Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences. 2(1): 63 – 66Evaluation of BcL–2 and PCNA Expression and Mitotic Index in Spontaneous Canine Tumours
J. Venkataravanappa Lokesh, Nitin. P. Kurade*, M.U. Shivakumar, Anil Kumar Sharma, Swapan K. Maiti
*Corresponding author: npkurade@yahoo.co.in
ARTICLE CITATION: Lokesh JV, Kurade NP, Shivakumar MU, Sharma AK and Maiti SK (2014). Evaluation of BcL–2 and PCNA expression and mitotic index in spontaneous canine tumours. Adv. Anim. Vet. Sci. 2 (1): 63 – 66.
Received:2013–10–21, Revised:2013–12-16, Accepted:2013-12-17
The electronic version of this article is the complete one and can be found online at (http://dx.doi.org/10.14737/journal.aavs/2.1.63.66) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
In the present study, the pathology of spontaneous canine tumours, their classifications, the expression of the BcL–2 as well as expression of the proliferation indices (mitotic and PCNA index) were investigated. A total of 27 canine tumour cases were studied and samples were collected in 10% buffered formalin. Organs wise distribution of tumour cases showed highest involvement of mammary gland (48.15%) followed by skin (29.62%), testicles (11.11%) and bone, muscle and eye (3.7% each). Histologically, 4 (14.81%) tumours were diagnosed as benign and 23 (85.19%) as malignant. Mitotic index was significantly low in benign tumours (0.3±0.00/hpf) compared to the malignant tumours (2.24±0.48/hpf). The highest Proliferating Cell Nuclear Antigen (PCNA) index was found to be in canine transmissible venereal tumour (92 %) followed by squamous cell carcinoma (86.6 %), anal adenocarcinoma (68.3±0.00), solid carcinoma (51.8±1.68), fibrosarcoma (31.5±1.32), carcinosarcoma (30.75±1.25) and tubulopapillary carcinoma (29.2±0.08). The BcL–2 immunostaining was membranous and cytoplasmic. It was mainly observed in non–proliferating and peripheral neoplastic cells. Out of 27 total tumours, only 9 showed the positive reaction to BcL–2 immunostaining. Benign tumour, fibroma showed high BcL–2 index (19.3±0.00) compared to malignant tumours (8.8±1.15). BcL–2 expression was noticed in both benign and malignant tumours and was prominent in comparatively less aggressive tumours showing mild PCNA immunostaining. Further studies, with more number of tumours, may elucidate the role of BcL–2 in progression of canine tumours.
Introduction
Cancer is the leading cause of mortality in pets (especially in dogs and cats), up to 41% in dogs (Bonnett et al 2005) and second most in humans (Jemal et al 2008). BcL–2 protein, a founding member of BcL–2 family of apoptosis regulator is encoded by BcL–2 gene. It is an oncoprotein product that plays a role in apoptosis by blocking programmed cell death and also discourages the promotion of cellular proliferation (Hockenberry et al 1990). BcL–2 protein is localized on the mitochondrial membrane, nuclear envelope, and endoplasmic reticulum (Tsujimoto et al 1985; Lu et al 1998). It is hypothesized that BcL–2 prevents apoptosis by stabilizing the mitochondrial membrane and inhibiting the release of cytochrome–c, which is the key element for activation of apoptotic pathway. BcL–2 also binds to the pro–apoptotic factor Apaf–1 and inhibits activation of caspase cascade. BcL–2 protein is found at inappropriately high levels in more than half of all cancers in humans and canines (Reed, 1995). There are very few reports for BcL–2 expression in spontaneous canine tumours (Yung et al 2006). In present study we have evaluated the expression of BcL–2 in comparison with expression of PCNA and mitotic index in spontaneous canine tumours.
MATERIALS AND METHODS
A total of 31 spontaneously occurring canine tumour cases presented at Referral Veterinary Polyclinic, IVRI, Izatnagar, during the period from September 2010 to June 2011 were included in present study. A total of 27 dogs underwent surgery and tumour samples were collected for pathological evaluation. Clinical and reproductive histories for all the cases were obtained from their owners. Detailed gross findings of individual tumours were recorded. Representative tumour samples were collected in 10% neutral buffered formalin for histological and immunohistochemical studies. One to 2 mm thick tissue samples were dehydrated in graded alcohol and cleared in xylene and embedded in paraffin blocks. Four–micrometer thick serial sections were subjected for Haematoxylin and Eosin staining (Luna, 1972). The stained sections of spontaneous canine tumours were evaluated and classified according to the WHO criteria (Goldschmidt et al 1998; Misdrop et al 1999).
Immunolabelling for PCNA and Bcl–2 antigen was performed on dewaxed sections by the streptavidin–biotin–peroxidase (ABC) complex method. The tissue sections were deparaffinised by heat at 60o C for 10 min, followed by three washes in xylene. After gradual hydration through graded alcohol, the slides were incubated in citrate buffer (pH 6.0) for two cycles of 5 min in microwave oven for antigen retrieval. The sections were allowed to cool for 20 min and then rinsed with phosphate buffered saline (PBS). The sections were treated 15 min each with 3% H2O2 in absolute methanol to inhibit endogenous peroxidase activity. Nonspecific antibody binding was reduced by incubating the sections with normal goat serum for 40 min. Mouse anti–BcL–2, clone 100 (Invitrogen; product no. 08 1193) and Mouse MAb PC 10 (Sigma Aldrich; product no. P8825) were used as primary antibodies specific against BcL–2 and PCNA antigens, respectively and the sections were incubated at 4oC overnight. The slides were washed with PBS and then incubated with anti–mouse biotin–labelled secondary antibody followed by streptavidin–biotin–peroxidase for 30 min each at room temperature. The immunoprecipitate was visualized by treating with 3,3– diaminobenzidine tetrahydrochloride (Sigma) and counterstaining with Mayer’s haematoxylin. Normal canine lymph node was kept as positive control for BcL–2. For negative control only 1% BSA was applied. The proportion of immunolabelled cells was scored as follows: – , < 5%; +, 5–19%; ++, 20–59%; +++, >60%. These scores were regarded as negative, slight, moderate and high, respectively. The IHC “labeling index” was defined as the ratio of labelled tumour cells to the total number of cells examined in 4–5 high–power fields (c. 1000 cells), expressed as a percentage.
Mitotic Index
Following Yu et al., (1992) method, mitotic figures were counted in 30 random high power (400x) fields (1 hpf = 0.159mm2) in H&E stained sections. Tumor sections having marked cellular activity were selected (field selection method) for counting mitotic figures. Mitotic index was recorded as mitotic figures per 1000 cells.
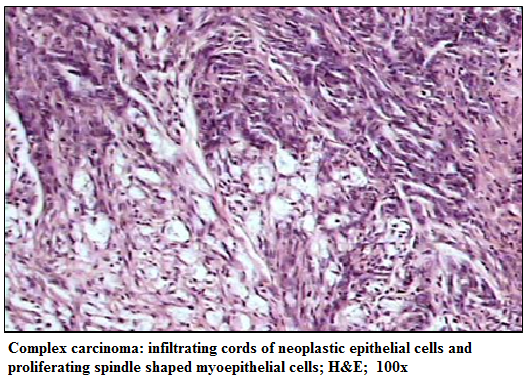
Figure 1:Complex carcinoma: infiltrating cords of neoplastic epithelial cells and proliferating spindle shaped myoepithelial cells; H&E; 100x
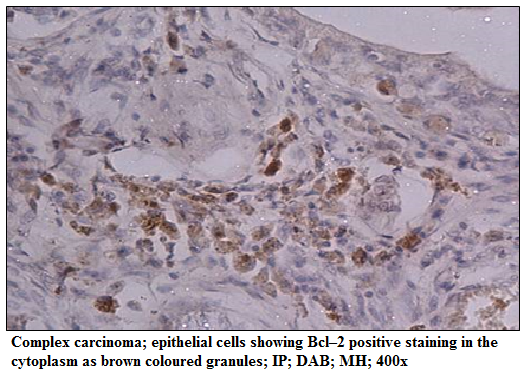
Figure 2: Complex carcinoma; epithelial cells showing Bcl–2 positive staining in the cytoplasm as brown coloured granules; IP; DAB; MH; 400x
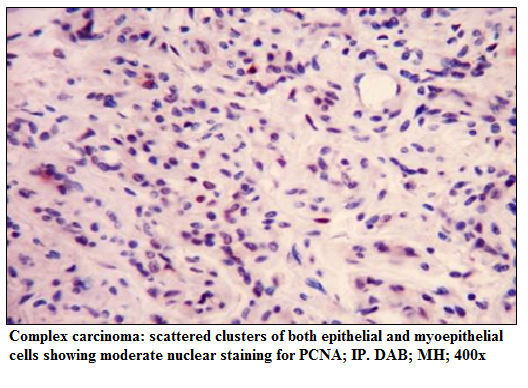
Figure 3: scattered clusters of both epithelial and myoepithelial cells showing moderate nuclear staining for PCNA; IP. DAB; MH; 400x
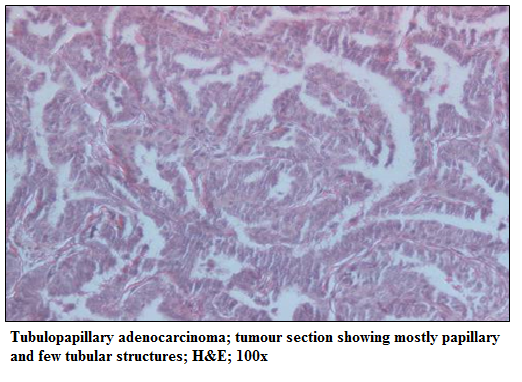
Figure 4: Tubulopapillary adenocarcinoma; tumour section showing mostly papillary and few tubular structures; H&E; 100x
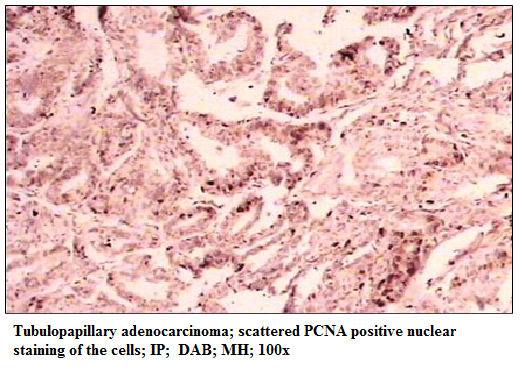
Figure 5: Tubulopapillary adenocarcinoma; scattered PCNA positive nuclear staining of the cells; IP; DAB; MH; 100x
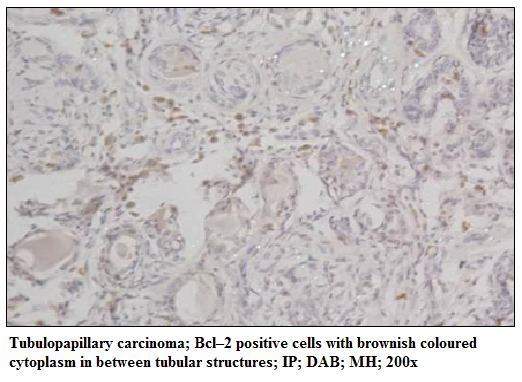
Figure 6: Tubulopapillary carcinoma; Bcl–2 positive cells with brownish coloured cytoplasm in between tubular structures; IP; DAB; MH; 200x
RESULTS
Age wise analysis showed maximum tumour cases in 6–8 year followed by 8–10 year, 4–6 years and more than 10 year with 37.04 %, 29.62%, 18.51% and 14.81%, respectively (Table 1). The mean age of tumour presentation was 8.4 years. Females (55.56%) had comparatively more tumours than in males (44.44%) (Table 1). All the tumour bearing animals were intact. Breed wise analysis showed highest tumour cases in Spitz and German shepherd (25.93%) (7/27) followed by Non–descript (ND), Labrador retriever, Rampur hound and Rottweiler with 22.22% (6/27), 14.81% (4/27), 7.41% (2/27) and 3.74% (1/27), respectively (Table 1). Organ wise distribution showed highest involvement of mammary gland (48.15%) followed by skin (29.62%), testicles (11.11%) and bone, muscle and eye (3.7% each). Mammary gland tumours more frequently encountered in left chain (53.33%) compared to right chain (46.67%). Grossly 40.74% (11/27) tumours were ulcerated compared to 59.26% (16/27) non ulcerated tumours. Histologically, 4 (14.81%) tumours were diagnosed as benign and 23 (85.19%) as malignant. There were 8 skin tumours, which include, benign tumour; Fibroma – (1 case, 3.7%), malignant tumours; Fibrosarcoma (3 cases, 11.11%), Malignant trichoepithelioma (1 case, 3.7%), Squamous cell carcinoma (1 case, 3.7%), Anal gland adenocarcinoma (1 case, 3.7%) and Canine transmissible venereal tumour (CTVT) – (1 case, 3.7%). Testicular tumours; benign; Sertoli cell tumour (1 case, 3.7%) and Granular cell tumour (1 case, 3.7%); malignant; Seminona (1 case, 3.7%). Muscle tumour; Rhabdomyosarcoma (1 case, 3.7%), Bone tumour; Osteosarcoma (1 case, 3.7%) and Eye tumour; Dermoid cyst (1 case, 3.7%). Histologically diagnosed mammary tumours were malignant in nature, which includes; Tubulopapillary adenocarcinoma (2 cases, 7.41%), Solid carcinoma (5 cases, 13.51%), Complex adenocarcinoma, (2 cases, 7.41%), Fibrosarcoma (1 case, 3.7%), Carcinosarcoma (2 cases, 7.41%). (Table 2)
In general mitotic index was significantly low in benign tumours (0.3±0.00/hpf) compared to the malignant tumours (2.24±0.48/hpf). Among malignant tumours, squamous cell carcinoma (5.6±0.00), canine transmissible venereal tumour (4.6±0.00), osteosarcoma (3.6±0.00) and solid mammary carcinoma (3.13±0.00) had high mitotic counts compared to cacinosarcoma (2.5±0.00), complex carcinoma (1.55±0.02) and tubulopapillary adenocarcinoma (1.35±0.00) (Table 2). PCNA immunostaining was nuclear and varied in intensity from 25% to 92%. Benign tumours; sertoli cell tumour and granular cell tumour did not show positive reaction to PCNA immunostaining. The highest PCNA index was found to be in canine transmissible venereal tumour (92%) followed by squamous cell carcinoma (86.6 %), anal adenocarcinoma (68.3±0.00), solid carcinoma (51.8±1.68), fibrosarcoma (31.5±1.32), carcinosarcoma (30.75±1.25) and tubulopapillary carcinoma (29.2±0.08) (Table 2). BcL–2 immunostaining was membranous and cytoplasmic. Out of 27 total tumours only 9 showed the positive reaction to BcL–2 immunostaining. Benign tumour, fibroma showed significantly high (p < 0.05) BcL–2 index (19.3±0.00) compared to malignant tumours (8.8±1.15) (Table 7). Among malignant tumours, BcL–2 index was more in tubulopapillary adenocarcinoma (11.78%), followed by carcinosarcoma (9.8±2.7), fibrosarcoma (9.4±1.3), solid carcinoma (8.25±1.25) and least in complex carcinoma (4.8±0.00) (Table 2).
DISCUSSION
Bcl–2 protein inhibits apoptotic cell death and also discourages the promotion of cellular proliferation (Hockenberry et al 1990). In present study, BcL–2 immunostaining was membranous and cytoplasmic. BcL–2 staining observed more in epithelial cells compared to the mesenchymal cells and the staining was observed mainly in the resting cells, which were not stained for PCNA, indicating more expression in non dividing neoplastic cells. Tumours having high PCNA index (>60) did not show any BcL–2 reactivity. Out of 27 total tumours only 9 tumours (33.34%) showed the positive reaction to BcL–2 immunostaining. This is very less compared to 61.5% (24/39) of MGTs (Yung et al 2006) and 17/21 (81%) breast tumours (Planas–Silva et al 2007). In the present study BcL–2 expression was noticed in both benign and malignant tumours. Benign tumour, fibroma showed high BcL–2 index (19.3±0.00) compared to malignant tumours (8.8±1.15) and similar finding also reported by Yung et al (2006) with 11.5±10.9 in benign and 8.6±11.6 in malignant tumours. Among malignant tumours, BcL–2 index was more in tubulopapillary adenocarcinoma (11.78%), followed by carcinosarcoma (9.8±2.7), fibrosarcoma (9.4±1.3), solid carcinoma (8.25±1.25) and least in complex carcinoma (4.8±0.00). these findings were in accordance with Yung et al (2006); tubulopapillary carcinoma (12.8±10.1) solid carcinomas (10±14.2), mixed mammary tumours (6.2±5.2) and complex carcinoma (0.2±0.00). The complex carcinomas showed more expression in the present study compared to the studies of Yung et al (2006). In present study, BcL–2 staining was not observed in osteosarcoma, rhabdomyosarcoma, seminoma and sertoli cell tumour, squamous cell carcinoma, canine transmissible venereal tumour, anal adenocarcinomas and granular cell tumours. Yung et al (2006) also reported BcL–2 negative in osteosacoma and squamous cell carcinoma. Tellechea et al (1993) and Swanson et al (1998) also reported BcL–2 negativity in squamous cell carcinoma. Fernandez–Flores and Valerdiz (2010) reported expression of BcL–2 in cutaneous granular cell tumours (grade +) in humans. In our study, no reactivity was observed which may be due to difference in the clone of primary antibody used. There are no reports of BcL 2 immunostaining for sertoli cell tumour, seminoma and canine transmissible venereal tumour. In present study, it appears that BcL–2 expression was prominent in comparatively less aggressive tumours showing mild PCNA immunostaining. Further studies are required to elucidate the role of BcL–2 in progression of canine tumours.
ACKNOWLEDGEMENTS
We are grateful to the Director, Indian Veterinary Research Institute for the facilities provided. We also thank Head, Division of Medicine and Coordinator, Polyclinic for help rendered.
REFERENCES
Bonnett B, Egenvall NA, Hedhammar A and Olson P (2005). Mortality in over 350000 insured Swedish dogs from 1995−2000: I. Breed, gender, age and cause specific rates, Acta Vet. Scand., 46(3); 105−120.
http://dx.doi.org/10.1186/1751-0147-46-105
PMid:16261924 PMCid:PMC1624819
Fernandez–Flores A and Valerdiz S (2010). Study of the immunoexpression of bcl–2 by a cutaneous granular cell tumour. Acta Dermatoven, 19; 11–19.
Goldschmidt MH, Dunstan RW, Stannard AA, Von Tscharner C, Walder EJ and Yager JA (1998). Histological classification of Epithelial and melanocytic tumours of the skin of domestic animals: WHO International Classification of Tumours of Domestic Animals. Publisher American Registry of Pathology, US.
Hockenbery D, Nunez G, Milliman C, Schreiber RD and Korsmeyer SJ (1990). Bcl–2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature, 348; 334.
http://dx.doi.org/10.1038/348334a0
PMid:2250705
Jemal A, Seigel R, Ward E, Hao Y, Xu J, Murray T and Thun MJ (2008). Cancer statistics 2008. CA: A Cancer J. Clin., 58; 71–96.
http://dx.doi.org/10.3322/CA.2007.0010
PMid:18287387
Lu J, Jiang C, Mitrenga T, Cutter G and Thompson HJ (1998). Pathogenetic characterization of 1–methyl–1–nitrosourea–induced mammary carcinomas in the rat. Carcinogenesis, 19(1); 223–227.
http://dx.doi.org/10.1093/carcin/19.1.223
Luna G (1968). Manual of Histological Staining Method of the Armed Forces Institute of Pathology. 3rd ed., McGraw–Hill Book Company, New York.
Misdorp W, Else RW, Hellmen E and Lipscomb TP (1999). Histological Classification of Mammary Tumours of the Dog and the Cat. In: WHO International Classification of Tumours of Domestic Animals. Publisher American registry of Pathology, US.
Moulton JE (1990). Tumours of the mammary gland. In: Tumours in Domestic Animals, 3rd Edit., J. E. Moulton, Ed., University of California Press, Berkeley, pp. 518–552.
Nowak Marcin, Madej A Janusz, Ciaputa Rafal. and Poradowski Dominik (2010). Manifestation of tumours in domestic animals in lower Silesia in 2005–2008. Bull. Vet. Inst. Pulawy, 54; 229–236.
Planas–Silva Maricarmen D, Richard D Bruggeman, Ronald T Grenko and Smith JS (2007). Overexpression of c–Myc and Bcl–2 during progression and distant metastasis of hormone–treated breast cancer. Exptl. and Molecular Path. 82: 85–90.
http://dx.doi.org/10.1016/j.yexmp.2006.09.001
PMid:17046747
Reed JC (1995). Regulation of apoptosis by bcl–2 family proteins and its role in cancer and chemo resistance. Curr. Opin. Oncol., 7; 541–546.
http://dx.doi.org/10.1097/00001622-199511000-00012
PMid:8547403
Tsujimoto Y, Finger LR, Yunis J, Nowell PC and Croce CM (1985). Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science, 226; 1097.
http://dx.doi.org/10.1126/science.6093263
Yung WY, Liu Ch, Chang CJ, Lee CC, Chang KJ and Lin CT (2006). Proliferative activity, apoptosis and expression of oestrogen receptor and Bcl–2 oncoprotein in canine mammary gland tumours. J. Comp. Path., 134, 70–79.
http://dx.doi.org/10.1016/j.jcpa.2005.07.002
PMid:16423573






