Susceptibility Evaluation of Different Legume Seed Genotypes against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) under Laboratory Conditions
Susceptibility Evaluation of Different Legume Seed Genotypes against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) under Laboratory Conditions
Muhammad Faizan1, Muhammad Nasir1, Muhammad Waqar Hassan1*, Ghulam Sarwar2 and Moazzam Jamil3
1Department of Entomology, Faculty of Agriculture and Environment, Islamia University of Bahawalpur, Bahawalpur-63100, Pakistan
2Department of Botany, Faculty of Science, Islamia University of Bahawalpur, Bahawalpur
3Department of Soil Science, Faculty of Agriculture and Environment, Islamia University of Bahawalpur, Bahawalpur-63100, Pakistan
Muhammad Faizan and Muhammad Nasir contributed equally to this work.
ABSTRACT
A study was conducted to assess the susceptibility of three different legumes types namely cow pea (host variety), mung bean (BRM-028, BRM-102 and BRM-106) and chickpea (BWP-white, Sadiq-2021 and Bittle-21) against the seed weevil Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) under laboratory conditions. Biology of beetles was assessed in the form of growth index (GI) which showed maximum GI values in cowpea and mung bean varieties and least in chick pea genotypes and because of this cowpea and mung bean genotypes were classified in susceptible category and chickpea varieties were classified in resistant category. Percent weight loss by C. maculatus was maximum in cow pea and mung bean genotypes and least percent weight loss was recorded in chickpea genotypes. Maximum eggs were laid on mung bean varieties, one chickpea genotype (Bitttle-16) followed by in cowpea in descending order and least eggs were laid in two chickpea varieties (Sadiq-21 and BWP-white). Study of factors which could affect egg output of C. maculatus included seed coat color, texture and see size and among which beetles laid more eggs due to dark color of seeds while there was no strong effect of seed texture and seed size on egg output by C. maculatus. Study of other factors included seed hardness which was maximum in chickpea genotypes followed by cowpea in descending order and least seed hardness was in mung bean genotypes. Correlation of factors with growth index, percent weight loss and mature insect exit hole showed seed hardness was negatively correlated with growth index, percent weight loss and mature insect exit hole area. Percent weight loss was positively correlated with growth index and exit hole area of beetles. Study of factors affecting egg output, growth index, percent weight loss and overall susceptibility in different legume seed types indicate the importance of seed coat color and seed hardness which should be given due care in legume seeds breeding programs for resistance development in new varieties against pulse beetles.
Article Information
Received 29 June 2022
Revised 18 July 2022
Accepted 01 August 2022
Available online 30 September 2022
(early access)
Published 16 October 2023
Authors’ Contribution
MF and MN conducted research. MWH is research supervisor, conceived research plan and wrote paper. GS performed analysis. MJ critically reviewed paper.
Key words
Seed beetles, Screening, Resistance, Pest management, Stored grain insect pests, Cowpea weevil
DOI: https://dx.doi.org/10.17582/journal.pjz/20220629190659
* Corresponding author: [email protected], [email protected]
030-9923/2023/0006-2805 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Pulses play a significant role to address food security and climate change challenges by providing proteins and lysine to complement cereals in human food, by contributing to diversified crop rotations, and by fixing nitrogen in the soil and thus reducing the use of fertilizers and greenhouse gas (GHG) emissions. Therefore, pulses improve food security while these help to mitigate the effects of climate change. In this connection, 68th UN General Assembly declared 2016 the International Year of Pulses (IYP) (Calles et al., 2019). In Pakistan, pulses production is much less than the requirement and the balance is maintained through imports. The reasons for low production and less yield of pulses comprise absence of innovative crop improvement programs and seed distribution system. At present, about 80% of the pulses are cultivated from the farmers own saved seed. Other key factors responsible for low production and less yield are abiotic (drought, heat, salinity) and biotic (weeds, diseases, and insect-pests) stresses, and factors related with soil, climate change, absence of crop-specific farm machinery, post-harvest losses and marketing issues (Ullah et al., 2020).
Pulse beetles in the family Bruchidae are severe pests of stored pulses which damage them to become unfit for human consumption if their attack is not prohibited. Different species belonging to the genus Callosobruchus are commonly found in different parts of the world including in Asian countries like Pakistan. These beetles are also known to attack the seeds in mature pods under field conditions before harvesting of the crop. Therefore, their attack begins in the field and it continues under storage conditions. Inside stores conditions are conducive for their multiplication and further attack. Compared with cereal grains, pulses suffer heavy losses under traditional storage conditions therefore pest control intervention is essential for pulses (Huis, 1991).
Insecticidal application for storage insect pest management is common but this is not without problems. Complications associated with insecticide applications comprise development of insecticide resistance in the targeted insect pests, residues in the commodities, environmental pollution and health risks for the people involved in insecticidal applications. Insect pest management has gained popularity all over the world while an important component of this modern technique is host plant resistance. This phenomenon involves selection and cultivation of cultivars which are resistant to insect pest attack in addition to their other suitable characters.
Resistant or less susceptible varieties are of particular interest for resource poor developing as well as developed grain exporting nations. Elite cultivars having good yields and acceptable storage characteristics are of worth exploitation as additional tool to grain protection strategies. Recently several authors have highlighted the variability in different legume seeds in Indo-Pak region for their preference for development of Callosobruchus spp. (Nisar et al., 2021; Satheesh et al., 2021; Singh and Boopathi, 2022).
Suitability of pulses genotypes for development of C. maculatus has been determined on the basis of growth index which is based on the idea that few offspring would emerge out of resistant genotypes and progeny development would take a longer time in resistant than in susceptible genotypes (Tripathi et al., 2015). Among the seed traits which can contribute to resistance against bruchids include seed color, texture, hardness, size and chemical constituents (War et al., 2017). Hardness has been determined as a primary factor responsible for resistance in seeds against different types of storage insect pests (Throne et al., 2000).
In this context there is always need to explore germplasms which are less suitable for insect growth under storage conditions and identification of the characters which can contribute to insect resistance in these seeds can help develop further resistant seeds by introducing those characters in new varieties.
MATERIALS AND METHODS
Insect source
Callosobruchus maculatus adults were collected from grain market infesting cowpea in Bahawalpur, Pakistan. These were reared on cowpea inside environment stability chamber (Organa International ESC-400L-TCH) with temperature and humidity maintained respectively as 28±2 oC and 65±5% R. H. Identification of adults and sex differentiation between male and female adults was done according to Rees (2004) for pulse beetles. Insects of this species are maintained since last ten generations in the laboratory.
Legume seeds
Three legume seed types were used in this study including one cowpea variety which also served as their rearing host, three mung bean varieties (BRM-28, BRM-102 and BRM-106) and three chickpea varieties (Bittle-16, BWP-white and Sadiq-2021). The seeds were obtained from Regional Agriculture Research Institute, Bahawalpur, Pakistan. Seeds of these seven genotypes were spread in trays in single file for several days under laboratory conditions to uniform the moisture content differences if any between varieties and with the laboratory conditions. Afterwards these seeds were used in experiments. Only healthy seeds (well filled and without any eggs on them) were used in experiments as seeds with any egg shows prior infestation.
Characteristics of legume seed types
Of the seven legume seed types, colors of legumes seeds showed green for mung bean varieties, off-white for cowpea while among the chickpea varieties, Bittle-16 has deep brown color, Sadiq-2021 has brown color and BWP-white has light brown color. Seed coat surfaces for seven seed types showed all three mung bean varieties and cowpea had smooth surface, chickpea variety BWP-white had less wrinkles on seed surface while Bittle-16 and Sadiq-2021 had more wrinkles on seed surface (Figs. 1 and 2).
Experimental set up
Biology of pulse beetles on different legume seeds was studied according to Nwanzs and Horber (1975) with slight modifications. This involved study of development duration from egg to adult stage, survival percentage (out of 15 individual seeds with single egg laid on each seed) and adult life span of emerging adults was studied on individual grains per each treatment. For this purpose, individual 15 seeds were placed in small vials (1 cm height and 1.5 cm diameter). One C. maculatus adult pair of 48 h age from the culture was released in each vial containing one seed for 24 h. Afterwards adult pair was removed from vial and a seed with one egg was left for development study in those vials. If a seed had more than one egg, other eggs were carefully wasted from the seed by gentle pricking with fine needle. In all there were fifteen such seeds with an egg glued on them by the beetles which were incubated for 40 + 5 d to measure the development time from egg to adult stage and percent survival or adult emergence percentage. When adults emerged from these seeds total development time was recorded. After the emergence of adults, adult life span till death was also recorded in all vials. Same procedure was followed for all seven legume seed treatments.
Growth index
Growth Index (GI) was calculated using the following formula (Howe, 1971; Jackai and Singh, 1988).

Where S is per cent adult emergence, while T is mean development time (days).
Egg output
Egg output by the beetles was assessed in another set of experiment in which three pairs of 48 h old C. maculatus adults were let to lay eggs on 15 grains together per vial (100 ml volume capacity) for three days. 15 healthy seeds for all treatments were selected as per criteria described above and were weighed. Microholes were created on the lid of plastic vials for air circulation and to restrict beetle movement out of vials. There were three such vials per each treatment as three replicates. After three days adults were removed from vials while eggs on all seeds were counted inside each vial to measure respective egg output for all treatments. To study the percent weight loss, these seeds with eggs were left in these vials for a period similar for the development study (40+5 days) to measure the final weight of grains after this period. Therefore, percent weight loss was calculated for all treatments by applying the formula.
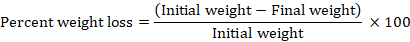
Seed size calibration
In order to measure and compare the individual seed size, the seeds of various varieties were fixed with UHU (glue) in big divisions (625mm2) of an ordinary graph paper. The width of seeds was arranged along one side and length along the other side of same square of graph paper (Figs. 1 and 2). The average size of individual seed can easily be measured by putting the values in the formula (625mm2/total number of seeds in a square) in mm2. The average length and width of each seed was measured by dividing 25 mm with number of seeds along one side of square. A standard foot rod was also kept along with seeds for calibration of divisions on graph paper (ISMA, 2019; Elias and Vieira, 2020).
Calibration of mature insect exit hole
Four seeds (showing adult insect exit holes) were selected randomly from each variety and pasted on graph paper by the help of UHU (transparent adhesive) in order to orientate the exit hole of mature insect for proper measurements and visual comparisons (Fig. 3). Those graph papers were photographed using mobile phone (Apple iPhone-12, 12 MP, f/1.6, 26mm (wide)) by putting a physical foot rod in order to calibrate the photography analysis software later on. Those photographs were transferred to laptop installed with T capture image analysis software (T Capture, 2017). The images were opened with T capture and software was calibrated by using the scale captured with those pictures. The exit holes of mature insects of three seeds for each variety under study were measured in order to get average values. These values were tabulated in Microsoft excel (2017) for further data analyses and graphical representations.
Seed hardness
To determine grain hardness three uniform size grains were taken from each seed type and their hardness was measured based on single kernel measurements. The grains were pressed in hardness tester (Monsanto) present in Department of Pharmacy of Islmia University of Bahawalpur, Pakistan. The pressure required to break or crush the kernels was recorded in Kg (Ram and Sirivastava,1974).
Data analysis
Analysis of data was done using SPSS software for windows (Version 28) by analysis of variance technique (1-Way ANOVA). Development time in days (from egg to adult), adult emergence percentage, insect growth index, adult life span, egg outputs, percent weight loss and seed characters including seed area, seed hardness (Kg) and mature insect exit hole dimensions (radius, area and perimeter) served as dependent variables while seven legume genotypes served as independent variable. Means were separated post hoc by Tukey HSD test at 5% level of probability. In order to know the effect of seed physical characteristics on outputs, a correlation (Pearson) was done between seed area and growth index of C. maculatus, seed area and percent weight loss and seed area and mature insect exit hole area, then seed hardness and growth index of C. maculatus, seed hardness and percent weight loss, seed hardness and mature insect exit hole area, then Growth index and percent weight loss and then hole area and percent weight loss.
RESULTS
Legume seed genotypes affected significantly development time (days) of combine immature stages (egg to adult stage) for C. maculatus. Maximum time from egg to adult stage was taken in chickpea Bittle-16 (38.93 d) followed by in Sadiq-2021 (37.93), 34.73 d for BWP-white in descending order. These days on chickpea varieties were significantly more than the days for development in cowpea and mung bean genotypes (P < 0.01). Percent adult emergence was significantly more in cowpea, mung bean BRM-102, BRM-28 (100%). It was followed by 93.33% in mung bean BRM-106. Percent adult emergence in chickpea genotypes including Bittle-16 (53%), Sadiq-2021 (66%) and BWP-white (73%) was significantly less then adult’s emergence in mung bean or cowpea (100-93%) (P < 0.01). Growth index which is calculated by applying the two factors namely development time and adult emergence percentage was significantly more for cow pea and mung bean varieties (GI ≥ 0.06) compared with the chickpea varieties (GI: 0.043 - 0.053) (P < 0.01). Adult life span on cowpea and mung bean varieties was significantly more than the adult life span on chickpea varieties Bittle-16 and Sadiq-2021 while adult life span was at par for BRM-28 and BWP-white varieties (Table I).
Based on growth index of C. maculatus, susceptibility classification of legume genotypes cowpea and mung bean varieties BRM-102, BRM-106 were classified as susceptible, mung bean genotypes BRM-28 as moderately susceptible while chickpea varieties Bittle-16 and Sadiq-2021 were classified as resistant while BWP-white as moderately resistant (Table II)
Egg counts by C. maculatus were significantly affected under the effect of different genotypes. Most eggs (46.67) were laid on mung bean BRM-28, followed by BRM-106 (44), chickpea variety Bittle-16 (42.67), 32.22 in BRM-102, 30.00 in Cowpea in descending order. Significantly less eggs were laid on Sadiq-2021 (17) and (14) on chickpea BWP-white (14) but statistically these were at par with mung bean BRM-102 and cowpea (P < 0.05). Percent weight loss was calculated when adults’ emergence stopped for all genotypes within the period of 40±5 d. Percent weight loss was affected significantly due to different genotypes of legumes. Maximum weight loss occurred in mung bean BRM-28 (52.52 %), followed by BRM-106 (48.76), cowpea (46.27 %), and mung bean BRM-102 (31.36). Significantly less weight loss occurred in all three chickpea genotypes (11-19 %) (Table III; P < 0.01).
Table I. Effect of different legume types on development period (d) and adult emergence of pulse beetles (n: 15).
|
Treatment |
N |
Development time (Egg to adult) |
Percent adult emergence |
Growth index |
Adult life span |
|
Cowpea (Host variety) |
15 |
31.33 ± 0.27 f |
100.00 ± 0.00 a |
.0627 ± 0.001 a |
8.13 ± 0.42 a |
|
Mung bean (BRM 28) |
15 |
33.27 ± 0.30 d |
100.00 ± 0.00 a |
.0600 ± 0.000 a |
6.80 ± 0.34 b |
|
Mung bean (BRM 102) |
15 |
32.00 ± 0.25 ef |
100.00 ± 0.00 a |
.0607 ± 0.001 a |
8.20 ± 0.29 a |
|
Mung bean (BRM 106) |
15 |
32.80 ± 0.31 de |
93.33 ± 0.00 b |
.0607 ± 0.001 a |
7.47 ± 0.48 ab |
|
Chick pea (Bittle 16) |
15 |
38.93 ± 0.30 a |
53.33 ± 0.00 e |
.0427 ± 0.001 c |
4.40 ± 0.37 c |
|
Chick pea (BWP-White) |
15 |
34.73 ± 0.59 c |
73.33 ± 0.00 c |
.0527 ± 0.001 b |
6.73 ± 0.43 b |
|
Chick pea (Sadiq 2021) |
15 |
37.93 ± 0.30 b |
66.67 ± 0.00 d |
.0500 ± 0.000 b |
4.73 ± 0.44 c |
|
Grand Mean |
105 |
34.42 ± 0.29 |
83.80 ± 1.74 |
.0556 ± 0.001 |
6.63 ± 0.20 |
|
Statistics |
F6, 104: 70.408; P < 0.000 |
F6, 104: 1.732 e + 32; P: 0.000 |
F6, 104: 75.308; P < 0.001 |
F6, 104: 14.343; P < 0.000 |
|
Table II. Categorization of susceptibility of pulse seed types on the basis of growth index of C. maculatus.
|
Treatment |
N |
Category of resistance for GI |
Growth index (GI) |
Resultant category |
|
None |
15 |
0.066-0.070 |
None |
Highly Susceptible |
|
Cowpea (Host variety) |
15 |
0.061-0.065 |
.063 |
Susceptible |
|
Mung bean (BRM 102) |
15 |
0.061-0.065 |
.061 |
Susceptible |
|
Mung bean (BRM 106) |
15 |
0.061-0.065 |
.061 |
Susceptible |
|
Mung bean (BRM 28) |
15 |
0.056-0.060 |
.060 |
Moderately susceptible |
|
Chick pea (BWP-White) |
15 |
0.051-0.055 |
.053 |
Moderately resistant |
|
Chick pea (Sadiq 2021) |
15 |
0.04-0.05 |
.050 |
Resistant |
|
Chick pea (Bittle 16) |
15 |
0.04-0.05 |
.043 |
Resistant |
Table III. Effect of different legume types on egg counts and percent weight loss to legumes by pulse beetles.
|
Treatments |
N |
Egg count |
Percent weight loss |
|
Cowpea (Host variety) |
3 |
30.00 ± 3.51 ab |
46.27 ± 2.56 ab |
|
Mung bean (BRM 28) |
3 |
46.67 ± 4.48 a |
52.52 ± 3.29 a |
|
Mung bean (BRM 102) |
3 |
32.33 ± 1.45 ab |
31.36 ± 3.12 bc |
|
Mung bean (BRM 106) |
3 |
44.00 ± 4.93 a |
48.76 ± 6.28 ab |
|
Chick pea (Bittle 16) |
3 |
42.67 ± 13.16 a |
11.43 ± 7.27 d |
|
Chick pea (BWP-white) |
3 |
14.00 ± 1.15 b |
14.48 ± 10.98 cd |
|
Chick pea (Sadiq 2021) |
3 |
17.00 ± 6.65 b |
19.19 ± 1.31 cd |
|
Total |
21 |
32.38 ± 3.38 |
32.00 ± 4.04 |
|
Statistics |
F 6, 20: 4.123; P: 0.014 |
F 6, 20: 8.686; P: 0.000 |
|
Table IV. Seed physical appearance (color, texture and area), hardness and adult emergence hole size in different legume seeds.
|
Varieties |
N |
Seed coat color |
Seed coat texture |
Seed area (Sq mm) |
Seed hardness (Kg) |
Emergence hole radius (mm) |
Emergence hole area (mm2) |
Emergence hole perimeter (mm) |
|
|
Cowpea (Host variety) |
5 |
Off-white |
Smooth |
52.06 ± 0.00a |
7.90 ± 0.47c |
0.91 ± 0.03ab |
2.58±0.19ab |
5.67 ± 0.21 ab |
|
|
Mung bean (BRM 28) |
5 |
Green |
Less smooth |
12.74 ± 0.00f |
3.73 ± 0.08e |
0.89 ± 0.04ab |
2.50±0.21ab |
5.58 ± 0.24 abc |
|
|
Mung bean (BRM 102) |
5 |
Green |
Less smooth |
17.85 ± 0.00d |
5.17 ± 0.33d |
0.93 ± 0.03a |
2.71±0.18a |
5.82 ± 0.19 a |
|
|
Mung bean (BRM 106) |
5 |
Green |
Smooth |
12.98 ± 0.00e |
3.17 ± 0.09e |
0.84±0.03abc |
2.25±0.19abc |
5.31 ± 0.22 abcd |
|
|
Chick pea (Bittle 16) |
5 |
Dark brown |
Wrinkled |
39.06 ± 0.00b |
9.33 ± 0.33b |
0.74±0.01d |
1.73±0.06d |
4.66 ± 0.07 cd |
|
|
Chick pea (BWP-White) |
5 |
Light brown |
Less wrinkled |
39.06 ± 0.00b |
11.90±0.83a |
0.76±0.04cd |
1.86±0.19cd |
4.80 ± 0.24 bcd |
|
|
Chick pea (Sadiq 2021) |
5 |
Brown |
Wrinkled |
31.25 ± 0.00c |
8.63±0.15bc |
0.81±0.04bcd |
2.07±0.18bcd |
4.48 ± 0.70 d |
|
|
Statistics |
F6, 34: 1.365 E + 34; P. 0.000 |
F6, 34: 61.861; P <.001 |
F6, 34: 4.854; P: 0.002 |
F6, 34: 4.364; P: 0.003 |
F6, 34: 2.707; P: 0.034 |
||||
Table V. Correlation between seed characteristics and outcomes (growth index, weight loss and hole area) caused by C. maculatus adults (Number of cases included 35 for each value).
|
Seed parameter |
Seed hardness |
Percent weight loss |
Growth index |
Exit hole area |
|
Seed area (mm2) |
r: 0.7662 P: 0.000 |
r: -0.3984 P: 0.0178 |
r: -0.3270 P: 0.0552 |
r: -0.2304 P: 0.1829 |
|
Seed Hardness (Kg) |
- |
r: -0.6634 P: 0.0000 |
r: -0.6000 P: 0.0001 |
r: -0.4726 P: 0.0041 |
|
Percent weight loss (%) |
- |
- |
r: 0.7646 P: 0.0000 |
r: 0.4599 P: 0.0054 |
|
Growth index |
- |
- |
- |
r: 0.6775 P:0.0000 |
All r values are result of 35 cases included in correlation analysis.
Seed physical characteristics were assessed to differentiate them among varieties (Table IV, Figs. 1-2). Study of seed external characteristics showed green color in mung bean varieties, dark brown in chickpea variety Bittle-16, brown in Sadiq-2021 and light brown in BWP-white while cowpea had off-white color. Texture of seed coat was smooth for cowpea, less smooth in mung bean varieties while there were wrinkles on surface of chickpea varieties Bittle-16 and Sadiq-2021 and there were less wrinkles on chickpea variety BWP-white. Seed area of mung bean varieties (12-17 Sq mm) was significantly less than chickpea varieties (31-39 Sq mm) and largest area in cowpea (52 Sq mm) (P < 0.01).
Seed hardness was significantly more in chickpea varieties (8-11 Kg) followed by cowpea (7 Kg) and least hardness was in mung bean varieties (3-5 Kg) (P < 0.01). Measurements of mature insects exit holes showed significantly more hole size (radius, area and perimeter) in mung bean and cowpea (2.71-2.25 Sq mm area) than in chickpea varieties (2.07-1.73 Sq mm area) (Table IV, Fig. 3; P < 0.01).
To see the effect of seed physical characteristics on C. maculatus biology and percent weight loss by beetles, correlation was done between seed physical characteristics and outcomes (Table V). Results showed positive correlation between seed size (area) and seed hardness. Seed area had negative correlation with growth index of beetles, emergence hole area and percent weight loss. Seed hardness showed negative correlation with growth index of C. maculatus, emergence hole area and percent weight loss caused by beetles. Percent weight loss on the other had positive correlation with growth index by beetles and mature insect emergence hole size.
DISCUSSION
Egg laying by C. maculatus was significantly affected due to legume seed types. Number of eggs deposited on mung bean varieties BRM-28 (46), BRM-106 (44), chickpea variety Bittle-16 (42), mung bean variety BRM-102 (32), cow pea (30), Sadiq 2021 (17) and BWP-white (14) in descending order. These results can be compared with those of Chakraborty and Mondal (2016) which stated that combination of some morphological characters such as texture, seed size, seed weight, volume of seed and seed color were responsible for ovipositional preference of bruchids to different pulses. They also reported that dark and brown-colored seeds were preferred most for oviposition over white seeds. These results are also in agreement with findings of earlier studies (Chavan et al., 1997; Chen et al., 2019) which reported that brown, black, grey and red colored seeds were more preferred for oviposition than white colored seeds. In our results most eggs were laid on mung bean varieties with green color and chickpea variety Bittle-16 with dark brown color and cowpea which had off-white color. Least eggs were deposited on chickpea varieties BWP-white and Sadiq-2021. In current experiment all mung bean varieties and chickpea variety Bittle-16 were dark colored however more eggs on cowpea with off-white color compared to chickpea varieties Sadiq-2021 and BWP-white which were of brown or light brown color might be because it was a host variety on which these beetles are being nourished for many generations. In our results seed coat texture did not affect egg output because cowpea variety with smooth surface received less egg than Mung bean varieties which had relatively less smooth surface than cowpea and chickpea variety Bittle-16 which received most eggs at par with mung bean varieties had wrinkles on its surface. Seed area also seemed to have no effect on egg output by beetles because most eggs were deposited on small seeded legumes like mung bean compared with cowpea with largest area while chickpea variety Bittle-16 also had less area than cowpea but it received more eggs than cowpea.
C. maculatus biology was affected significantly due to different legume types. Development time from egg to adult stage was shortest in mung bean varieties and cowpea compared with chickpea varieties while adult emergence percentage was also maximum on these varieties compared with chickpea varieties. This resulted in more growth index values for these genotypes than chickpea varieties. Growth index value which is assessed on the basis of development time and adult emergence percentage determines the suitability of a host as food source for development of larvae of species under study (Sulehrie et al., 2007). According to growth index values of legume types and comparison with the susceptibility scale, mung bean varieties and cowpea were classified as susceptible while chickpea varieties were classified as resistant. Similarly, adult life span on these genotypes ranged as minimum on chickpea varieties (4.40-6.73) and longest on mung bean genotypes and cowpea (6.80-8.20) days. Percent weight loss which was calculated at the end of experiment was maximum in case of mung bean varieties and cowpea (31-52 %) while minimum percent weight loss was caused in chickpea varieties (11-19 %). These results can be compared with those of Falke et al. (2021) which reported the susceptibility of different legumes to pulse beetle on the basis of adult population, per cent seed damage and seed weight loss in descending order as moth bean > green gram > cowpea > pigeon pea > chickpea > and black gram respectively.
Study of seed hardness revealed hardness was maximum in chickpea varieties followed by cowpea and least hardness was in mung bean varieties. These results can be compared with those of Falke et al. (2021) which showed maximum hardness in chickpea (18.98 Kg) followed by cowpea (17.56 Kg) and least hardness was in mung bean (12.64 Kg) which was only more than moth bean (8.73 Kg) which had least hardness in their test.
According to correlation analysis seed area had positive correlation with seed hardness. As seeds size increased their hardness increased except cowpea which had highest seed area but its hardness was less than chickpea varieties. Biggest seeds were of cowpea followed by chickpea and smallest seeds were of mung bean varieties. Seed hardness was negatively correlated with growth index, percent weight and exit hole size. These results can be compared with those of Holay et al. (2018) who reported that hardness of seeds affected negatively adult emergence and development period of pulse beetles while grain damage and weight loss caused by pulse beetles also decreased with increase in seed hardness. These results are also in agreement with study reports of Throne et al. (2000) which reported seeds of varieties of wheat, corn and sorghum which exhibited more hardness were comparatively resistant to attack by different primary storage insect pests. Hard seeds of chickpea resulted in less emerged adults which took more time for development and resulted in less growth index for C. maculatus. Cow pea seeds are harder than mung bean varieties but their exit holes size is at par with mung bean varieties. Maximum growth index was observed in cow pea and mung bean varieties and significantly more weight loss occurred in these pulse genotypes. Percent weight loss in seeds was positively correlated with growth index of beetles and their emergence hole size. These results are in agreement with earlier findings (Tripathi et al., 2012; Soumia et al., 2017) which reported correlation between growth index and other growth parameters of pulse beetle on different accessions indicated that growth index had negative relationship with mean developmental period, and significant positive relationship with adult emergence and weigh loss.
CONCLUSION
Based on growth index of C. maculatus on different pulse genotypes, cowpea was classified as susceptible, mung bean two varieties were classified as susceptible and one as moderately susceptible. Chickpea varieties were classified as moderately susceptible to resistant. However, none of genotypes under this study was classified as highly resistant to C. maculatus. Percent weight loss was significantly more in susceptible varieties including Cow pea and Mung bean varieties than in chickpea varieties. Study of physical characteristics revealed egg laying was more on varieties with dark color seeds compared with those of light color seeds. Other physical characteristic which affected growth index was seed hardness. All chickpea genotypes were harder than rest of legume seed types and their hardness resulted in least growth index of C. maculatus. Less hardness in mung bean on the other hand resulted in higher growth index of C. maculatus in Mung bean genotypes. Although cow pea seeds were harder than mung bean genotypes but adult exit holes size in them were at par with mung bean genotypes showing their susceptibility for C. maculatus alongside mung varieties compared with resistant chickpea varieties.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Calles, T., del Castello, R., Baratelli, M., Xipsiti, M. and Navarro, D.K., 2019. The international year of pulses - final report. FAO, Rome. pp. 40. Licence: CC BY-NC-SA 3.0 IGO.
Chakraborty, S. and Mondale, P., 2016. Physico-chemical parameters of pulses affecting the bruchid (C. chinensis L.) infestation. Asian J. Sci. Technol., 7: 2554-2560.
Chavan, P.D., Yeshbir S. and Singh, S.P., 1997. Ovipositional preference of Callosobruchus chinensis for cowpea lines. Indian J. Ent., 59: 295-303.
Chen, Q., Ma, J.J., Yang, H., Gong, J., Gong, X. and Weng, Q., 2019. Seed-coat color affects oviposition in the bean beetle, Callosobruchus maculatus (Coleoptera: Chrysomelidae). Ann. Zool. Fenn., 56: 199-205. https://doi.org/10.5735/086.056.0116
Elias, P.S. and Vieira, D.L.M., 2020. Growth and survival of Campinarana seedlings subject to drought and flooding: Implications for ecological restoration. Pl. Ecol., 221: 459–474. https://doi.org/10.1007/s11258-020-01025-0
Falke, A.D., Patil, S.K. and Sonkamble, M.M., 2021. Studies on host preference of selected pulses to pulse beetle during storage. J. Pharm. Innov., 10: 322-327.
Holay, P.P., Patil, S.K., Kulkarni, S.R. and Lokhande, P.K., 2018. Physio-chemical parameters of pigeonpea seed affecting the infestation of pulse beetle, Callosobruchus maculatus (Fabricius). J. Pharmacogn. Phytochem., 7: 484-487.
Howe, R.W., 1971. A parameter for expressing the suitability of an environment for insect development. J. Stored Prod. Res., 7: 63-65. https://doi.org/10.1016/0022-474X(71)90039-7
Huis, V.A., 1991. Biological methods of bruchid control in the tropics: A review. Int. J. Trop. Insect Sci., 12: 87-102. https://doi.org/10.1017/S1742758400020579
ISMA, 2019. (International Seed Morphology Association). Method for seed size measurement. ISMA Editorial Board for Seed Identification Guide (2019) https://www.idseed.org
Jackai, L.E.N. and Singh, S.R., 1988. Screening techniques for host plant resistance to insect pests of cowpea. Trop. Gr. Legume Bull., 35: 2-18.
Nisar, M.S., Haq, I.U., Ramzan, H., Aljedani, D.M., Qasim, M., Islam, W. and Khan, K.A., 2021. Screening of different legumes for the developmental preference of Callosobruchus maculatus (Bruchidae: Coleoptera). Int. J. Trop Insect. Sci., 41: 3129–3136. https://doi.org/10.1007/s42690-021-00507-6
Nwanze, F.K. and Horber, E., 1975. Laboratory techniques f or screening cowpea for resistance lo Callosobruchus maculatus F. Environ. Ent., 4: 415-419. https://doi.org/10.1093/ee/4.3.415
Nwanze, F.K. and Horber, E., 1976. Seed coats of Cowpeas affect oviposition and larval. development of Callosobruchus maculatus. Environ. Ent., 5: 213-218. https://doi.org/10.1093/ee/5.2.213
Ram, H.H. and Srivastava, J.P., 1974. Inheritance of grain hardness in wheat. Cereal Res. Commun., 2: 129–139. http://www.jstor.org/stable/23777579
Rees, D., 2004. Insects of stored products. CSIRO Publishing, Australia. https://doi.org/10.1071/9780643101128
Satheesh, N.S.J., Lamichaney, A., Bohra, A., Mishra, R.K., Singh, F., Datta, D., Singh, I.P. and Singh, N.P., 2021. Identification of tolerant genotypes against pulse beetle as a source to reduce post-harvest losses in pigeonpea [Cajanus cajan (L.) Millisp.]. Legume Res., 44: 480-485.
Singh, D. and Boopathi, T., 2022. Callosobruchus chinensis (Coleoptera: Chrysomelidae): Biology, life table parameters, host preferences, and evaluation of green gram germplasm for resistance. J. Stored Prod. Res., 95: https://doi.org/10.1016/j.jspr.2021.101912
Soumia, P.S., Srivastava, C., Dikshit, H.K. and Pandi, G.G.P., 2017. Screening for resistance against pulse beetle, Callosobruchus analis (F.) in green gram (Vigna radiata (L.) Wilczek accessions. Proc. natl. Acad. Sci., India Sect. B Biol. Sci., 87: 551–558. https://doi.org/10.1007/s40011-015-0635-5
Sulehrie, M.A.Q., Golob, P., Moss, C. and Tran, B.M.D., 2007. The oviposition and development of a Pakistani biotype of Callosobruchus macultus (F.) (Coleoptera: Bruchidae) on different host legumes. In: Proceedings of the 7th international working conference on stored-product protection (eds. Z. Jin, Q. Liang, Y. Liang, X. Tan and L. Guan), 14-19 October 1998, Beijing, China. Sichuan Publishing House of Science and Technology, Chengdu, China, 1999. pp. 43-50.
T Capture., 2017. Software version 3.9, build 5001 in 2017. Tucsen Photonics Co. Ltd., Fuzhou, Fujian, China
Throne, J.E., Baker, J.E., Messina, F.J., Kramer, K.J. and Howard, J.A., 2000. Varietal resistance. In: Alternatives to pesticides in stored-product IPM (eds. B. Subramanyam and D.W. Hagstrum). Springer, Boston, MA., https://doi.org/10.1007/978-1-4615-4353-4_7
Tripathi, K., Bhalla, S., Prasad, T.V. and Srinivasan, K., 2012. Differential reaction of cowpea (Vigna Unguiculata) genotypes to pulse-beetle (Callosobruchus maculatus). Int. J. Pl. Res., 25: 367–374.
Tripathi, K., Chauhan, S.K., Gore, P.G., Prasad, T.V., Kalyani, S. and Bhalla, S., 2015. Screening of cowpea [Vigna unguiculata (L.) Walp.] accessions against pulse beetle, Callosobruchus chinensis (L.). Legume Res., 38: 675-680. https://doi.org/10.18805/lr.v38i5.5949
Ullah, A., Shah, T.M. and Farooq, M., 2020. Pulses production in Pakistan: Status, constraints and opportunities. Int. J. Pl. Prod., 14: 549–569. https://doi.org/10.1007/s42106-020-00108-2
War, A.R., Murugesan, S., Boddepalli, V.N., Srinivasan, R. and Nair, R.M., 2017. Mechanism of resistance in mungbean [Vigna radiata (L.) R. Wilczek var. radiata] to Bruchids, Callosobruchus spp. (Coleoptera: Bruchidae). Front. Pl. Sci., 8: 1031. https://doi.org/10.3389/fpls.2017.01031
To share on other social networks, click on any share button. What are these?









