. J. appl. Ecol., 44: 983–992. https://doi.org/10.1111/j.1365-2664.2007.01352.x
Chick, J.H. and McIvor, C.C., 1994, Patterns in the abundance and composition of fishes among beds of different macrophytes: viewing a littoral zone landscape, Canad. J. Fish. aqua. Sci., 51: 2873-2882. https://doi.org/10.1139/f94-286
Getzner, M., 2002. Investigating public decisions about protecting wetlands. J. environ. Manage., 64: 237-246. https://doi.org/10.1006/jema.2001.0471
Grant, P.R. and Grant, O.R., 1987. The extraordinary El Nino event of 1982-1983: effects on Darwin’s Finches on Island Genovesa. Galapagos. Oikos, 49: 55-66. https://doi.org/10.2307/3565554
Grewal, B., Harvey, V. and Pfister, O., 2002. A photographic guide to the birds of India. Periplus Edition (HK) Ltd., Singapore.
Grimmett, R. and Inskipp, T., 2007. Birds of Southern India. Om Book International, New Delhi, India. Helam field guides, 240.
Grimmett, R., Inskipp, C. and Inskipp, T., 2011. Birds of the Indian subcontinent. Oxford University Press, India.
Harisha, M.N. and Hosetti, B.B., 2018. Status and conservation issues of wetland birds in Komaranahalli Lake, Davanagere District, Karnataka, India. J. Threat. Taxa, 10:11290-11294. https://doi.org/10.11609/jott.2809.10.2.11290-11294
Hartke, K.M., Kriegel, K.H., Nelson, G.M. and Merendino, M.T., 2009. An abundance of wigeongrass Ruppiamaritima during winter and use by herbivorous waterbirds in a Texas coastal marsh. Wetlands, 29: 288–293. https://doi.org/10.1672/07-206.1
Hilden, O., 1965. Habitat selection in birds: A review. Annls. Zool. Fenni., 2: 53–75.
Jones, J., 2001. Habitat selection studies in avian ecology: A critical review. The Auk, 118: 557-562. https://doi.org/10.2307/4089822
Jones, J.J. and Drobney, R.D., 1986. Winter feeding ecology of scaup and common goldeneye in Michigan. J. Wildl. Manage., 50: 446-452. https://doi.org/10.2307/3801102
Joyner, D.E., 1980. Influence of invertebrates on pond selection by ducks in Ontario. J. Wildl. Manage., 44: 700-705. https://doi.org/10.2307/3808023
Kaminski. R.M. and Prince, H.H., 1981. Dabbling duck and aquatic macroinvertebrate responses to manipulated wetland habitat. J. Wildl. Manage., 45: 1-15. https://doi.org/10.2307/3807868
Kumar, A., Sati, J.P., Tak, P.C. and Alfred, J.R.B., 2005. Handbook on Indian wetland birds and their conservation. Zoological Survey of India. pp. 472.
Lakshmi, B.B., 2006. Avifauna of Gosthani estuary near Visakhapatnam, Andhra Pradesh. J. Nat., 18: 291–304
Li, Z.W. and Mundkur T., 2004. Numbers and distribution of waterbirds and wetlands in the Asia-Pacific region. Results of the Asian Waterbird Census: 1997–2001. Wetlands International, Kuala Lumpur, Malaysia.
Masero, J.A., Perez-Hurtado, A., Castro, M. and Arroyo, G.M., 2000. Complimentary use of intertidal mudflats and adjacent salinas by foraging waders. Ardea, 88:177–191.
Mishra, H., Kumar, V. and Kumar, A., 2016. Diversity and population status of waders (Aves) of Bakhira Tal, a natural wetland in District Sant Kabir Nagar, Uttar Pradesh, India. Biodiv. J., 7: 331–336
Mishra, H., Kumar, V. and Kumar, A., 2019. Resource Partitioning between Two Species of Migratory Waders, Common Redshank Tringa totanus (Linnaeus, 1758) and Little Stint Calidris minuta (Leisler, 1812) (Scolopacidae): A Behavioural Comparison in a Wetland Ecosystem in Bakhira Tal, Uttar Pradesh, India. Acta zool. Bulg., 71: 103-111.
Mishra, S. and Narain, S., 2010. Floristic and ecological studies of Bakhira wetland, Uttar Pradesh, India. Indian Forester, 136: 375-381.
Mitsch, W.I. and Gosselink, I.G., 1986. Wetlands. Van Nostrand Reinhold, New York.
Moore, F.R., Smith, R.J. and Sandberg, 2005. Stopover ecology of intercontinental migrants: En route problems and consequences for reproductive performance. In: Birds of two worlds: The ecology and evolution of migration (eds. R. Greenberg and P.P. Marra). Johns Hopkins University Press, Baltimore, Maryland, pp. 251-261
Murkin, H.R., Kaminski, R.M. and Titman, R.D., 1982. Responses by dabbling ducks and aquatic invertebrates to an experimentally manipulated cattail marsh. Canad. J. Zool., 60: 2324-2332. https://doi.org/10.1139/z82-299
Nagarajan, R. and Thiyagesan, K., 1996. Waterbirds and substrate quality of the Pichavaram wetlands, southern India. Ibis, 138: 710-721. https://doi.org/10.1111/j.1474-919X.1996.tb04773.x
Oksanen, L., 1987. Interspecific competition and the structure of bird guilds in boreal Europe: the importance of doing fieldwork in the right season. Trends Ecol. Evol., 2: 376–379. https://doi.org/10.1016/0169-5347 (87)90140-6
Prasad, S.N., Ramachandra, T.V, Ahalya, N., Sengupta, T., Kumar, A., Tiwari, A.K., Vijayan V.S. and Vijayan, L., 2002. Conservation of wetlands of India- A review. Trop. Ecol., 43: 173-186.
Rahmani, A.R. and Soni, R.G., 1997. Avifaunal changes in the Indian Thar desert. J. Arid Environ., 36: 687-703. https://doi.org/10.1006/jare.1996.0242
Roshnath, R. and Shruthi, V., 2015. Habitat utilization by wetland birds of Munderikadavu, a proposed bird sanctuary in northern Kerala, India. J. Threat. Taxa, 7: 7870-7878. https://doi.org/10.11609/JoTT.o3999.7870-8
Sharma, D., Vishwakarma, D. and Yadav, K.C., 2014. The water birds of Gidhwa and Parsada Wetlands, Nandghat, Bemetara, Chhattisgarh (India). Int. J. scient. Res. Publ., 4:1-8.
Sibley, C.G. and Monroe, B.L., 1990. Distribution and taxonomy of birds of the world. Yale University Press, New Haven, CT.
Sillett, T.S. and Holmes, R.T., 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol., 71: 296–308. https://doi.org/10.1046/j.1365-2656.2002.00599.x
Skagen, S.K., Sharpe, P.B., Waltermire, R.G. and Dillon, M.B., 1999. Biogeographical profiles of shorebird migration in midcontinental North America (No. USGS/BRD/BSR--2000-0003). Geological Survey Fort Collins Biological Resources Division CO.
Swanson, G.A., Meyer, M.I. and Serie J.R., 1974. Feeding ecology of breeding blue-winged teals. J. Wildl. Manage., 38: 396-407. https://doi.org/10.2307/3800869
Taft, O.W. and Haig, S.M., 2005. The value of agricultural wetlands as invertebrate resources for wintering shorebirds. Agri. Ecosys. Environ., 110: 249–256. https://doi.org/10.1016/j.agee.2005.04.012
Terborgh, J., 1985. Habitat selection in Amazonian birds, In: (eds. M.L. Cody). Habitat Selection in Birds. Academic Press, London, pp. 311–340
Wang, Y., Fan, B.L., Ding, Y.R. and Pang, S.S., 2011. The current situation and discussion on wetland ecological restoration of the middle and lower Yangtze River. China Water Res., 13: 4–6.
Warnock, S.E. and Takekawa, J.Y., 1995. Habitat preferences of wintering shorebirds in a temporally changing environment: Western Sandpipers in the San Francisco Bay estuary. Auk, 112: 920–931. https://doi.org/10.2307/4089023
Xiang, G.E. and Wang, K.F., 2005. Research on the conservation and sustainable utilization of Shengjin Lake wetland resources. Territ. Nat. Resour. Stud., 1: 40–41.
Zhao, F., Zhou, L. and Xu, W., 2013. Habitat utilization and resource partitioning of wintering hooded cranes and three goose species at Shengjin Lake. Chinese Birds, 4: 281-290. https://doi.org/10.5122/cbirds.2013.0032
Zhu, X. and Zou, X.P., 2001. The herons, egrets and bitterns in China. China Forestry Publishing House, Beijing.
To share on other social networks, click on any share button. What are these?










