Quality and Proportion of X and Y Sperm After Sexing Process using Percoll Density Gradient Centrifugation Method on Different Gradients and Diluents in Belgian Blue Cross Bull
Research Article
Quality and Proportion of X and Y Sperm After Sexing Process using Percoll Density Gradient Centrifugation Method on Different Gradients and Diluents in Belgian Blue Cross Bull
Aulia Puspita Anugra Yekti, Ani Atul Arif, Putri Utami, Habib Asshidiq Syah, Anggita Dian Pramudhita, Rr. Ani Rizqianti, Trinil Susilawati*
Faculty of Animal Science, Universitas Brawijaya, Indonesia.
Abstract | This study aimed to evaluate the success rate of the sexing method using Percoll density gradient centrifugation on various gradients and diluents in Belgian Blue bull. The research material used was a semen sample of Belgian Blue cross bull with progressive motility of fresh semen of≥ 70%. The research used a Completely Randomized Design (CRD) with four treatments and ten replications. Data were analyzed using the Tukey test. The treatment in the study was T0: sexing PDGC 10 fractions using Andromed® diluent. T1: sexing PDGC 10 fractions using tris aminomethane diluent + 20% egg yolk. T2: sexing PDGC 5 fractions using Andromed® diluent. T43: sexing PDGC 10 fractions using tris aminomethane diluent + 20% egg yolk. Parameters measured include motility, viability, abnormalities, concentration, Total motile sperm, and proportion of sperm. The results of the analysis of variance showed significant differences in the upper layer for motility, concentration, and Total motile sperm (P<0.01), as well as in the lower layer for Motility and Total motile sperm (P<0.05). There were no significant differences in concentrations between treatments. Viability and abnormality showed significant differences in both layers (P<0.01). The proportion of sperm produced met the expected value of 70%. In conclusion, Andromed® diluent and tris aminomethane + 20% egg yolk can maintain the quality and proportion of sperm, and further analysis needs to be carried out regarding the use of Andromed® diluent and tris aminomethane + 20% egg yolk in the sexing semen freezing process.
Keywords | Percoll density gradient centrifugation, Proportion, Quality, Sexing sperm, Tris aminomethane+20% egg yolk
Received | May 24, 2024; Accepted | June 22, 2024; Published | October 05, 2024
*Correspondence | Trinil Susilawati, Faculty of Animal Science, Universitas Brawijaya; Email: [email protected]
Citation | Yekti APA, Arif AA, Utami P, Syah HA, Pramudhita AD, Rizqianti RA, Susilawati T (2024). Quality and proportion of x and y sperm after sexing process using percoll density gradient centrifugation method on different gradients and diluents in belgian blue cross bull. Adv. Anim. Vet. Sci. 12(11): 2211-2220.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.11.2211.2220
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Artificial insemination is a reproductive technology widely applied on farms because it can increase reproductive efficiency and improve the genetic quality of livestock. Artificial Insemination technology is currently developing by using sexing semen to obtain the offspring sex as expected. In the beef cattle industry, male calves are preferred over females because they can grow faster and have high prices. Meanwhile, in dairy cattle, female calves are needed more as replacement stock, and the price is also higher than that of male calves. The sex of a calf is determined by the presence of X and Y chromosomes, which have differences in size, shape, weight, motility, and biochemical content on its surface (Prakash, 2014) and DNA content (Rahman and Pang, 2019). Those differences are factors that affected the separation of sperm X and Y. Methods of sexing sperm have been widely reported previously, including using flow cytometry (Garner et al., 2013) swim-up (Hikmawan et al., 2016) (Utomo et al., 2021), H-Y antigen (Yadav et al., 2017), albumin sedimentation and Percoll density gradient centrifugation (Kusumawati et al., 2019) and the SexedULTRATM method (Vishwanath and Moreno, 2018).
The Percoll density gradient centrifugation (PDGC) sexing method was developed in Indonesia and has been commercialized by an artificial insemination center. The PDGC sexing method creates Percoll gradient layers with different concentrations in a tube and then centrifuges at a certain speed and time. The principle of the sexing method using Percoll centrifugation is that the difference in density between sperm carrying X and Y. X-carrying sperm weight is higher than Y-carrying sperm, causing X sperm to reach the lower fraction more quickly. In contrast, Y sperm will remain in the upper fraction (Kusumawati et al., 2019).
Sexing frozen semen using the PDGC method is currently produced using ten gradients, which has a motility of 44.60 in sexing X and 42.10 in sexing Y (Yekti et al., 2023). Even though it has a good quality of frozen semen, making a gradient with many layers is difficult and takes a long time. (Promthep et al., 2016) It was revealed that using seven-layer personal density gradients with a percoll concentration of 40-80% can separate the sperm about 65-70%, which produces 60.75% X sperm and motility of 95.86 ± 0.46%. Then, the resulting sex ratio was 71.4% of female offspring with X-sexing semen and 50% with unsexed semen. In comparison, the results of in vitro fertilization (IVF) and embryo transfer with sexing semen produced 67.86% female offspring. The sexing success of the PDGC method varies between 86 – 94% (Silva et al., 2017).
Sexing sperm requires a diluting medium that can protect and provide optimal environmental conditions for sperm so that the quality of sexing results can be maintained (Murphy et al., 2017). The commercial diluent Andromed® is more widely used for sexing sperm because its application is more accessible and produces high fertility, being able to maintain good sperm quality and preventing sperm from experiencing early capacitation after the sexing process (Juniandri et al., 2014). However, Andromed® diluent is more expensive, causing the high cost of producing sexing semen and has a short duration of use. On the other hand, the diluent tris aminomethane + 20% egg yolk is also one of the diluents widely used at an affordable price and can maintain good sperm quality. The previous research conducted by (Kusumawati et al., 2019) sexing was performed by using 10 gradient and andromed diluent, while (Yekti et al., 2023) using 10 gradient with Tris aminomethane egg yolk 20% diluent. Furthermore, the research of sexing PDGC sexing using 5 and 3 gradients are not fully studied. Therefore, this study aims to evaluate sperm separation using the sexing method with different gradients and diluents.
MATERIALS AND METHODS
Ethical Approval
The authors confirmed that no ethical issues were involved in this study, all the procedure was followed the standard procedure in artificial insemination center Singosari Malang with ISO 9001: 2015 and supervised by a veterinarian from AI Center Singosari, Malang.
Animals and Semen Collection
Belgian Blue Cross mature bulls aged 6–7 years were raised at an artificial insemination center in Singosari, Malang, Indonesia. The bulls weighed 700–800 kg and had body conditioning scores (BCS) ranging from 3–4. The procedure of collecting semen followed the standard operational procedure of AIC. Fresh semen was collected once a week, routinely using an artificial vagina. The fresh semen with progressive motility exceeding 70% and abnormal sperm below 20% were processed prior to the experiments.
Production of Sexed Semen with Centrifugation Gradient Percoll Density
The sexed semen was produced with centrifugation gradient percoll using 10 and 5 gradients. The percentage of percoll gradient included ten gradients (20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%) and five gradients (20%, 30%, 40%, 50%, 60%) respectively. The fresh semen that meets the requirements is then diluted into diluent with a ratio of semen, and the diluent was 1:1, in this experiment using 0,5 ml semen: 0,5 ml diluent. The diluent was using Andromed® and Tris Aminomethan + 20% egg yolk. According to (Susilawati, 2013), Tris aminomethane diluent + 20% egg yolk in 100 ml consists of Tris Aminomethan 1.363 g, citric acid 0.762 g, Lactose 1.5 g, Fructose 0.5 g, egg yolk 20 ml, Raffinosa 2.7 g, Streptomycin 0.1 g, penicillin 0.1 g aquadest 80 g, while andromed is diluted using distilled water 1: 4. The centrifugation was performed at 45 G for 5 minutes. The treatment was divided into four treatments with ten replications each. The following treatments were:
T0: 10 percoll gradients with Andromed® diluent
T1: 10 percoll gradients with Tris aminomethane diluent + 20% egg yolk
T2: 5 percoll gradients with Andromed® diluent
T3: 5 percoll gradients with Tris aminomethane diluent + 20% egg yolk
Parameters observed
The semen was evaluated after the sexing process for some parameters including:
Individual motility (%): The percentage of individual motility is measured based on the sperm movement which is classified into forward (progressive), backward and circular movements (Susilawati, 2013). One drop of sexing semen is placed on an object glass, then covered with a cover glass. Furthermore, semen was observed using an Olympus CX-23 light microscope with 400x magnification. Individual motility is assessed in a minimum of five view fields.
Sperm Viability (%): One drop of semen was placed on a glass object, and then one drop of eosin nigrosine was homogenized gently. Make a smear of the sample along the surface of the object glass then air dry. The viability was observed using an Olympus CX-23 binocular light microscope with 400x magnification. The live and dead sperm were calculated minimum for a total of 200 sperm viability is calculated by the formula (Susilawati et al., 2022):
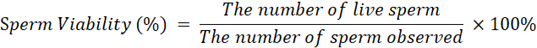
Sperm Concentration: placed 990 μl of 3% NaCl into the microtube. Then, add 10μl of sexing semen and homogenize gently. Take the 10μl of semen that has been diluted with 3% NaCl in the counting chamber and cover with a cover glass. Sperm were observed using an Olympus CX 23 binocular light microscope and counted in 5 boxes in the upper right and left corners, lower right and left corners, and the middle. Sperm concentration can be calculated with the formula (Yekti et al., 2023):
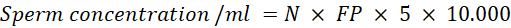
Description:
N: Average number of sperm in chambers A and B
FP: Diluent Factor (1:100)
5: Correction Factor (chamber room)
10.000: Correction Factor (chamber depth 0.0001 ml)
Abnormal sperm (%): Abnormal sperm was observed from sperm with abnormal shapes such as no head, large head, small head, no tail, and circular tail. The observations were observed on a minimum of 200 sperm, then calculated by the formula (Yekti et al., 2023):
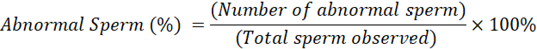
Total Motile Sperm (TMS): TMS is calculated by multiplying the percentage of individual motility and sperm concentration in a million/ml (Susilawati et al., 2022; Aldini et al., 2022). The following formula calculates TSM:
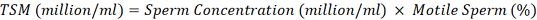
Identification of X and Y Sperm,
identification of the proportion of X and Y sperm was carried out using morphometric tests. The morphometric test was carried out by measuring the sperm head, including the length and width, using Olympus LC Micro software.
Observations were made on 1000 sperm in fresh semen samples to determine the fresh semen proportion (50:50) and the average head area of sperm as a comparison to determine X and Y sperm. If the sperm head is larger or equal to the average (fresh semen), it is categorized as X-chromosome sperm. If the size of the sperm head is smaller than the average, it is categorized as Y sperm (Kusumawati et al., 2019). The percentage proportion of X and Y sperm can be calculated using the formula:
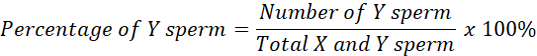
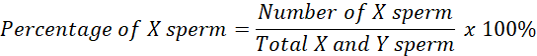
Data analysis
The research design used was a Completely Randomized Design (CRD) with four treatments and ten replications. The data obtained was then analyzed using analysis of variance, and then the Tukey test was carried out to compare treatments directly. The results of this test provide confidence intervals and p values for each pair of groups, allowing specific differences between treatment groups to be identified (Sudarwati et al., 2019). Parameters observed included individual motility, viability, abnormality, concentration, total motile sperm (TMS), and proportion of sperm. Calculations of sperm proportions were analyzed using the chi-square test to evaluate the significance of differences between treatment groups and assess accuracy in meeting the expected value of 70%. The data was then tabulated and analyzed statistically using IBM SPSS (Statistical Program for Social Science) version 25 software.
RESULTS AND DISCUSSION
Quality of Belgian Blue Cross Bull Fresh Semen
The quality of fresh semen is the leading indicator in estimating the fertility capacity of sperm and determining the suitability of semen for processing, whether diluted, frozen, or used for sexing. The quality of fresh semen is evaluated macroscopically and microscopically. Macroscopic evaluation includes semen volume, color, pH, odor, and semen consistency, while microscopic evaluation includes mass motility, individual motility, viability, abnormalities, and concentration (Garner and Hafez, 2000). Fresh semen that is suitable for further processing has motility≥70%. The results of research evaluating the quality of fresh semen from Belgian blue cross bull are as follows.
Table 1: Average Quality of Fresh Semen for Belgian Blue Cross Bull.
|
Parameters |
Average ± SD |
|
Macroscopic |
|
|
Volume (ml) |
9,5 ± 2,35 |
|
Color |
Milky White |
|
pH |
6,3 ± 0,26 |
|
Odor |
Specific |
|
Consistency |
moderate |
|
Microscopic |
|
|
Mass Motility |
++ |
|
Individual motility (%) |
80,67 ± 2,94 |
|
Viability (%) |
86,24 ± 2,50 |
|
Concentration (106 /ml) |
1425,00 ± 470,66 |
|
Abnormality (%) |
|
|
TSM (106/ml) |
10620,61 ± 3529,49 |
Based on Table 1, it can be seen from the macroscopic and microscopic evaluation results of fresh semen that Belgian Blue cross bull have an average fresh semen volume of 9.5 ± 2.35 ml. According to (Filipcík et al., 2023), generally, the average volume of fresh sperm is 8.72 mL. (Susilawati, 2011) stated that the volume of fresh semen production in cows ranges from 1-15 ml/ejaculate. The fresh semen produced from Belgian Blue cross bull is milky white and has a distinctive odor. The characteristics of fresh cow semen are dark white to milky white (Bintara, et al., 2021; Susilawati, 2013). Belgian Blue cross bull has an average pH of 6.3 ± 0.26, which means the pH of Belgian Blue cross bull semen is within the normal limits. According to (Nongbua et al., 2020), a sperm pH of 6.2 can be categorized as normal. A pH of fresh sperm in cows that is too acidic can cause a decrease in individual motility and sperm capacity, which increases the possibility of infertility (Zhou et al., 2015).
The average percentage of individual Motility for Belgian Blue cross bull is 80.67 ± 2.94%. These results are not different from the research results of (Lagu et al., 2020), which stated that the average individual motility of cow semen was 80.72 ± 6.34%. According to Indonesian national standard, fresh sperm can be processed into frozen sperm with a minimum criterion of 70% motility. Thus, the motility of fresh sperm from Belgian Blue cross bull can be categorized as good.
Viability parameters are related to the viability of sperm in terms of the condition of the sperm cell membrane. Based on the research results, it is known that the average percentage of viability of fresh semen from Belgian Blue cross bull is 86.24 ± 2.50%. It is known that the average viability of fresh cow semen is 83.09 ± 2.22% (Nugroho et al., 2014). The average concentration of fresh semen from Belgian Blue cross bull is 1,425.00 ± 470.66 million/ml. This value is higher than the research results of (Nugroho et al., 2014) and (Aisah et al., 2014), respectively. The average concentration of fresh cow semen was 1197.20 ± 335.25 million/ml and 1043.36 ± 225.86 million/ml. The average percentage of abnormalities in fresh semen from Belgian Blue cross bull is 5.16 ± 1.25%; this value is lower than the results of research by (Nugroho et al., 2014), which states that the average abnormality in fresh cow semen is 10.40 ± 0.73%. High levels of abnormalities in sperm can affect sperm fertility (Rosyada et al., 2020).
Progressive Motility, Concentration, and Total Motile Sperm After Sexing
Progressive Motility and total motile sperm are essential factors for sexing semen quality because progressive motility is vital in fertilization success. The total value of motile sperm is influenced by the average individual motility value and the concentration of sexed semen produced (Yekti et al., 2023).Table 2 showed the percentage of pro
Table 2: The Value of Progressive Motility, Concentration, and Total Motile Sperm after sexing in all treatments.
|
Parameters |
Treatment |
|||||||
|
Upper Layer |
Lower Layer |
|||||||
|
T0 (Mean) |
T1 (Mean) |
T2 (Mean) |
T3 (Mean) |
T0 (Mean) |
T1 (Mean) |
T2 (Mean) |
T3 (Mean) |
|
|
Motility (%) |
68 ± 4.9ab |
72 ± 4.8a |
59 ± 13.7b |
60 ± 9.8b |
69 ± 3.5 |
71 ± 5.2 |
56 ± 9.6 |
52 ± 9.8 |
|
Concentration(106/ml) |
228 ± 58.4 |
271 ± 101.7 |
61 ± 41.6 |
190 ± 39.5 |
256 ± 98.4 |
269 ± 123.8 |
215 ± 83.7 |
268 ± 99.4 |
|
Total Motile Sperm (106/ml) |
151 ± 30.4ab |
175 ± 65.5a |
65 ± 34.3c |
74 ± 56.5b |
174 ± 64.8AB |
188 ± 81.7A |
98 ± 52B |
138 ± 67.8AB |
Significant discrepancies are indicated by dissimilar superscripts within rows. In this context, a and b denote the upper layer, while A and B represent the lower layer.
gressive motility, concentration, and total motile sperm after the sexing process in all treatments.
Table 2 showed the results of the variance analysis for all treatments. Significant differences existed in the upper layer for motility, concentration, and TMS (P<0.01). On the other hand, in the lower layer, there were very significant differences in motility parameters between treatments, while the total parameters of motile sperm showed significant differences (P<0.05). However, there were no significant differences between treatments in concentration parameters. The percentage of progressive motility on ten gradients, both in the upper and lower layers, was higher than five gradients, about more than 65%, which meets the requirements for the freezing process. Meanwhile, the percentage of sperm motility with Tris aminomethane diluent + 20% egg yolk was higher than Andromed diluent in the upper layer on T0-T4 values of 68%, 72%, 59%, and 60%, respectively. (Kusumawati et al., 2019) The Motility of gradient SGDP 10 sexing semen with Andromed® diluent in the top layer was 61%, and the motility in the bottom layer was 67.5%.
The individual Motility sexing semen, both the top and bottom layers in 10 gradients, are still suitable for processing into the frozen process. The decrease in motility values in sexed semen is caused by the centrifugation process during sperm separation, causing damage to the sperm plasma membrane. The integrity of the sperm plasma membrane is related to motility and fertilization ability, which must maintain membrane integrity (Aslam et al., 2014). Damage of the plasma structure membrane due to centrifugal force while separating sperm disrupts metabolic and physiological processes, resulting in decreased movement and death of sperm. (Kusumawati et al., 2017) also stated that reduced motility occurs due to various treatments, including the separation and washing process, which causes sperm to require much energy to maintain their physiological condition; the processing and storage period of sperm also affects the quality of sperm membranes, due to the metabolic activity of sperm which produces adverse effects in the form of production. ROS, where ROS production can reduce motility values, is characterized by damage to the sperm membrane.
Furthermore, Tris aminomethane + 20% egg yolk diluent can maintain the individual motility of sexing semen in both the upper and lower layers compared to Andromed® diluent because of egg yolk addition to the tris aminomethane diluent, and egg yolk contains lecithin and lipoprotein which function to maintain and protect the sperm plasma membrane during the separation process by centrifugation. (Tethool et al., 2022) also stated that egg yolk contains amino acids, and lipoprotein plays a role in maintaining the integrity of the sperm membranes. The carbohydrate, vitamin, and mineral content maintains the life of sperm; glucose and vitamins in egg yolk are also readily soluble in water, so they are beneficial for sperm.
Based on Table 2, the average concentration in this study between the upper layer and bottom layer is balanced, showing that the separation of sperm was successful. Concentrations in ten and five gradients in the diluent andromed and tris aminomethane + 20% egg yolk showed a significant difference in the upper layer (P<0.01). In contrast, in the lower layer, there was no significant difference (P>0.05). (Juniandri et al., 2014) stated that sperm concentration in each layer of sexing semen is a primary factor influencing the TMS calculation. Based on the Indonesian (National Standards, 2021), the total number of motile sperm obtained for frozen semen is a minimum of 10 million/straw, obtained by multiplying the standard motility of 40% by the standard concentration of 25 million/straw.
The concentration value in this study is not different from the study of (Fatahillah et al., 2016), which states that the TMS of PDGC 10 gradient sexing semen with a centrifugation time of 5 minutes in the upper and lower layers, respectively, about 210 x 106/ml and 170 x 106/ml, while the TMS of PDGC 10 gradient sexing semen with centrifugation time 7 minutes on the top and bottom layers respectively, namely 151 x 106/ml and 210 x 106/ml. Based on the results of TMS calculations for sexing semen in this study, it is known that the quality of PDGC 10 gradient sexing semen using Andromed® diluent and tris aminomethane + 20% egg yolk in terms of TMS parameters is still suitable for freezing. (Fatahillah et al., 2016) explained that the total number of motile sperm for the AI standard is 40 million sperm/ml. The average TMS of the upper and lower layers of sexed semen in T1 is higher than T0, presumably because the sperm in T1 has more energy than the sperm in T0 because the diluent composition is tris aminomethane + 20% egg yolk which contains more energy sources including fructose, lactose, egg yolk, and raffinose, where raffinose is a trisaccharide composed of galactose, glucose, and fructose (Pubiandara et al., 2016). Meanwhile, in Andromed® diluent, the energy source is only obtained from fructose (Arif et al., 2022).
Viability and Abnormality Sperm after Sexing
Table 3 presents the average results of the percentage of viability of sperm from sexing by the percoll density gradient centrifugation method at 10 and 5 gradients of Belgian Blue cross bull using Andromed® diluent, tris aminomethane + 20% egg yolk. Table 3.
The analysis of variance showed highly significant differences in both the upper and lower layers in terms of viability and abnormality parameters (P<0.01). These results indicate that the treatments applied had a considerable
Table 3: The Viability and Abnormality of sperm after sexing in all treatments.
|
Parameters |
Treatment |
|||||||
|
Upper Layer (Mean) |
Lower Layer (Mean) |
|||||||
|
T0 |
T1 |
T2 |
T3 |
T0 |
T1 |
T2 |
T3 |
|
|
Abnormality (%) |
5 ± 1.7ab |
4 ± 1.7a |
8 ± 3.2b |
8 ± 4.0b |
4 ± 1.8A |
4 ± 1.2A |
8 ± 4.5B |
7 ± 3.3AB |
|
Viability (%) |
85 ± 2.2b |
87 ± 1.8b |
96 ± 3.9a |
95 ± 3.8a |
87 ± 1.6B |
86 ± 2.5B |
95 ± 7.7A |
95 ± 4.2A |
Dissimilar superscripts within rows indicate significant discrepancies. In this context, a and b denote the upper layer, while A and B represent the lower layer.
impact on the viability and abnormality levels of sperm, both on the upper and lower surfaces. This significant difference may be due to variations in environmental conditions such environmental temperature and components contained in the medium used or treatments applied to the two layers. The percentage of viability in sexed sperm of Belgian Blue bull using ten gradients and five gradients as well as a combination of diluents, namely andromed and tris-aminomethane + egg yolk, showed a more significant percentage of viability at five gradients in the T2 and T3 treatments in both the upper and lower layers, namely 96±3. 9, 95±3.8 (in the upper layer), and 95±7.7, 95±4.2 (in the lower layer), while in the ten gradients, the percentage of viability in treatments T0 and T1 was 85±2.2, 87±1.8 (in the upper layer), and 87±1.6, 86±2.5 (in the lower layer) respectively (P<0.01).
Using fewer gradients allows for more stable sperm separation, reducing the mechanical and osmotic stress that often occurs at higher gradients (Koh and Marcos, 2015). This was reflected in the higher viability in treatments T2 and T3, compared to T0 and T1, which used 10 gradients. (Tethool et al., 2022) explain that dead sperm have plasma membranes that are no longer active or semipermeable so that acidic eosin can be absorbed and color acidophilic parts of the tissue, such as the cytoplasm, red. The cytoplasm, which stores substances needed for cell metabolism, has acidic properties that cause acidic substances to stick in it (Hossain et al., 2011).
Sperm in the upper layer tend to run out of reserve energy faster and die faster because of their higher mobility than sperm in the lower layer. This aligns with research (Kusumawati et al., 2017), which showed that the viability percentage of PDGC-sexed sperm in Ongole Crossbred bull was 85.15% in the upper layer and 88.64% in the lower layer. The results of sexing research with ten gradient density percoll centrifugation with tris aminomethane + 20% egg yolk diluent had a viability of 77.74% in the lower layer and 74.9% in the upper layer, while Andromed® diluent had a viability of 79.8% in the lower layer and 76.25% in the upper layer.
(Kumar et al., 2019) support these findings by showing that lower gradients reduce cellular damage and maintain sperm membrane integrity, which is essential for optimal fertility. In addition, using less gradient allows for a more even distribution of nutrients and buffers, helping to maintain ideal physiological conditions for sperm (Leung et al., 2022). Using a combination of android and tris-aminomethane + egg yolk diluents provided additional support in maintaining sperm viability. Tris-aminomethane + egg yolk diluent is known to have the ability to protect sperm from oxidative damage and provide nutrients essential for cell survival (Pahlevy et al., 2022). The egg yolk in the diluent can protect the sperm membrane from damage due to refrigeration and freezing. This supports the results of this study, where higher viability and lower abnormality were observed in treatments with this diluent combination.
Sperm viability is also related to sperm abnormality. Abnormality is one sign of sperm quality due to abnormal cell structure and can cause problems or difficulties during fertilization, even pregnancy failure (Rasad et al., 2020). The percentage of abnormality between the 10 and 5 gradient treatments, as well as between andromed diluent and tris aminomethane + egg yolk, showed a very significant difference (P < 0.01), the abnormality in the T0, T1 treatment was smaller than the abnormality in the T2 and T3 treatments in the upper layer, vice versa in the lower layer the abnormality in the T0 and T1 treatments was smaller than the abnormality in the T2 and T3 treatments, this indicates that in the ten gradient, the abnormality is smaller than the abnormality in the five gradient. This is in line with research (Yekti et al., 2023), which states that the percentage of sexed semen abnormality in Friesian Holstein cows ranges from 5-7%. Several factors can cause sperm abnormalities, including oxidative stress, osmotic imbalance, and damage during the handling and separation (Agarwal et al., 2014). In this context, proper use of diluents such as andromed and tris-aminomethane + egg yolk also reduces abnormalities. Egg yolk in the diluent serves as a protective agent that protects the plasma membrane of sperm from damage (Watson, 2000). Andromed diluent is also known to provide a more stable environment for sperm, which helps to reduce abnormality rates (Medeiros et al., 2002). Oxidative stress during cold storage is one of the leading causes of sperm damage. Free radicals, primarily reactive oxygen species (ROS), can damage lipids, proteins, and DNA in sperm cells, which increases morphological abnormalities and reduces viability. According to (Koppers et al,. 2008), oxidative stress can cause DNA fragmentation and membrane damage in sperm, which negatively affects fertilization ability.
Proportion of X and Y sperm after Sexing
Sexing uses the Percoll density gradient centrifugation method based on the difference in weight or head size of X sperm being more significant than that of Y sperm. The proportion of X and Y sexing sperm was confirmed by measuring the heads of sperm morphometrically, namely based on the principle of differences in the characters of X and Y sperm (Yekti et al., 2023) . The proportion of X and Y sperm in all treatments can be seen in (Tables 4) (upper layer) and 5 (lower layer). (Table 4, 5) (Figure 1).
Table 4: The Proportion of X and Y sperm after sexing in the upper layer in all treatments.
|
Proportion |
Fresh Semen |
After Sexing |
|||
|
Upper Layer |
|||||
|
T0 |
T1 |
T2 |
T3 |
||
|
X (%) |
49.1 |
25.10 |
25.80 |
23.90 |
16.90 |
|
Y (%) |
50.9 |
74.90 |
74.20 |
76.10 |
83.10 |
Based on chi-square analysis on calculating the proportion of X and Y sperm, it was found that in the upper and lower layers, all treatments met the expected value of 70% for the separation of X and Y sperm (P<0.01). These findings indicate that the distribution of X and Y sperm in each treatment did not differ significantly from the previously determined expected value. This confirms the accuracy and consistency in separating X and Y sperm in this study.
Table 5: The Proportion of X and Y sperm after sexing in the Lower layer in all treatments.
|
Proportion |
Fresh Semen |
After Sexing |
|||
|
Lower Layer |
|||||
|
T0 |
T1 |
T2 |
T3 |
||
|
X (%) |
49.1 |
85.80 |
84.10 |
83.00 |
85.10 |
|
Y (%) |
50.9 |
14.20 |
15.90 |
17.00 |
14.90 |
This study found that the average head area of Belgian blue cross bull sperm was 35.79 μm with a head length ranging from 7.19 - 10.41μm and a head width ranging from 3.14 - 5 .0 μm (Figure 1). This result per the opinion of (Susilawati, 2014), who states that the length and width of the sperm head in cattle is approximately 8-10 μm and 4.0 - 4.50 μm. The identification of X sperm is known to be larger than average, while Y sperm are smaller than average (fresh semen); this is to the statement of (Kusumawati et al., 2019) that the sperm head is larger or equal to the average (fresh semen), then it is categorized as X chromosome sperm, and if the sperm head size is smaller than average, then it is categorized as Y sperm. From the results of measuring the length and width of the sperm head, it was found that X and Y sperm had a percentage of 49.1% and 50.9%, respectively.
This study showed that the proportion of sexing semen in the upper layer using Andromed® diluent and tris aminomethane + 20% egg yolk was not significantly different; the Y sperm was 74.90% and 74.20%, respectively. These results show a higher value compared to the research of (Kusumawati et al., 2019), which stated that the percentage of Y sperm in Ongole crossbred cattle resulting from PDGC sexing, with Andromed® diluent in the top layer, was 72%, whereas in the results of research by (Susilawati, 2014) using the same sexing method the percentage of Y sperm in the top layer was obtained 73 .1%. (Rasad et al., 2019) stated that sexing PDGC 10 gradients in Etawa goats also produced a higher percentage of the Y chromosome, namely 76.10%
The proportion of sexing semen in the lower layer using Andromed® diluent and tris aminomethane + 20% egg yolk showed no significant difference; the X sperm was 85.80% and 84.10%, respectively. This result is no different from the study of (Susilawati, 2014), that sexing PDGC 10 gradients with a centrifugation speed of 44.5 G for 5 minutes produced 83.1% of X sperm in the lower layer. However, compared to (Fatahillah et al., 2016), which has a higher value using the PDGC 10 gradient sexing method for 5 minutes, it was found that the percentage of X sperm in the lower layer was 78.6%.
Several factors that can influence the size of the proportion of sperm in the PDGC 10 gradient sexing method include the molecular weight of the density gradient (personal, diluent), centrifugation time, and speed. In this study, the molecular weight of Andromed® diluent and tris aminomethane + 20% egg yolk does not have a significant difference of 1.07 g/ml and 1.05 g/ml, respectively. (Susilawati, 2014) stated that percoll has a density of 1.13 ± 0.005 g/ml and can form a density gradient below 1.13 g/ml. The speed and time of centrifugation affect the percentage of X and Y sperm after centrifugation.
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, the differences in gradients and diluents used during the sexing process significantly influence the quality of sperm. The best results of sperm motility were achieved when using ten density gradients with tris aminomethane diluent plus 20% egg yolk. Furthermore, the sexing process in all treatments has proportions above 70%, as expected. This finding showed that five gradient and diluents of tris aminomethane + 20% egg yolk can be used for further sexing method.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Research Grant Universitas Brawijaya for the research grant, contract number 2044.15/UN10.F05/PN/2023.
NOVELTY STATEMENT
The novelty of this study is the development of 5 gradients percoll in the sexing process of centrifugation density percoll method and also using tris aminomethane diluent, which has not been widely studied before. This approach demonstrated the potential for increased viability and reduced sperm abnormality, resulting in a separation yield proportion of more than 70%. These findings significantly contribute to reproductive technology and livestock breeding, offering a more effective and efficient method to improve the quality and quantity of sperm sexing results.
AUTHOR’S CONTRIBUTION
APA, conceptualization, collecting data, interpreting data, and drafting the original manuscript; ADP, RAR, collecting data, drafting the original manuscript; PU, AAA, HAS, analyzing and interpreting data, drafting the original manuscript; TS, conceptualization, supervision, collecting data, interpreting data, and drafting the original manuscript.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Aisah S, Isnaini N, Wahjuningsih S (2014). Kualitas Semen Segar dan Recovery Rate Sapi Bali pada Musim Yang Berbeda. J. Ilmu-Ilmu Peternakan, 27 (1): 63 – 79. https://doi.org/10.21776/ub.jiip.2017.027.01.06
Agarwal A, Mulgund A, Hamada A, Chyatte MR (2015). A unique view on male infertility around the globe. Reprod. Biol. Endocrinol., 13: 1-9. https://doi.org/10.1186/s12958-015-0032-1
Agarwal A, Virk G, Ong C, du Plessis SS (2014). Effect of oxidative stress on male reproduction. World J Mens Health, 32(1):1-17. doi: 10.5534/wjmh.2014.32.1.1
Aldini SA, Isnaini N, Yekti APA, Susilawati T (2022). Study of The Quality and Integrity of Sperm Acrosome Caps in Frozen Sexing Semen Friesian Holstein Cattle. J.Ilmu-Ilmu Peternakan, 32 (2): 233 – 240. https://doi.org/10.21776/ub.jiip.2022.032.02.09
Arif AA, Maulana T, Kaiin EM, Purwantara B, Arifiantini RI (2022). The quality of frozen semen of limousin bull in various semen diluents. Trop. Anim. Sci. J., 45(3): 284-290. https://doi.org/10.5398/tasj.2022.45.3.284
Aslam HA, Dasrul, Rosmaidar (2014). Pengaruh Penambahan Vitamin C dalam Pengencer Andromed® Terhadap Persentase Motilitas dan Membran Plasma Utuh Sperm Sapi Aceh Setelah Pembekuan. J. Medika Ve., 8 (1): 10 – 26.
Bintara S, Panjono P, Aji RN (2021). Motility and Viability of Sperm of Belgian Blue Crossbreeds with the Addition of Tomato (Solanum lycoperiscum) Extract in Egg Yolk Citrate Diluent. Adv. Biol. Sci. Res., 18 (1): 243 – 246. https://doi.org/10.2991/absr.k.220207.050
Fatahillah, Susilawati T, Isnaini N (2016). Pengaruh Lama Sentrifugasi Terhadap Kualitas dan Proporsi Sperm X-Y Sapi Limousin Hasil Sexing Dengan Gradien Densitas Percoll Menggunakan Pengencer CEP-2 + 10% KT. J. Ternak Trop., 17 (1): 86 – 97. https://doi.org/10.21776/ub.jtapro.2016.017.01.10
Filipčík R, Rečková Z, Pešan V, Konoval O, Kopec T (2023). Evaluation of semen parameters from Fleckvieh–Simmental bulls and the influence of age and season of collection. Arch. Anim. Breeding, 66(1): 113-120. https://doi.org/10.5194/aab-66-113-2023
Garner DLESE, Hafez (2000). Sperm and Seminal Plasma In: Reproduction in Farm Animals Edit by E. S. E. Hafez. 7th Edition. Lippincott Williams and Wilkins: Maryland, USA. https://doi.org/10.1002/9781119265306.ch7
Garner DL, Evans KM, Seidel GE (2013). Sex-sorting sperm using flow cytometry/cell sorting. Methods Mol. Biol., 927: 279-295. https://doi.org/10.1007/978-1-62703-038-0_26
Hikmawan SW, Ciptadi G, Wahyuningsih S (2016). Kualitas Sperm Swim Up Kambing Peranakan Etawah Hasil Pembekuan Menggunakan Metode Vitrifikasi Dengan Persentase Gliserol Yang Berbeda. J. Ternak Trop., 17(1): 42-48. https://doi.org/10.21776/ub.jtapro.2016.017.01.5
Hossain MS, Johannisson A, Wallgren M, Nagy S, Siqueira AP, Rodriguez-Martinez, H (2011). Flow cytometry for the assessment of animal sperm integrity and functionality: state of the art. Asian j. androl., 13(3): 406. https://doi.org/10.1038/aja.2011.15
Juniandri, Susilawati T, Isnaini N (2014). Perbandingan Pengencer Andromed® dan CEP-2 Terhadap Kualitas Sperm Sapi Hasil Sexing dengan Sentrifugasi Gradien Densitas Percoll. J. Vet., 15 (2): 252 – 262.
Koh JBY, Marcos (2015). The study of sperm and sorting in relation to human reproduction. Microfluid. Nanofluid., 18: 755-774. https://doi.org/10.1007/s10404-014-1520-x
Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ (2008). Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in sperm. J. Clin. Endocrinol. Metab., 93(8): 3199-3207. https://doi.org/10.1210/jc.2007-2616
Kumar A, Prasad JK, Srivastava N, Ghosh SK (2019). Strategies to minimize various stress-related freeze-thaw damages during conventional cryopreservation of mammalian sperm. Biopreserv. biobanking, 17(6): 603-612. https://doi.org/10.1089/bio.2019.0037
Kusumawati ED, Isnaini N, Yekti APA, Luthfi M, Affandhy M, Pamungkas D, Kuswati, Ridhowi A, Sudarwati H, Susilawati T, Rahayu S (2017). The Quality of Sexed Semen on Filial Ongole Bull using Percoll Density Gradient Centrifugation Method. J. Microb. Biotechnol. Environ. Sci., 19 (1): 189 – 199.
Kusumawati ED, Isnaini N, Yekti APA, Luthfi M, Affandhy L, Pamungkas D, Kuswati, Ridhowi A, Sudarwati H, Rahadi S, Rahayu S, Susilawati T (2019). The Motility and Ratio of X and Y Sperm Filial Ongole Cattle Using Different Sexed Semen Methods. Am. J. Anim. Vet. Sci., 14(2): 111-114. https://doi.org/10.3844/ajavsp.2019.111.114
Lagu BE, Pudjihastuti E, Paputungan U, Adiani S (2020). Kualitas Semen Sapi Pejantan Simmental Dan Limousin Yang Dipelihara Dalam Tipe Kandang Yang Berbeda di Balai Inseminasi Buatan Lembang. Zootec., 40 (2): 439 – 449. https://doi.org/10.35792/zot.40.2.2020.28438
Leung ET, Lee CL, Tian X, Lam KK, Li RH, Ernest, HY, Ng, Yeung WSB, Chiu PC (2022). Simulating nature in sperm selection for assisted reproduction. Nat. Rev. Urol., 19(1): 16-36. https://doi.org/10.1038/s41585-021-00530-9
Medeiros CMO, Forell F, Oliveira ATD, Rodrigues JL (2002). Current status of sperm cryopreservation: why isn’t it better?. Theriogenology, 57(1): 327-344. https://doi.org/10.1016/S0093-691X(01)00674-4
Murphy EM, Murphy CO, Meara C, Dunne G, Eivers B, Lonergan P, Fair S (2017). A comparison of semen diluents on the in vitro and in vivo fertility of liquid bull semen. J. Dairy Sci., 100(2): 1541-https://doi.org/10.3168/jds.2016-11646
National Standardization Agency (2021). SNI Frozen Semen-Part 1: Bulls. BSN.
Nugroho Y, Susilawati T, Wahjuningsih S (2014). Kualitas Semen Sapi Limousin Selama Pendinginan Menggunakan Pengencer Cep-2 Dengan Penambahan Berbagai Konsentrasi Kuning Telur Dan Sari Buah Jambu Biji (Psidium guajava). J. Ternak Tropika, 15 (1): 31 – 42.
Nongbua T, Utta A, Am-In N, Suwimonteerabutr J, Johannisson A, Morrell JM (2020). Effects of season and single layer centrifugation on bull sperm quality in Thailand. Asian-Australasian j. anim. Sci., 33(9); 1411-1420. https://doi.org/10.5713/ajas.19.0624
Pahlevy JR, Ratnani H, Fikri F, Restiadi TI, Saputro AL, Agustono B (2022). The addition of vitamin C in tris–egg yolk extender maintained Sapera goat semen quality in 5 C storage. Ovozoa: J. Anim. Reprod., 11(1): 1-8. https://doi.org/10.20473/ovz.v11i1.2022.1-8
Prakash MA (2014). Sexing of Sperm in Farm Animals: a Mini Review. Adv. Anim. Vet. Sci., 2(4): 226-232. https://doi.org/10.14737/journal.aavs/2014/2.4.226.232
Promthep K, Satitmanwiwat S, Kitiyanant N, Tantiwattanakul P, Jirajaroenrat K, Sitthigripong R, Singhapol C (2016). Practical use of percoll density gradient centrifugation on sperm sex determination in commercial dairy farm in Thailand. Indian J. Anim. Res., 50(3): 310-313. https://doi.org/10.18805/ijar.8427
Pubiandara S, Suharyati S, Hartono M (2016). Pengaruh Penambahan Dosis Rafinosa Dalam Pengencer Sitrat Kuning Telur Terhadap Motilitas, Persentase Hidup Dan Abnormalitas Spermatozoa Sapi Ongole. J. Ilmiah Peternakan Terpadu, 4(4): 292-299.
Rahman MS, Pang MG (2019). New Biological Insights on X and Y Chromosome-Bearing Sperm. Front Cell Dev. Biol., 21(7): 388. https://doi.org/10.3389/fcell.2019.00388
Rasad SD, Setiawan R (2019). Solihati, N. Widyastuti, R. Nugraha, I. Derajat Pemulihan dan Persentase Sperm X dan Y Kambing Peranakan Etawah Setelah Separasi dengan Gradient Percoll. J. Vet., 20 (1): 14 – 19. https://doi.org/10.19087/jveteriner.2019.20.1.14
Rasad SD, Solihati N, Winangun K, Yusrina A, Avicenna F (2020). Effect of Incubation Time During Sperm Sexing Process on Sperm Quality of Pasundan Bull. J. Ilmu Ternak Dan. Vet., 25(3): 112–119. https://doi.org/10.14334/jitv.v25i3.2494
Rosyada ZNA, Ulum MF, Tumbelaka LITA, Purwantara B (2020). Sperm protein markers for Holstein bull fertility at national artificial insemination centers in Indonesia. Vet. World, 13(5): 947–955. https://doi.org/10.14202/vetworld.2020.947-955
Silva JCF, Moura MT, Basto SRL, Oliveira LRS, Caldas ELC, Filho MLS, Oliveira MAL (2017). Use Of Percoll Density Centrifugation for Sperm Sexing In Small Ruminants. Glob. J. Sci. Rront. D. Agrc. Vet., 6 (1): 55 – 59.
Sudarwati H, Natsir MH, Nurgiatiningsih VMA (2019). Statistika dan Rancangan Percobaan (Penetapan dalam Bidang Peternakan). Malang: UB Press. ISBN: 978-602-432-641-8.
Susilawati T (2011). Spermatologi. Malang: UB Press. ISBN: 978-602-8960-04-5.
Susilawati T (2013). Pedoman Inseminasi Buatan Pada Ternak. Malang: UB Press. ISBN: 978-602-203-458-2.
Susilawati T (2014). Sexing Sperm Hasil Penelitian Laboratorium dan Aplikasi pada Sapi dan Kambing. Malang: UB Press. ISBN: 978-602-203-711-8.
Susilawati T, Suyadi MN, Ihsan S, Wahjuningsih N, Isnaini A, Rachmawati APA, Yekti dan P, Utami (2022). Manajemen Reproduksi dan Inseminasi Buatan. Malang: UB Press. ISBN: 978-623-296-624-6.
Tethool AN, Ciptadi G, Wahjuningsih S, Susilawati T (2022). Karakter dan Jenis Pengencer Semen Sapi Bali: Riview. J. Ilmu Peternakan dan. Vet. Tropis, 12 (1): 45 – 57. https://doi.org/10.46549/jipvet.v12i1.214
Utomo B, Rimayanti, Lokapirnasari WP (2021). Molecular Confirmation Test of Sexing Method on Limousin Cattle Sperm with Swim Up Technique. J. Hunan Uni. (Nat. Sci.), 48(4): 187-194.
Vishwanath R, Moreno JF (2018). Review: Semen sexing - current state of the art with emphasis on bovine species. Animal, 12(1): 85-96. https://doi.org/10.1017/S1751731118000496
Watson PF (2000). The causes of reduced fertility with cryopreserved semen. Animal reproduction science, 60: 481-492. https://doi.org/10.1016/S0378-4320(00)00099-3
Yadav SK, Gangwar DK, Singh J, Tikadar CK, Khanna VV, Saini S, Dholpuria S, Palta P, Manik RS, Singh MK, Singla SK (2017). An immunological approach of sperm sexing and different methods for identification of X- and Y-chromosome bearing sperm. Vet. World, 10(5): 498-504. https://doi.org/10.14202/vetworld.2017.498-504
Yekti APA, Rahayu S, Ciptadi G, Susilawati T (2023). The Quality and Proportion of Sperm X and Y in Sexed Frozen Semen Separated with Percoll Density Gradient Centrifugation Method on Friesian Holstein Bull. Adv. Anim. Vet. Sci., 11(3): 371-378. https://doi.org/10.17582/journal.aavs/2023/11.3.371.378
Zhou JL. Chen J, Li H, Li Z, Hong M, Xie S, Chen (2015). The semen pH affects sperm motility and capacitation. PLoS One, 10: 1–15. https://doi.org/10.1371/journal.pone.0132974
To share on other social networks, click on any share button. What are these?





