Supplementation of Snakehead Fish Extracts into Tris Egg-Yolk Extender on Mortality and Motility of Limousin Bull Sperm
Supplementation of Snakehead Fish Extracts into Tris Egg-Yolk Extender on Mortality and Motility of Limousin Bull Sperm
Haris Setiawan1, Aris Winaya1,*, Herwintono Herwintono1, Mulyoto Pangestu2 and Yayuk Kholifah3
1Department of Animal Science, Faculty of Agriculture and Animal Science, University of Muhammadiyah Malang, Jl. Raya Tlogomas 246, Malang 65144, East Java, Indonesia; 2Department of Obstetrics and Gynaecology, Faculty of Medicine, Nursing and Health Sciences, Block F, level 5 Monash Medical Centre 246 Clayton Road, Clayton VIC 3168, Monash University, Australia; 3Singosari National Artificial Insemination Center, Ministry of Agriculture Republic of Indonesia, Ngujung, Toyomarto, Singosari, Malang, East Java, Indonesia.
Abstract | Freezing process reduces sperm quality due to membrane damage. Antioxidants protects the membrane of spermatozoa. This study aimed to observe the effects of Snakehead Fish (Channa striata [Bloch 1793]) Extract (SFE) supplementation in tris Egg-yolk extender on mortality and motility of spermatozoa of Limousin bull (Bos primigenius f. taurus [Linnaeus, 1758]) collected from Singosari Artificial Insemination Center, Indonesia and commercial SFE. Semen from the same bull were collected and processed according to the protocol at Singosari AI Centre. Semen extender were supplemented with SFE, G0 = 0 % SFE, G1 = 2 % SFE, G2 = 4 % SFE, G3 = 6 % SFE and G4 = 8 % SFE. Each of the treatments was repeated three times. The sperm mortality and motility data from SFE treatments were analyzed using Analysis of Variance (ANOVA). The results showed no significant effect on the mortality and motility of spermatozoa (P > 0.05) amongst the treatment. However, there was reduction on mortality and tended to increase on the motility of Limousin bull spermatozoa compared to the non-supplemented extender. Furthermore, the SFE can use in egg-yolk extender with some modification for the efficiency of sperm preservation.
Received | January 16, 2021; Accepted | March 12, 2021; Published | May 21, 2021
*Correspondence | Aris Winaya, Department of Animal Science, Faculty of Agriculture and Animal Science, University of Muhammadiyah Malang, Jl. Raya Tlogomas 246, Malang, East Java, Indonesia; Email: [email protected]
Citation | Setiawan, H., A. Winaya, H. Herwintono, M. Pangestu and Y. Kholifah. 2021. Supplementation of snakehead fish extracts into Tris egg-yolk extender on mortality and motility of limousin bull sperm. Sarhad Journal of Agriculture, 37(Special issue 1): 35-40.
DOI | https://dx.doi.org/10.17582/journal.sja/2021/37.s1.35.40
Keywords | Animal breeding, Artificial insemination, Bovine serum albumin, Channalbumin, Cryopreservation.
Introduction
The Indonesian government has declared that Indonesia will reach meat sufficiency in 2026. Increase population through reproduction is the key role. Also reproductive technology needs to be used widely. Consequently, the utilization of frozen semen through artificial insemination is very important. Artificial insemination (AI) is one of the reproductive biotechnologies which has been scientifically proven to improve the genetic quality of livestock (Patel et al., 2017) and most effective tools for improving the productivity of cattle (Thundathil et al., 2016; Ugur et al., 2019). However, the implementation of AI technology relies on sperm quality (Morell et al., 2018). The quality of sperm will reduce gradually during the freezing process, especially in motility and viability (Khalil et al., 2016). The sperm quality maintenance can be done by adding extenders that provides a suitable element to protect spermatozoa from freezing stress.
The study by Wahana et al. (2014) showed that the addition of Bovine Serum Albumin (BSA) in phosphate egg-yolk extender significantly increased progressive motility of Turkey’s spermatozoa after storage at 4 °C compare to control. BSA is in liquid phase contains high protein (5 g per 500 mL) and 20 different amino acid. BSA also known as extracellular cryo-protectants and supply reserve energy during the semen preservation and cryopreservation.
However, BSA has limited availability since it is imported product, expensive, and difficult to obtain . Albumin protein is also found in some types of fish, although slightly different in structure, such as sharks and stingrays. Albumin in mammal- (HSA and BSA) structures is simple compared to sharks and stingrays, which are more glycoprotein structures (Andreeva, 2010). Asikin and Kusumaningrum (2018) reported that the albumin content of snakehead fish was around 13.95 % to 19.61 %. Previous study reported that BSA could be substituted with other compounds, like Snakehead fish (Channa striata Bloch 1793) extract that also rich in minerals, essential and non-essential amino acids that similar to BSA (Chasanah et al., 2015).
This study aimed to observe the supplementation of snakehead fish extract (SFE) into semen extender in the sperm freezing. Evaluation and observation will be made on motility and mortality.
Materials and Methods
This study was conducted at the National Artificial Insemination Center (BBIB – Balai Besar Inseminasi Buatan) Singosari, Malang, Indonesia. The materials were fresh semen from Limousin bull (Bos primigenius f. taurus [Linnaeus, 1758]), and the commercial product snakehead fish (Channa striata [Bloch 1793]) extract (Pro Albumin©). The treatments are G0 = 0 % SFE (control); G1 = 2 % SFE; G2 = 4 % SFE; G3 = 6 % SFE and G4 = 8 % SFE. Each of SFE (Snakehead Fish Extracts) treatment was repeated three times within the same ejaculate. Macroscopic observations (volume, pH, consistency, color, and smell) were conducted on fresh ejaculate. While microscopic observations (mortality and motility) were made on fresh ejaculate and after freezing.
Macroscopic observation
The macroscopic observation was applied to spermatozoa before the treatments, including volume, pH, consistency, color, and smell.
Microscopic observation
Mortality
The sperm mortality was observed by preparing 100 µL of semen suspension added with 1 µL to 2 µL drops of eosin 1 % on the object-glass and then mix them. Smear of the suspension was made on an object-glass and dried. Observation was made using a microscope (Olympus, CX 21) with 400× magnification. The spermatozoa that absorbed color indicates that the sperm is dead, while the spermatozoa that did not absorbed color is determined as life sperm (Susilawati, 2011). Percentage of mortality was calculated using the equation (1):
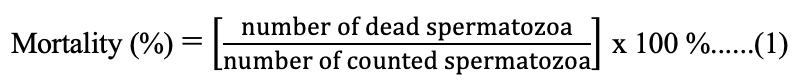
Motility
The sperm motility was observed by dripping 10 µL of the semen on the object-glass and covered using coverslip. Observation was made under a microscope (Olympus, CX 21) with 400× magnification at 37 °C. Only spermatozoa with progressively motility were calculated.
Concentration
The sperm concentration measured using spectrophotometer (Minitube, SDM 5). Fresh semen (3.5 µL) was mixed with 3.5 mL NaCl 0.9 % and the suspension was vortexed (Velp Scientifica, Wizaed Advance 1 500 g) until homogen.
Statistical analysis
The data were analyzed using Analysis of Variance (ANOVA) and followed by Least Significant Difference (LSD) test if the ANOVA has any significant or very significant effect.
Results and Discussion
Fresh semen evaluation
Macroscopic and microscopic assessments of fresh semen were done immediately after collection (Table 1). According to Feradis (2010), the average ejaculate volume of semen bull ranges from 5 mL to 8 mL. In this study, the Limousin semen ejaculate volume was 6.4 mL. This is similar to Mostari et al. (2019) that Limousin breed semen volume around 5.16 mL; Zamuna et al. (2016) was 7.2 mL, and Isnaeni et al. (2019) was 5.48 mL.
Table 1: Fresh semen data from Limousin bull.
|
Parameter |
Information |
|
General condition |
|
|
Name |
Daren |
|
Code |
808101 |
|
Type of cow |
Limousin |
|
Age |
11 year old |
|
Weight (kg) |
960 |
|
Macroscopic |
|
|
Color |
White |
|
Volume (ml) |
6.4 |
|
pH |
6.8 |
|
Consistency |
Aqueous |
|
Microscopic |
|
|
Individual motility ( %) |
54 |
|
Mortality ( %) |
50 |
|
Concentration (106 mL–1) |
538 |
Sperm concentration from fresh ejaculate was 0.538 × 106 mL–1, lower than average concentration from other bull. Ma’ruf (2018) reported average of sperm concentration on a 12-year-old of Limousin bull was 0.718 × 106 mL–1. While Isnaeni et al. (2019) result was 1.2 × 106 mL–1 and Mostari et al. (2019) was 3.62 × 106 mL–1.
The difference quality of the ejaculate are influenced by a different breed of cattle (Rahman et al., 2012), season, breeds, and age or year of semen collection and post thaw sperm quality (D’andre et al., 2017). There is a relationship between sperm concentration and consistency (Sunami et al., 2018).
The semen concentration which is measured by using spectrophotometer also can use to calculate the sperm consistency. By using spectrophotometer we can get the information about the data of analysis, sample number, sperm concentration, volume of ejaculate, spermatozoon concentration in a straw, quantity of the necessary extender, and the number of straws received from the semen (Barszcz et al., 2012).
The white color and milky in fresh ejaculate indicated that the sperm has a normal color (Azizunnesa et al., 2013; Jha et al., 2018). Susilawati (2013) stated that the color of normal ejaculate is white or yellowish-white due to the riboflavin effect. Meanwhile, the reddish-yellow of abnormal sperm caused by the presence of water, pus, and blood.
The mortality of frozen semen
Figure 1 illustrated the SFE supplementation on semen extender. The highest number of mortality was found in G1 (79.67 %), while the lowest sperm mortality was found in G2 (69 %). The mortality on G3 was higher than G4. The analysis of variance showed no significant effect (P > 0.05) on frozen sperm mortality. However, sperm mortality tends to decrease with increasing levels of SFE supplementation (Fig. 1B). This means that the treatment of SFE on extender of frozen semen could reduce the mortality of sperm after thawing.
Freezing process reduces sperm motility. It has been known that the freezing process changes the structure and function of spermatozoa due to membrane protein denaturation, shrinkage, and irreversible membrane collapse (Khalil et al., 2018). Therefore, managing the phospholipid and cryoprotective agents, as well as optimal dilution rate, equilibration, and cooling procedures, can avoid cold shock, reduce ice crystal
formation, and minimize sperm damage. Supplementation of SFE extract as an alternative protein sources in semen extender may not optimal in protecting the sperm plasma membrane during cryopreservation. Heating process during the SFE production, may damage the albumin and amino acid content and reduces its protecting ability. Protein denatures at 50 °C to 60 °C and 10 °C to 15 °C. The denaturation process does not damage the peptide bond in the primary structure but changes its folding shape (Kunsah, 2016).
The motility of frozen semen
The effect of SFE supplementation into the sperm motility after thawing was shown in Figure 2. In G0 treatment, sperm motility was 13.33 % and increased to 15 % at G1 treatment. It was then followed by G2 treatment which reach the peaks at 18.33 %. It decreased to 13.33 % in G3 treatment before then increased again to 18.33 % in G4 treatment. Sperm motility after thawing semen was fluctuated (Fig. 2A), but based on data trend analysis, it showed any incremental trend up to 8 % (Fig. 2B).
Table 2: Analysis of variance.
|
Source |
Sum of squares |
df |
Mean square |
F |
Sig. |
|
Between groups |
191.600 |
4 |
47.900 |
1.126 |
0.398 |
|
Within groups |
425.333 |
10 |
42.533 |
||
|
Total |
616.933 |
14 |
Sig. > 0.05 SFE treatments had no signification effect.
The mortality and motility of semen were observed after fresh Limousin semen treated with SFE supplementation and stored in deep freeze by -180 °C as long as 24 h.
The data of the frozen-thawed sperm after treatments is shown in Table 4. The statistical analysis showed that supplementation of SFE in tris egg-yolk extender has no significant effect (P > 0.05) to sperm motility after thawing (Table 5). However, there was a tendency of increasing motility compared to the control (non-supplemented extender). SFE supplementation up to 8 % was not enough to influence the motility and mortality of frozen sperm after thawing. One of the factors that can reduce motility, viability, and morphological damage in sperm after freezing is reactive oxygen species (ROS) (Wagner et al., 2017). Addition anti-oxidant reduces the ROS reaction. SFE contains antioxidant but it was not enough to influence the motility and mortality of sperm at a significant level.
Table 4: The motility of frozen-thawed sperm after treatments
|
Treatments |
Motility (%) |
|
G0 |
13.33 ± 5.77 |
|
G1 |
15.00 ± 5.00 |
|
G2 |
18.33 ± 5.77 |
|
G3 |
13.33 ± 2.88 |
|
G4 |
18.33 ± 2.88 |
Notes: G0 = 0 % SFE (control); G1 = 2 % SFE; G2 = 4 % SFE; G3 = 6 % SFE and G4 = 8 % SFE
Table 5: Analysis of variance of the sperm motility after thawing
|
Source |
Sum of squares |
df |
Mean square |
F |
Sig. |
|
Between groups |
76.667 |
4 |
19.167 |
0.885ns |
0.507 |
|
Within groups |
216.667 |
10 |
21.667 |
||
|
Total |
293.333 |
14 |
Note: ns = not significant
The albumin content of SFE may not provide sufficient nutrition and give protection of the sperm membrane during cryo-preservation processes. Widodo (2016) stated that protein could be broken down into amino acids by releasing its amino groups through deaminase oxidative or pyruvate and acetyl coenzyme-A formed before entering Crab’s cycle to form energy. Amino acids, such as alanine, cysteine, glycine, threonine, serine, and hydroxyproline, are converted into pyruvate. All amino acids that made to pyruvate can be converted to acetyl coenzyme-A, except five amino acids without through pyruvate forms, namely phenylalanine, tyrosine, tryptophan, lysine, and leucine. These amino acids are not directly utilized by spermatozoa for motility due to its long-chain-break down. The variation of sperm motility might due to age, breed of bull, inadequate nutrition, and poor management (Akhter et al., 2013).
Conclusions and Recommendations
Supplementation of snakehead fish extract (SFE) until 8 % into tris egg yolk extender was not a significant effect on mortality and motility of Limousin bull semen after thawing of stored semen. However, the data analysis showed mortality reduction and an motility improvement. This means that the SFE can use as an alternative for protein source for cryopreserved bull semen with corresponding to substitute BSA protein
Acknowledgements
The authors would like to thanks the Rector of University of Muhammadiyah Malang that gave the opportunity to conduct this research successfully. Extended thank is to the Head of National Artificial Insemination Center (BBIB – Balai Besar Iseminasi Buatan), Ministry of Agriculture, the Republic of Indonesia for the permission of conducting the experiment and use laboratory facilities for spermatozoa assessments.
Novelty Statement
The use of Snakehead fish (Channa striata, [Bloch 1793]) extract as an extender in the frozen semen is still not revealed until recently, especially on Limousin bull (Bos primigenius f. taurus [Linnaeus, 1758]) semen. Thus, this initial study could be as an important information related to the potency of Snakehead fish extract as an alternative substitution of Bovine Serum Albumin (BSA) for extender dilution in freezing semen processes.
Author’s Contribution
HS and AW conceived and designed the study, carried out definition of intellectual content, performed literature search, experimental studies, data acquisition, data analysis, statistical analysis, and manucript preparation. HH, MP and YK elaborated intellectual content, performed literature search, experimental studies, and manuscript editing and review. AW and MP perfomed guarantor. All authors read and approved the final manuscript.
Conflict of interest
The authors declares that there is no conflict of interests regarding the publication of this article.
References
Akhter, M.S., M.A.K. Azad, M.Z. Rahman and A. Ashraf, A. 2013. Study on the quality of semen of different genetic groups of bull from Khulna Region of Bangladesh. Int. J. Pharm. Biomed. Res., 1: 19–23.
Andreeva, A.M. 2010. Structure of fish serum albumins. J. Evol. Biochem. Physiol., 46: 135–144. https://doi.org/10.1134/S0022093010020018
Asikin, A.N. and I. Kusumaningrum. 2018. Albumin profile of snakehead fish (Channa striata) from East Kalimantan, Indonesia. IOP Conf. Ser. Earth Environ. Sci., 144: 1–5. https://doi.org/10.1088/1755-1315/144/1/012035
Azizunnesa, B., F. Zohara, F.Y. Bari and M.G.S. Alam. 2013. Effects of concentrate supplementation on reproductive performances and semen quality of indigenous rams in Bangladesh. J. Embryo Trans., 28: 325–335. https://doi.org/10.12750/JET.2013.28.4.325
Barszcz, K., D. Wiesetek, M. Wąsowicz and M. Kupczyńska. 2012. Bull semen collection and analysis for artificial insemination. J. Agric. Sci., 4: 1–10. https://doi.org/10.5539/jas.v4n3p1
Chasanah, E., M. Nurimala, A.R. Purnamasari and D. Fitriani. 2015. Chemical composition, albumin content and bioactivity of crude protein extract of native and cultured of Channa striata fish. J. Pascapanen Bioteknol. Kelautan Perikanan, 10: 123–132. https://doi.org/10.15578/jpbkp.v10i2.364
D’Andre, H.C., K.D. Rugira, A. Elyse, I. Claire, N. Vincent, M. Celestin, M. Maximillian, M. Tiba, N. Pascal, N.A. Marie, K. Christine and G. Daphrose. 2017. Influence of breed, season and age on quality bovine semen used for artificial insemination. Int. J. Livest. Prod., 8: 72–78. https://doi.org/10.5897/IJLP2017.0368
Feradis. 2010. Reproductive biotechnology on livestock. Penerbit Alfabveta, Bandung, Indonesia.
Jha, P.K., G.S. Alam, A. Al Mansur, T. Islam and F.Y. Bari. 2018. Selection of breeding rams by evaluating semen quality. J. Appl. Anim. Sci., 11: 9–20.
Isnaini, N., S. Wahjuningsih, A. Ma’ruf and D.A. Witayanto. 2019. Effects of age and breed on semen quality of beef bull sires in an Indonesian artificial insemination center. Livest. Res. Rural. Dev., 31: Article 78.
Khalil, W.A., M.A. El-Harairy, A.E.B. Zeidan, M.A.E. Hassan and O. Mohey-Elsaeed. 2018. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. Int. J. Vet. Sci. Med., 6: S49–S56. https://doi.org/10.1016/j.ijvsm.2017.11.001
Kunsah, B. 2016. Protein analysis of Gallus domesticus egg storage at temperature 12 to 15 °C. J. Muhammadiyah Med. Lab. Technol., 2: 54–63.
Ma’ruf, A. 2018. The comparison of fresh semen quality and quantity and recovery rate from Limousin and Bali Bull. Thesis, Brawijaya University, Malang.
Morrell, J.M., A.S. Valeanu, N. Lundeheim and A. Johannisson. 2018. Sperm quality in frozen beef and dairy bull semen. Acta Vet. Scand., 60: s13028. https://doi.org/10.1186/s13028-018-0396-2
Mostari, M.P., M.S. Islam, M.Y.A. Khan and M. Morshed, M. 2019. Evaluation of different crossbreed Beef Bulls based on physical and bio-chemical properties of semen at BLRI cattle research farm. IOSR J. Agric. Vet. Sci., 12: 59–71.
Patel, G.K., N. Haque, M. Madhavatar, A.K. Chaudhari, D.K. Patel, N. Bhalakiya, N. Jamnesha, P. Patel and R. Kumar. 2017. Artificial insemination: A tool to improve livestock productivity. J. Pharmacogn. Phytochem., 1: 307–313.
Rahman, M.A., N.S. Juyena, J.U. Ahmed, R.N. Ferdousy, S. Chakma, M.Z. Rine and A.M.M. Tarif. 2014. Evaluation of semen for breeding soundness of four different breeds of bull used for artificial insemination. IOSR J. Agric. Vet. Sci., 7: 28-34. https://doi.org/10.9790/2380-07922834
Sunami, S., N. Isnaini, and S. Wahjuningsih. 2017. Fresh semen quality and recovery rate of Limousin cow in different season. J. Ternak Tropika, 18: 36–50. https://doi.org/10.21776/ub.jtapro.2017.018.01.6
Susilawati, T. 2011. Spermatology. Universitas Brawijaya Press, Malang, Indonesia.
Susilawati, T. 2013. Artificial insemination guideline on livestock. Universitas Brawijaya Press, Malang, Indonesia.
Thundathil, J.C., A.L. Dance and J.P. Kastelic. 2016. Fertility management of bulls to improve beef cattle productivity. Theriogenology, 86: 397–405. https://doi.org/10.1016/j.theriogenology.2016.04.054
Ugur, M.R., A.S. Abdelrahman, H.C. Evans, A.A. Gilmore, M. Hitit, R.I. Arif, B. Purwantara, A. Kaya and E. Memili, E. 2019. Advances in cryopreservation of bull sperm. Front. Vet. Sci., 6: 268. https://doi.org/10.3389/fvets.2019.00268
Wagner, H., J.W. Cheng and Y.K. Edmund. 2017. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J. Urol., 16: 35-43. https://doi.org/10.1016/j.aju.2017.11.001
Wahana, A.G., M.K. Budiasa and Bebas, W. 2014. The addition of bovine serum albumin to maintain progressive motility of Turkeys spermatozoa storage at temperature 4 °C. Indonesia Med. Vet., 3: 317–322.
Widodo, W. 2016. Poultry nutrition science. Universitas Muhammadiyah Malang Press, Malang, Indonesia.
Zamuna, A.A.K.M., T. Susilawati and G. Ciptadi 2016. Evaluation of different breeds of beef cattle bull’s capacity in producing frozen sperms. Res. Zool., 6: 8–10.
To share on other social networks, click on any share button. What are these?






